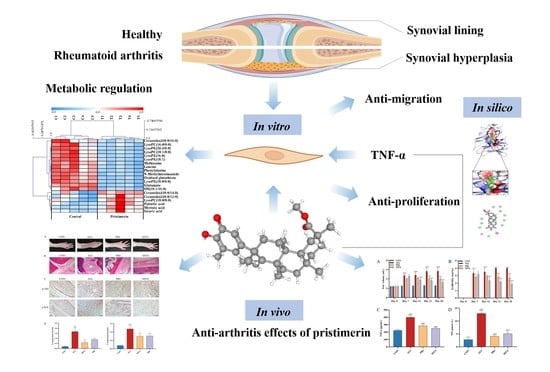
UPLC-LTQ-Orbitrap-Based Cell Metabolomics and Network Pharmacology Analysis to Reveal the Potential Antiarthritic Effects of Pristimerin: In Vitro, In Silico and In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effects of Pristimerin on TNF-α-Stimulated MH7A Cells
2.1.1. Cell Culture
2.1.2. Cell Viability Assay
2.1.3. Transwell and Wound Healing Migration Assay
2.1.4. Cell Metabolomics
- Sample preparation:
- UPLC-LTQ-Orbitrap-MS analysis:
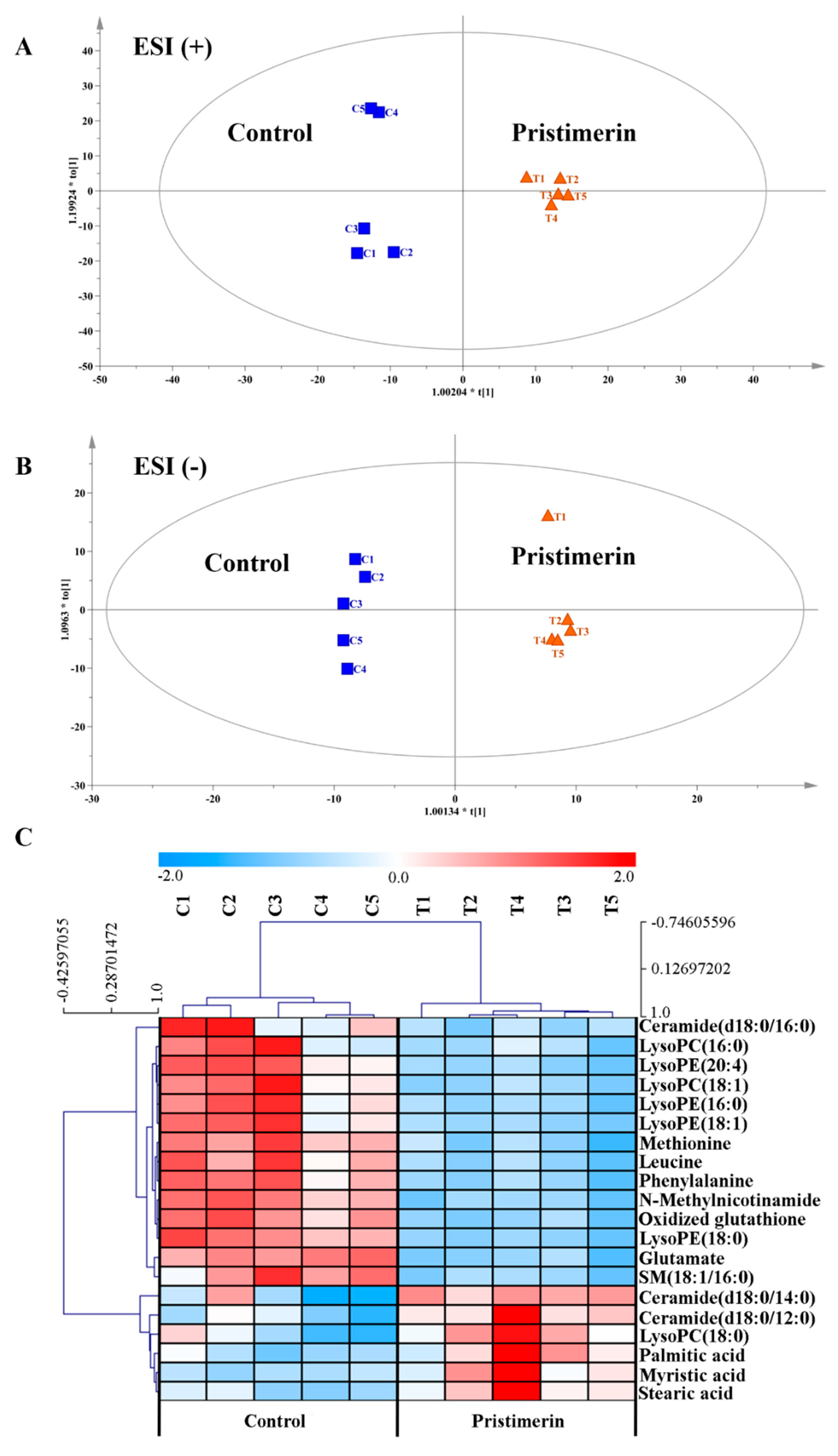
- Data processing and potential biomarker screening:
2.1.5. Network Pharmacology Analysis
2.1.6. Western Blotting Assay
2.1.7. Molecular Docking Study
2.2. Effects of Pristimerin on Adjuvant-Induced Arthritis (AIA) Rats
2.2.1. AIA Rat Model and Experimental Protocols
2.2.2. Histopathological Examination and Immunohistochemical Examination of Ankle Joints
2.3. Statistical Analysis
3. Results
3.1. Effects of Pristimerin on TNF-α-Stimulated MH7A Cells
3.1.1. Inhibition of Cell Viability and Migration by Pristimerin in TNF-α-Stimulated MH7A Cells
3.1.2. Metabolic Regulation by Pristimerin in TNF-α-Stimulated MH7A Cells
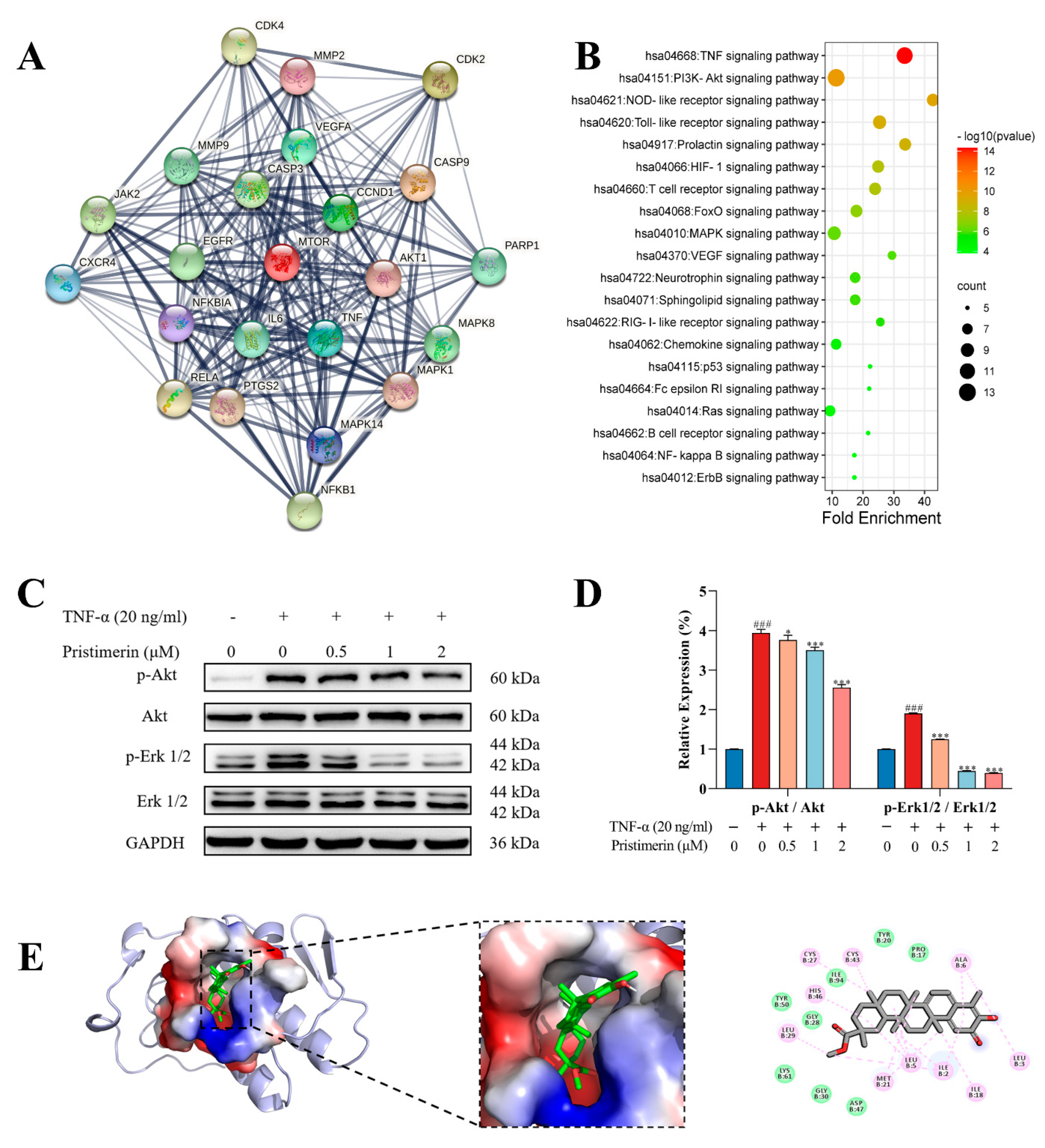
3.1.3. Potential Targets of Pristimerin for RA Treatment
3.1.4. Inhibition of p-Akt and p-Erk Expressions by Pristimerin in TNF-α-Stimulated MH7A Cells
3.1.5. Interaction of Pristimerin with Potential Targets Regarding Metabolic Pathways
3.2. Therapeutic Effects of Pristimerin on AIA Rats
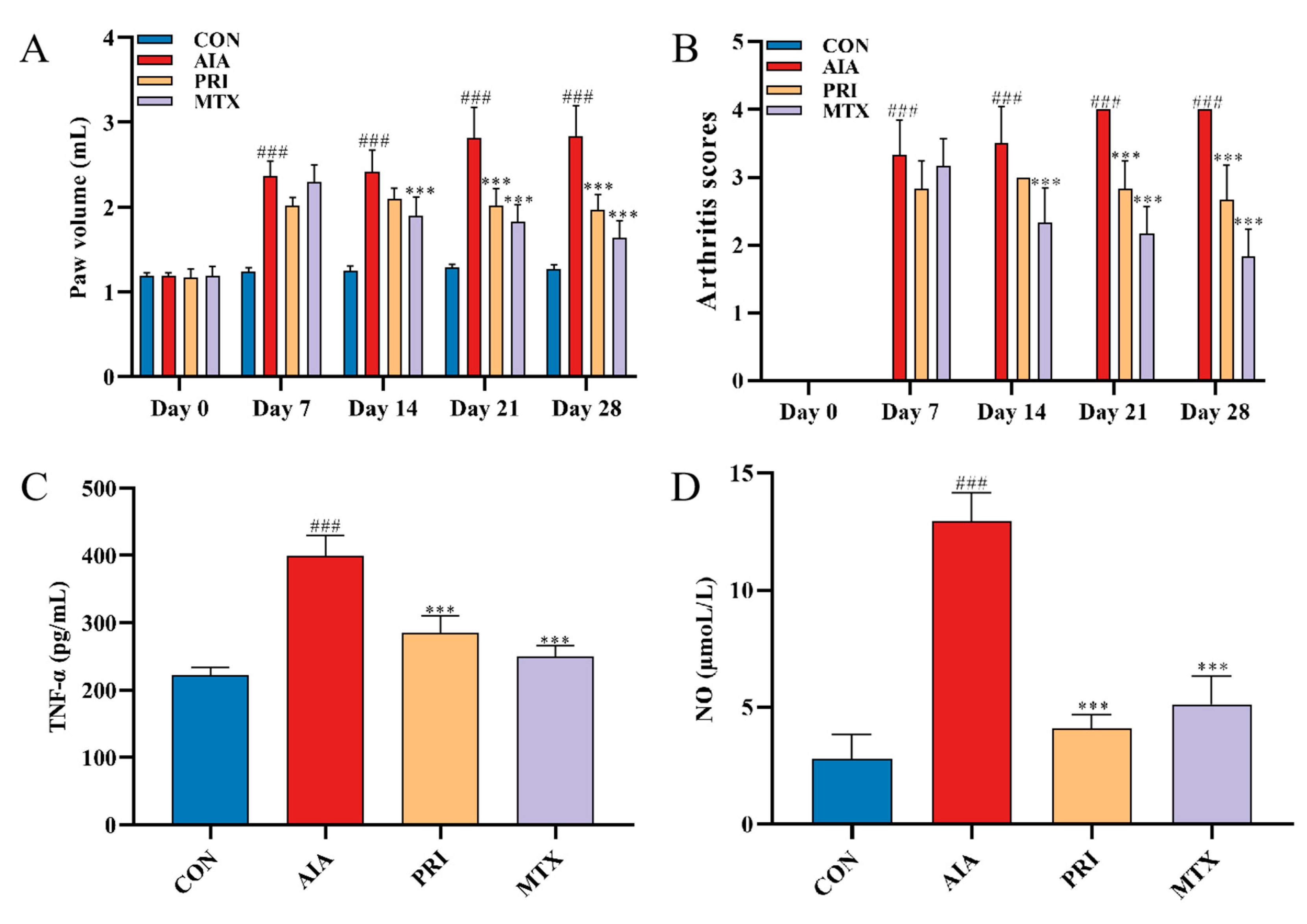
3.2.1. Effects of Pristimerin on Degree of Paw Swelling, Arthritis Scores and Serum Levels of TNF-α and NO
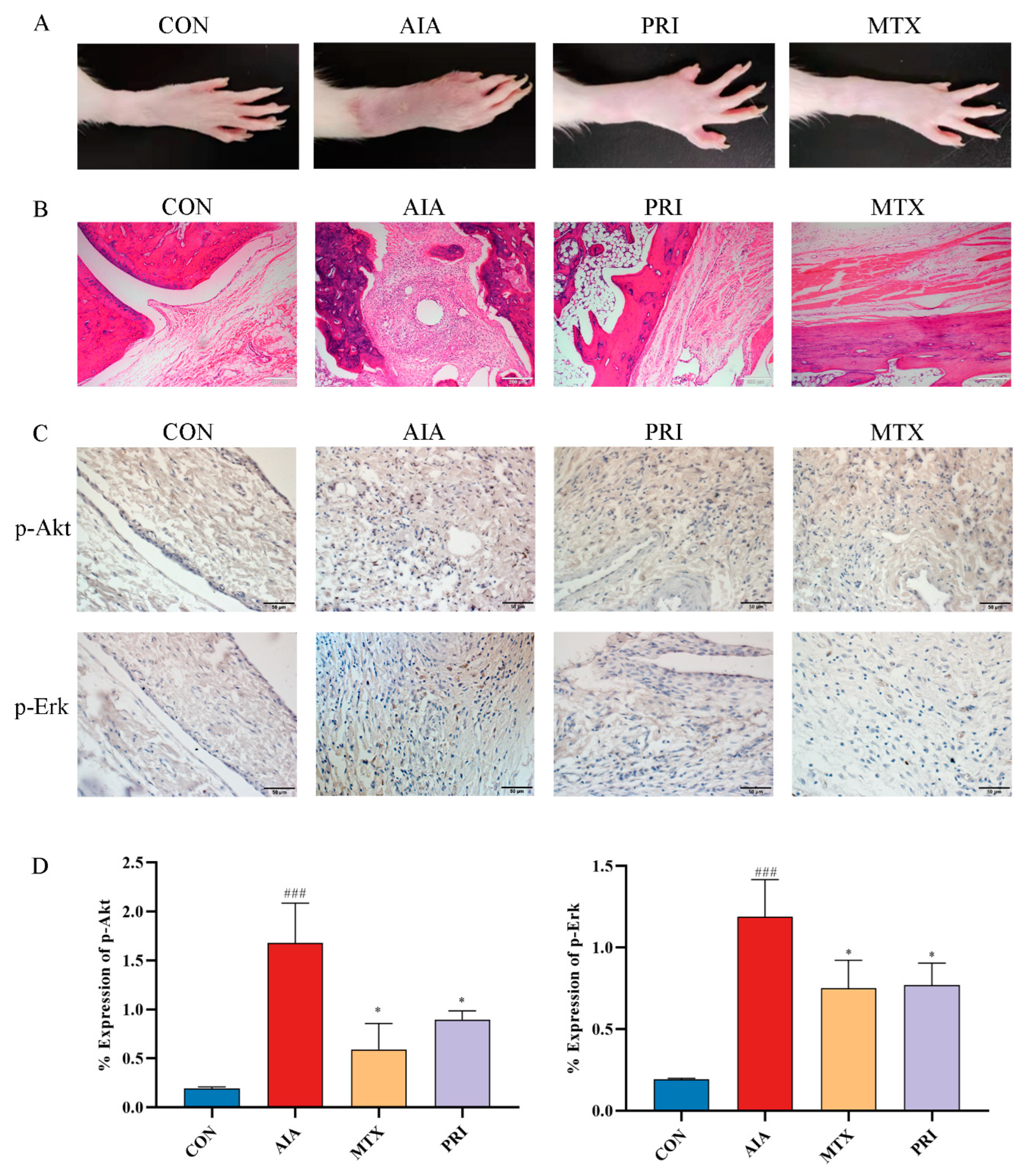
3.2.2. Effects of Pristimerin on Histopathological Changes of Ankle Joints and Immunochemical Expression of p-Akt and p-Erk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 2021, 41, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.F.; Garcia-Carbonell, R.; Whisenant, K.D.; Guma, M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Nizer, W.; Ferraz, A.C.; Moraes, T.F.S.; Lima, W.G.; Santos, J.P.D.; Duarte, L.P.; Ferreira, J.M.S.; de Brito Magalhães, C.L.; Vieira-Filho, S.A.; Andrade, A.; et al. Pristimerin isolated from Salacia crassifolia (Mart. Ex. Schult.) G. Don. (Celastraceae) roots as a potential antibacterial agent against Staphylococcus aureus. J. Ethnopharmacol. 2021, 266, 113423. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, Y.; Zhao, Y.; Liu, Y.; Tao, L. Pristimerin synergizes with gemcitabine through abrogating Chk1/53BP1-mediated DNA repair in pancreatic cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 147, 111919. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Yan, Y.Y.; Sun, H.M.; Liu, Y.; Su, C.Y.; Chen, H.B.; Zhang, J.Y. Anti-Cancer Effects of Pristimerin and the Mechanisms: A Critical Review. Front. Pharmacol. 2019, 10, 746. [Google Scholar] [CrossRef]
- Deng, Q.; Bai, S.; Gao, W.; Tong, L. Pristimerin inhibits angiogenesis in adjuvant-induced arthritic rats by suppressing VEGFR2 signaling pathways. Int. Immunopharmacol. 2015, 29, 302–313. [Google Scholar] [CrossRef]
- Yousef, B.A.; Hassan, H.M.; Zhang, L.Y.; Jiang, Z.Z. Anticancer Potential and Molecular Targets of Pristimerin: A Mini—Review. Curr. Cancer Drug Targets 2017, 17, 100–108. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Zhong, J.; Bi, Y.; Liu, Y.; Ren, Z.; Li, X.; Jia, J.; Yu, M.; Yu, X. Pristimerin induces apoptosis and autophagy via activation of ROS/ASK1/JNK pathway in human breast cancer in vitro and in vivo. Cell Death Discov. 2019, 5, 125. [Google Scholar] [CrossRef]
- Tong, L.; Nanjundaiah, S.M.; Venkatesha, S.H.; Astry, B.; Yu, H.; Moudgil, K.D. Pristimerin, a naturally occurring triterpenoid, protects against autoimmune arthritis by modulating the cellular and soluble immune mediators of inflammation and tissue damage. Clin. Immunol. 2014, 155, 220–230. [Google Scholar] [CrossRef]
- Bai, S.; Deng, W.G.Q.; Lin, X.; Zheng, J.; Chen, Y.; Tong, L. Pristimerin Inhibits Adjuvant Arthritis Fibroblast Like Synoviocytes Cell Proliferation through Cell Cycle Arrest and Induction of Apoptosisa. Indian J. Pharm. Sci. 2020, 82, 819–827. [Google Scholar] [CrossRef]
- Achudhan, D.; Liu, S.C.; Lin, Y.Y.; Huang, C.C.; Tsai, C.H.; Ko, C.Y.; Chiang, I.P.; Kuo, Y.H.; Tang, C.H. Antcin K Inhibits TNF-α, IL-1β and IL-8 Expression in Synovial Fibroblasts and Ameliorates Cartilage Degradation: Implications for the Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 790925. [Google Scholar] [CrossRef]
- Jing, M.; Yang, J.; Zhang, L.; Liu, J.; Xu, S.; Wang, M.; Zhang, L.; Sun, Y.; Yan, W.; Hou, G.; et al. Celastrol inhibits rheumatoid arthritis through the ROS-NF-κB-NLRP3 inflammasome axis. Int. Immunopharmacol. 2021, 98, 107879. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Zierer, J.; Valdes, A.M.; Spector, T.D. Mixing omics: Combining genetics and metabolomics to study rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.H.R.; Mendes, L.F.; de Carvalho, F.V.; de Paula, E.; Duarte, I.F. Comparative Metabolomics Study of the Impact of Articaine and Lidocaine on the Metabolism of SH-SY5Y Neuronal Cells. Metabolites 2022, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, X.; Guo, R.; Jing, W.; Lu, H. Cell Metabolomics Reveals Berberine-Inhibited Pancreatic Cancer Cell Viability and Metastasis by Regulating Citrate Metabolism. J. Proteome Res. 2020, 19, 3825–3836. [Google Scholar] [CrossRef]
- Müller-Ladner, U.; Ospelt, C.; Gay, S.; Distler, O.; Pap, T. Cells of the synovium in rheumatoid arthritis. Synovial fibroblasts. Arthritis Res. Ther. 2007, 9, 223. [Google Scholar] [CrossRef]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef]
- Li, T.; Wei, Z.; Kuang, H. UPLC-orbitrap-MS-based metabolic profiling of HaCaT cells exposed to withanolides extracted from Datura metel.L: Insights from an untargeted metabolomics. J. Pharm. Biomed. Anal. 2021, 199, 113979. [Google Scholar] [CrossRef]
- Lv, M.; Chen, J.; Gao, Y.; Sun, J.; Zhang, Q.; Zhang, M.; Xu, F.; Zhang, Z. Metabolomics based on liquid chromatography with mass spectrometry reveals the chemical difference in the stems and roots derived from Ephedra sinica. J. Sep. Sci. 2015, 38, 3331–3336. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Japonicone A and related dimeric sesquiterpene lactones: Molecular targets and mechanisms of anticancer activity. Off. J. Eur. Histamine Res. Soc. 2022, 71, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Rigsby, R.E.; Parker, A.B. Using the PyMOL application to reinforce visual understanding of protein structure. Biochem. Mol. Biol. Educ. 2016, 44, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Qu, F.; Sreeharsha, N.; Sharma, S.; Mishra, A.; Gubbiyappa, S.K. Antiarthritic effect of chitosan nanoparticle loaded with embelin against adjuvant-induced arthritis in Wistar rats. IUBMB Life 2020, 72, 1054–1064. [Google Scholar] [CrossRef]
- Moases Ghaffary, E.; Abtahi Froushani, S.M. Immunomodulatory benefits of mesenchymal stem cells treated with Caffeine in adjuvant-induced arthritis. Life Sci. 2020, 246, 117420. [Google Scholar] [CrossRef]
- Luo, S.; Li, H.; Liu, J.; Xie, X.; Wan, Z.; Wang, Y.; Zhao, Z.; Wu, X.; Li, X.; Yang, M.; et al. Andrographolide ameliorates oxidative stress, inflammation and histological outcome in complete Freund’s adjuvant-induced arthritis. Chem. Biol. Interact. 2020, 319, 108984. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Kedia, A.K.; Mohansundaram, K.; Goyal, M.; Ravindran, V. Safety of long-term use of four common conventional disease modifying anti-rheumatic drugs in rheumatoid arthritis. J. R. Coll. Physicians Edinb. 2021, 51, 237–245. [Google Scholar] [CrossRef]
- Singh, J.A. Treatment Guidelines in Rheumatoid Arthritis. Rheum. Dis. Clin. North Am. 2022, 48, 679–689. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D. Rheumatoid arthritis therapy reappraisal: Strategies, opportunities and challenges. Nat. Rev. Rheumatol. 2015, 11, 276–289. [Google Scholar] [CrossRef]
- Yi, O.; Lin, Y.; Hu, M.; Hu, S.; Su, Z.; Liao, J.; Liu, B.; Liu, L.; Cai, X. Lactate metabolism in rheumatoid arthritis: Pathogenic mechanisms and therapeutic intervention with natural compounds. Phytomed. Int. J. Phytother. Phytopharm. 2022, 100, 154048. [Google Scholar] [CrossRef]
- Chen, R.Z.; Yang, F.; Zhang, M.; Sun, Z.G.; Zhang, N. Cellular and Molecular Mechanisms of Pristimerin in Cancer Therapy: Recent Advances. Front. Oncol. 2021, 11, 671548. [Google Scholar] [CrossRef]
- Renda, G.; Gökkaya, İ.; Şöhretoğlu, D. Immunomodulatory properties of triterpenes. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2022, 21, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lyu, L.; Zhu, H.; Huang, X.; Xu, W.; Xu, W.; Feng, Y.; Fan, Y. Serum Metabolome Mediates the Antiobesity Effect of Celastrol-Induced Gut Microbial Alterations. J. Proteome Res. 2021, 20, 4840–4851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Chen, Y.T.; Feng, X.; Li, L.Y.; Song, K.W.; Sun, Y.P.; Zhang, G.H.; Zhang, L.T. A comprehensive study of celastrol metabolism in vivo and in vitro using ultra-high-performance liquid chromatography coupled with hybrid triple quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2022, 45, 1222–1239. [Google Scholar] [CrossRef]
- Abu Bakar, M.H.; Nor Shahril, N.S.; Mohamad Khalid, M.S.F.; Mohammad, S.; Shariff, K.A.; Karunakaran, T.; Mohd Salleh, R.; Mohamad Rosdi, M.N. Celastrol alleviates high-fat diet-induced obesity via enhanced muscle glucose utilization and mitochondrial oxidative metabolism-mediated upregulation of pyruvate dehydrogenase complex. Toxicol. Appl. Pharmacol. 2022, 449, 116099. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Q.; Xiao, X.; Yang, R.; Hu, D.; Zhu, X.; Gonzalez, F.J.; Li, F. Modulation of Lipid Metabolism by Celastrol. J. Proteome Res. 2019, 18, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Park, J.; Yun, W.; Kang, P.J.; Son, D.; Jang, J.; Kim, I.Y.; You, S. Inhibitory effect of celastrol on adipogenic differentiation of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2018, 507, 236–241. [Google Scholar] [CrossRef]
- Yang, L.; Liu, R.; Fang, Y.; He, J. Anti-inflammatory effect of phenylpropanoids from Dendropanax dentiger in TNF-α-induced MH7A cells via inhibition of NF-κB, Akt and JNK signaling pathways. Int. Immunopharmacol. 2021, 94, 107463. [Google Scholar] [CrossRef]
- Li, T.P.; Zhang, A.H.; Miao, J.H.; Sun, H.; Yan, G.L.; Wu, F.F.; Wang, X.J. Applications and potential mechanisms of herbal medicines for rheumatoid arthritis treatment: A systematic review. RSC Adv. 2019, 9, 26381–26392. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, S.; Dong, Q. Nobiletin suppresses IL-21/IL-21 receptor-mediated inflammatory response in MH7A fibroblast-like synoviocytes (FLS): An implication in rheumatoid arthritis. Eur. J. Pharmacol. 2020, 875, 172939. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Guo, J.; Yao, X.; Jing, X.; Guo, F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr. Cartil. 2020, 28, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Liang, Q.; Wan, X.; Wang, Z.; Qian, Y.; Xiang, J.; Luo, Z.; Ni, T.; Jiang, W.; Wang, W.; et al. Metabolomics and molecular docking-directed antiarthritic study of the ethyl acetate extract from Celastrus orbiculatus Thunb. J. Ethnopharmacol. 2022, 294, 115369. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Schiller, J.; Wagner, U.; Häntzschel, H.; Arnold, K. The phosphatidylcholine/lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: Investigations by 31P NMR and MALDI-TOF MS. Clin. Biochem. 2005, 38, 925–933. [Google Scholar] [CrossRef]
- McGrath, C.M.; Young, S.P. Lipid and Metabolic Changes in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2015, 17, 57. [Google Scholar] [CrossRef]
- Falconer, J.; Murphy, A.N.; Young, S.P.; Clark, A.R.; Tiziani, S.; Guma, M.; Buckley, C.D. Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 984–999. [Google Scholar] [CrossRef]
- Friday, S.C.; Fox, D.A. Phospholipase D enzymes facilitate IL-17- and TNFα-induced expression of proinflammatory genes in rheumatoid arthritis synovial fibroblasts (RASF). Immunol. Lett. 2016, 174, 9–18. [Google Scholar] [CrossRef]
- Zhou, G.; Lu, J.; Xu, T.; Lu, Y.; Chen, W.; Wang, J.; Ke, M.; Shen, Q.; Zhu, Y.; Shan, J. Clinical lipidomics analysis reveals biomarkers of lipid peroxidation in serum from patients with rheumatoid arthritis. Microchem. J. 2021, 169, 106607. [Google Scholar] [CrossRef]
- Alisik, M.; Alisik, T.; Nacir, B.; Neselioglu, S.; Genc-Isik, I.; Koyuncu, P.; Erel, O. Erythrocyte reduced/oxidized glutathione and serum thiol/disulfide homeostasis in patients with rheumatoid arthritis. Clin. Biochem. 2021, 94, 56–61. [Google Scholar] [CrossRef]
- Phull, A.R.; Nasir, B.; Haq, I.U.; Kim, S.J. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem. Biol. Interact. 2018, 281, 121–136. [Google Scholar] [CrossRef]
- Pan, T.; Han, D.; Xu, Y.; Peng, W.; Bai, L.; Zhou, X.; He, H. LC-MS Based Metabolomics Study of the Effects of EGCG on A549 Cells. Front. Pharmacol. 2021, 12, 732716. [Google Scholar] [CrossRef]
- Arra, M.; Swarnkar, G.; Adapala, N.S.; Naqvi, S.K.; Cai, L.; Rai, M.F.; Singamaneni, S.; Mbalaviele, G.; Brophy, R.; Abu-Amer, Y. Glutamine metabolism modulates chondrocyte inflammatory response. Elife 2022, 11, e80725. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.K.; Kim, S.; Hwang, J.; Kim, J.; Kim, K.H.; Cha, H.S. GC/TOF-MS-based metabolomic profiling in cultured fibroblast-like synoviocytes from rheumatoid arthritis. Jt. Bone Spine 2016, 83, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Che, N.; Xu, L.; Zhang, Q.; Wang, Q.; Tan, W.; Zhang, M. LC-MS-based serum metabolomics reveals a distinctive signature in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 1493–1502. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, M.; Liang, Q.; Luo, Z.; Han, B.; Ni, T.; Wang, Y.; Tao, L.; Lyu, W.; Xiang, J.; Liu, Y. UPLC-LTQ-Orbitrap-Based Cell Metabolomics and Network Pharmacology Analysis to Reveal the Potential Antiarthritic Effects of Pristimerin: In Vitro, In Silico and In Vivo Study. Metabolites 2022, 12, 839. https://doi.org/10.3390/metabo12090839
Lv M, Liang Q, Luo Z, Han B, Ni T, Wang Y, Tao L, Lyu W, Xiang J, Liu Y. UPLC-LTQ-Orbitrap-Based Cell Metabolomics and Network Pharmacology Analysis to Reveal the Potential Antiarthritic Effects of Pristimerin: In Vitro, In Silico and In Vivo Study. Metabolites. 2022; 12(9):839. https://doi.org/10.3390/metabo12090839
Chicago/Turabian StyleLv, Mengying, Qiaoling Liang, Zhaoyong Luo, Bo Han, Tengyang Ni, Yang Wang, Li Tao, Weiting Lyu, Jie Xiang, and Yanqing Liu. 2022. "UPLC-LTQ-Orbitrap-Based Cell Metabolomics and Network Pharmacology Analysis to Reveal the Potential Antiarthritic Effects of Pristimerin: In Vitro, In Silico and In Vivo Study" Metabolites 12, no. 9: 839. https://doi.org/10.3390/metabo12090839
APA StyleLv, M., Liang, Q., Luo, Z., Han, B., Ni, T., Wang, Y., Tao, L., Lyu, W., Xiang, J., & Liu, Y. (2022). UPLC-LTQ-Orbitrap-Based Cell Metabolomics and Network Pharmacology Analysis to Reveal the Potential Antiarthritic Effects of Pristimerin: In Vitro, In Silico and In Vivo Study. Metabolites, 12(9), 839. https://doi.org/10.3390/metabo12090839