Iron Metabolism in the Disorders of Heme Biosynthesis
Abstract
1. Introduction
2. Role of Iron in the Biosynthesis of Heme
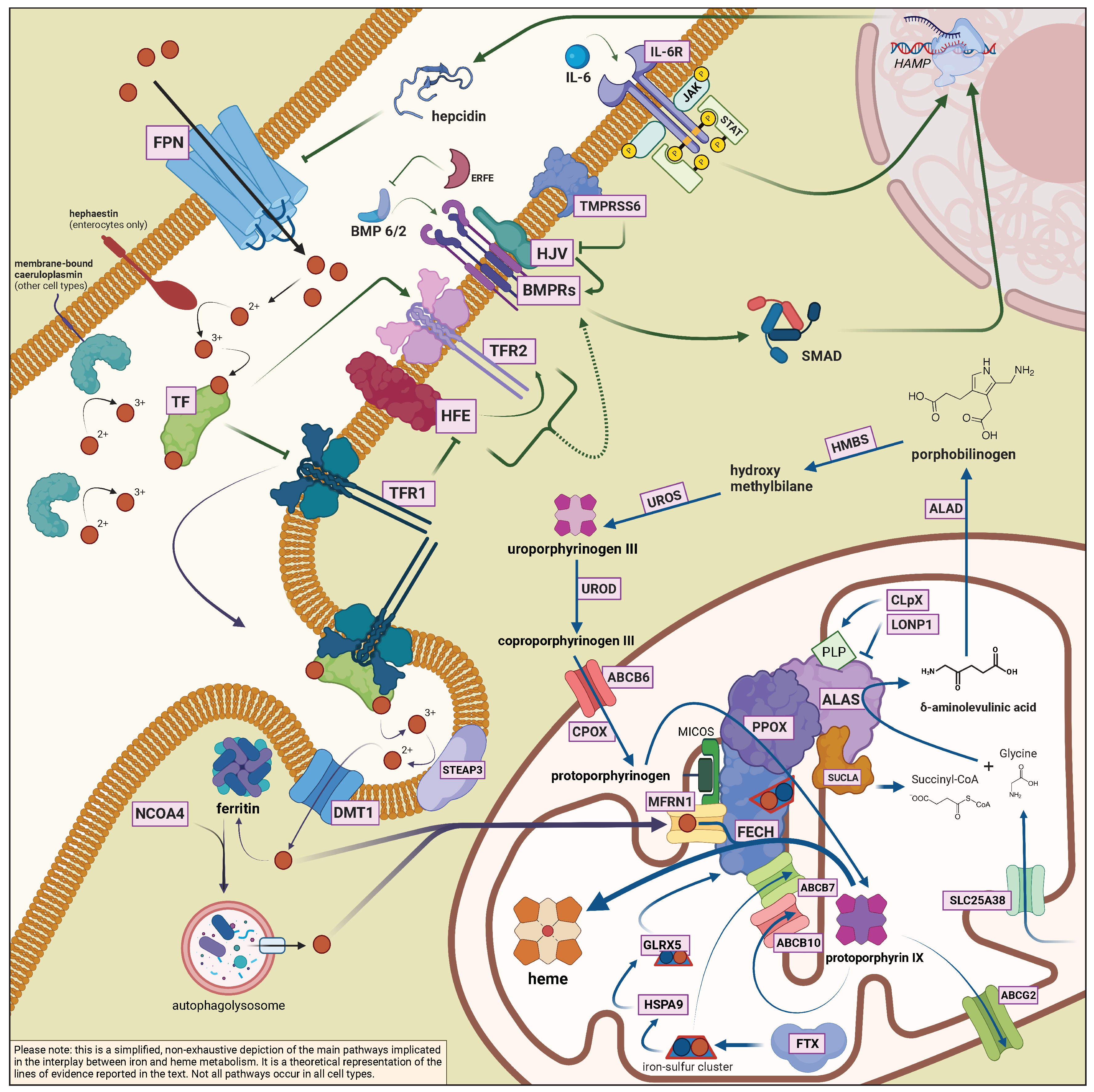
2.1. General Aspects of Iron Metabolism in Mammals
2.2. The Interplay between Heme Biosynthesis and Iron
| Protein | Gene | Function | Associated Diseases |
|---|---|---|---|
| Bone morphogenetic protein 2 | BMP2 | Ligand of the BMP-SMAD signaling pathway regulating hepcidin expression in response to iron | |
| Bone morphogenetic protein 6 | BMP6 | Ligand of the BMP-SMAD signaling pathway regulating hepcidin expression in response to iron | BMP6-associated iron-overload |
| Caeruloplasmin | CP | Soluble/membrane-bound copper-carrying ferroxidase | Aceruloplasminemia |
| Divalent metal transporter 1 | DMT1 or SLC11A2 | Ferrous iron importer | DMT1 deficiency |
| Duodenal cytochrome B | DCYTB | Reduces dietary ferric iron to ferrous form at the apical border of enterocytes | |
| Erythroferrone | ERFE | Hepcidin inhibitor, produced by the bone marrow in response to erythropoietin | |
| Ferritin heavy chain | FTH1 | Subunit of ferritin, with ferroxidase activity | FTH-related iron-overload |
| Ferritin light chain | FTL | Subunit of ferritin, with iron storage properties | Hyperferritinemia-cataract syndrome Hereditary benign hyperferritinemia Neuroferritinopathy L-ferritin deficiency [66] |
| Ferroportin | SLC40A1 | Ferrous iron exporter | Ferroportin disease (loss-of-function)SLC40A1-related hemochromatosis (gain-of-function) |
| Frataxin | FXN | Iron carrier, participates in iron-sulfur cluster biogenesis | Friedreich’s ataxia |
| Haephastin | HEPH | Membrane-bound ferroxidase | |
| Hemojuvelin | HJV or HFE2 | BMP co-receptor, involved in the iron-sensing pathway which regulates hepcidin | HJV-related hemochromatosis |
| Hepcidin | HAMP | Iron regulating hormone; internalises ferroportin | HAMP-related hemochromatosis |
| Human homeostatic iron regulator protein | HFE | Protein involved in the iron-sensing pathway which regulates hepcidin | HFE-related HH |
| IRE binding protein 1 | IREB1 or IRP1 or ACO1 | Iron-sensing regulator of translation; aconitase activity in the presence of iron | |
| IRE binding protein 2 | IREB2 or IRP2 | Iron-sensing regulator of translation | IRP2-related protoporphyria |
| Matriptase | TMPRSS6 | Cleaves membrane-bound HJV; negative regulator of hepcidin in response to iron deficiency | Iron-deficient iron refractory anaemia (IRIDA) |
| Mitoferrin-1 | MFRN1 | Intramitochondrial iron carrier | |
| Nuclear receptor coactivator 4 | NCOA4 | Delivers ferritin to autophagolysosomes (ferritinophagy) | |
| Six-transmembrane epithelial antigen of prostate 3 | STEAP3 | Membrane-bound metalloreductase | Sideroblastic anaemia with primary hypogonadism |
| Transferrin | TF | Ferric iron carrier | Hypo/Atransferrinemia |
| Transferrin receptor | TFRC | Receptor for endocytosis-mediated iron uptake; one of the plasma iron sensors | TFRC-related combined immunodeficiency [67] |
| Transferrin receptor 2 | TFR2 | Protein involved in the iron-sensing pathway which regulates hepcidin | TFR2-related hemochromatosis |
| Protein | Gene | Function | Associated Diseases |
|---|---|---|---|
| -aminolaevulinate dehydratase | ALAD | Dehydrates ALA to yield PBG | ALAD-deficiency (Doss) porphyria |
| -aminolaevulinate synthase 1 | ALAS1 | Condenses glycine and succinyl-CoA to yield ALA | |
| -aminolaevulinate synthase 2 | ALAS2 | Condenses glycine and succinyl-CoA to yield ALA (erythroid-specific isoform) | X-linked congenital sideroblastic anaemia (loss-of-function) X-linked erythropoietic protoporphyria (gain-of-function) |
| ATP-binding cassette super-family B member 6 | ABCB6 | Imports porphyrins into mithocondria | Phenotype modifier in porphyrias [68] |
| ATP-binding cassette super-family B member 7 | ABCB7 | Mitochondrial [Fe-S] cluster exporter | X-linked sideroblastic anaemia with ataxia |
| ATP-binding cassette super-family B member 10 | ABCB10 | Mitochondrial exporter with putative roles in porphyrin or iron transport; complexes with MFRN1 and FECH to enhance heme biosynthesis | |
| ATP-binding cassette super-family G member 2 | ABCG2 | Cytosolic and mitochondrial exporter of protoporphyrin IX; also involved in the export of heme | |
| caseinolytic mitochondrial matrix peptidase chaperone subunit X | CLpX | Mitochondrial protein with ATP-dependent protease and unfoldase activity; regulates ALAS turnover; activates ALAS catalyzing PLP insertion | Phenotype modifier in protoporphyria (see Section 3.3) |
| coproporphyrinogen III oxidase | CPOX | Eliminates two carboxyl groups from coproporphyrinogen III side chains to yield protoporphyrinogen IX | Hereditary coproporphyria (autosomal dominant) Harderoporphyria (autosomal recessive) |
| ferrochelatase | FECH | Chelates iron into protoporphyrin IX to yield heme | Erythropoietic protoporphyria |
| glutaredoxin 5 | GLRX5 | Mitochondrial protein with thiol reductase activity; involved in [Fe-S] cluster assembly | Autosomal recessive sideroblastic anaemia |
| hydroxymethylbilane synthase | HMBS | Condensates four PBG molecules into HMB | Acute intermittent porphyria (AIP)Autosomal recessive AIP |
| heme oxygenase 1 | HO-1 | Cleaves heme into biliverdin IX, releasing CO and ferrous iron (inducible isoform) | HO-1 deficiency |
| heme oxygenase 2 | HO-2 | Cleaves heme into biliverdin IX, releasing CO and ferrous iron (constitutive isoform); involved in the CO signalling pathway | |
| hemopexin | HPX | Heme scavenger in the plasma | |
| heat shock protein family A member 9 | HSPA9 | Mitochondrial protein with chaperone activity for [Fe-S] clusters | Autosomal recessive sideroblastic anaemia |
| lon peptidase 1, mitochondrial | LONP1 | ATP-dependent protease involved in the turnover of mitochondrial matrix protein | CODAS (Cerebral, Ocular, Dental, Auricular and Skeletal) syndrome [69] |
| protoporphyrinogen oxidase | PPOX | Dehydrogenates protoporphyrinogen IX to yield protoporphyrin IX | Variegate porphyria (VP) Autosomal recessive VP |
| mitochondrial solute carrier family 25 member A38 | SLC25A38 | Mitochondrial glycine transporter | Autosomal recessive sideroblastic anaemia |
| succinyl-CoA synthase | SUCLA | Controls the flux of heme precursors catalyzing a reversible conversion from succinate + coenzime A to succynil-CoA (precursor of ALA) | |
| uroporphyrinogen III decarboxylase | UROD | Eliminates four carboxyl groups from uroporphyrinogen III side chains to yield coproporphyrinogen III | Porphyria cutanea tarda (sporadic or familial)Hepatoerythropoietic porphyria |
| uroporphyrinogen III synthase | UROS | Converts linear PBG to cyclic uroporphyrinogen III | Congenital erythropoietic porphyria (Günther disease) |
3. Clinical and Experimental Aspects of the Role of Iron in Porphyrias
3.1. Porphyria Cutanea Tarda
3.2. Congenital Erythropoietic Porphyria
3.3. Erythropoietic and X-Linked Protoporphyria
IRP2-Related Protoporphyria
3.4. Acute Hepatic Porphyrias
4. Clinical and Experimental Aspects of the Role of Iron in Congenital Sideroblastic Anaemias
4.1. ALAS2-Related X-Linked Sideroblastic Anaemia
4.1.1. X-Linked Sideroblastic Anaemia with Ataxia
4.2. Autosomal Recessive Sideroblastic Anaemias
4.2.1. SLC25A38 Mutations
4.2.2. GLRX5, HSPA9, HSCB Mutations
4.2.3. STEAP3-Related Sideroblastic Anaemia with Primary Hypogonadism and DMT1 Deficiency
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-UTR | 5 untranslated region |
| ABCB | adenosine triphosphate-binding cassette subfamily B |
| ACTR | activin receptor |
| AIP | acute intermittent porphyria |
| AHPs | acute hepatic porphyrias |
| ALA | -aminolevulinic acid |
| ALA· | enoyl (ALA) radical |
| ALAD | ALA dehydratase |
| ALAS1 | ALA synthase 1 |
| ALAS2 | ALA synthase 2 |
| ALK | activin receptor-like kinase |
| APA | acute porphyric attack |
| ARCSA | autosomal recessive sideroblastic anaemia |
| BMP | bone morphogenetic protein |
| CEP | congenital erythropoietic porphyria |
| ClpX | caseinolytic mitochondrial matrix peptidase chaperone subunit X |
| CP | ceruloplasmin |
| CPOX | coproporphyrinogen III oxidase |
| CSA | congenital sideroblastic anaemias |
| CYP1A2 | cytochrome 1A2 of the P450 family |
| DCYTB | duodenal cytochrome B |
| DFO | deferoxamine |
| DMT1 | divalent metal transporter 1 |
| DOVA | 4,5-dioxovaleric acid |
| EPP | erythropoietic protoporphyria |
| EPO | erythropoietin |
| ERFE | erythroferrone |
| Fe | iron |
| FECH | ferrochelatase |
| Fe-S cluster | iron-sulfur cluster |
| F-PCT | familial porphyria cutanea tarda |
| FPN1 | ferroportin (protein) |
| FTH1 | ferritin heavy chain |
| FTL | ferritin light chain |
| FXN | frataxin |
| GATA1 | GATA-binding factor 1 |
| GLRX5 | glutaredoxin 5 |
| GDF15 | growth/differentation factor 15 |
| GRX | glutaredoxin |
| HAMP | hepcidin gene |
| HCP | hereditary coproporphyria |
| HC | hemochromatosis |
| HEPH | hephaestin |
| HJV or HFE2 | hemojuvelin gene |
| HFE | human homeostatic iron regulator protein |
| HMB | hydroxymethylbilane |
| HMBS | HMB synthase |
| HO | heme oxygenase |
| HSCB | heat-shock cognate B |
| HSPA9 | heat-shock protein family A member 9 |
| IL-6 | interleukin 6 |
| IRE | iron responsive element |
| IRIDA | iron deficiency-iron refractory anaemia |
| IRP1 | IRE-binding protein 1 |
| IRP2 | IRE-binding protein 2 |
| LIC | liver iron content |
| LONP1 | lon peptidase 1, mitochondrial |
| LSEC | liver sinusoidal endothelial cell |
| Mfrn1 | mitoferrin 1 |
| MtF | mitochondrial ferritin |
| NCOA4 | nuclear receptor coactivator 4 |
| PBG | porphobilinogen |
| PCBP1 | poly rC–binding protein 1 |
| PCT | porphyria cutanea tarda |
| PLP | pyridoxal phosphate |
| PPOX | protoporphyrinogen oxidase |
| RISC | RNA-induced silencing complex |
| ROS | reactive oxygen species |
| S-PCT | sporadic porphyria cutanea tarda |
| SLC40A1 | ferroportin (gene) |
| SMAD | suppressor of mothers against decapentaplegic |
| STAT3 | signal transducer and activator of transcription 3 |
| STEAP3 | six-transmembrane epithelial antigen of prostate 3 |
| TF | transferrin |
| TFR1 | transferrin receptor 1 (protein) |
| TFR2 | transferrin receptor 2 |
| TFRC | transferrin receptor (gene) |
| TMPRSS6 | transmembrane protease serine 6 |
| UROD | uroporphyrinogen decarboxylase |
| UROS | uroporphyrinogen III synthase |
| UTR | untranslated region |
| VP | variegate porphyria |
| XLP | X-linked protoporphyria |
| XLSA | X-linked sideroblastic anaemia |
| XLSA/A | XLSA with ataxia |
References
- Pietrangelo, A. Mechanisms of iron hepatotoxicity. J. Hepatol. 2016, 65, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Desnick, R.J. The porphyrias: Advances in diagnosis and treatment. Blood J. Am. Soc. Hematol. 2012, 120, 4496–4504. [Google Scholar]
- Camaschella, C. Hereditary sideroblastic anemias: Pathophysiology, diagnosis, and treatment. Semin. Hematol. 2009, 46, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, E.; Granata, F. Nutrients and Porphyria: An Intriguing Crosstalk. Int. J. Mol. Sci. 2020, 21, 3462. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Ventura, P.; Corradini, E. Iron in Porphyrias: Friend or Foe? Diagnostics 2022, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Schmitt, C.; Moulouel, B.; Mirmiran, A.; Puy, H.; Lefèbvre, T.; Gouya, L. Iron, Heme Synthesis and Erythropoietic Porphyrias: A Complex Interplay. Metabolites 2021, 11, 798. [Google Scholar] [CrossRef]
- West, A.R.; Oates, P.S. Mechanisms of heme iron absorption: Current questions and controversies. World J. Gastroenterol. WJG 2008, 14, 4101. [Google Scholar] [CrossRef]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Aschemeyer, S.; Qiao, B.; Stefanova, D.; Valore, E.V.; Sek, A.C.; Ruwe, T.A.; Vieth, K.R.; Jung, G.; Casu, C.; Rivella, S.; et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood 2018, 131, 899–910. [Google Scholar] [CrossRef]
- Katsarou, A.; Pantopoulos, K. Basics and principles of cellular and systemic iron homeostasis. Mol. Asp. Med. 2020, 75, 100866. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Alfaro-Magallanes, V.M.; Babitt, J.L. Physiological and pathophysiological mechanisms of hepcidin regulation: Clinical implications for iron disorders. Br. J. Haematol. 2021, 193, 882–893. [Google Scholar] [CrossRef]
- Andriopoulos Jr, B.; Corradini, E.; Xia, Y.; Faasse, S.A.; Chen, S.; Grgurevic, L.; Knutson, M.D.; Pietrangelo, A.; Vukicevic, S.; Lin, H.Y.; et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Genet. 2009, 41, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef]
- Fisher, A.L.; Babitt, J.L. Coordination of iron homeostasis by bone morphogenetic proteins: Current understanding and unanswered questions. Dev. Dyn. 2022, 251, 26–46. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta BBA Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Ryu, M.S.; Zhang, D.; Protchenko, O.; Shakoury-Elizeh, M.; Philpott, C.C. PCBP1 and NCOA4 regulate erythroid iron storage and heme biosynthesis. J. Clin. Investig. 2017, 127, 1786–1797. [Google Scholar] [CrossRef]
- Zhang, A.S.; Sheftel, A.D.; Ponka, P. Intracellular kinetics of iron in reticulocytes: Evidence for endosome involvement in iron targeting to mitochondria. Blood 2005, 105, 368–375. [Google Scholar] [CrossRef]
- Sheftel, A.D.; Zhang, A.S.; Brown, C.; Shirihai, O.S.; Ponka, P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood J. Am. Soc. Hematol. 2007, 110, 125–132. [Google Scholar] [CrossRef]
- Pantopoulos, K. Iron Metabolism and the IRE/IRP Regulatory System: An Update. Ann. N. Y. Acad. Sci. 2004, 1012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schranzhofer, M.; Schifrer, M.; Cabrera, J.A.; Kopp, S.; Chiba, P.; Beug, H.; Müllner, E.W. Remodeling the regulation of iron metabolism during erythroid differentiation to ensure efficient heme biosynthesis. Blood 2006, 107, 4159–4167. [Google Scholar] [CrossRef]
- Pietrangelo, A. Hereditary hemochromatosis: Pathogenesis, diagnosis, and treatment. Gastroenterology 2010, 139, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Corradini, E.; Buzzetti, E.; Pietrangelo, A. Genetic iron overload disorders. Mol. Asp. Med. 2020, 75, 100896. [Google Scholar] [CrossRef] [PubMed]
- Zoller, H.; Schaefer, B.; Vanclooster, A.; Griffiths, B.; Bardou-Jacquet, E.; Corradini, E.; Porto, G.; Ryan, J.; Cornberg, M. EASL Clinical Practice Guidelines on haemochromatosis. J. Hepatol. 2022, 77, 479–502. [Google Scholar] [CrossRef]
- Ramsay, A.J.; Quesada, V.; Sanchez, M.; Garabaya, C.; Sardà, M.P.; Baiget, M.; Remacha, A.; Velasco, G.; López-Otín, C. Matriptase-2 mutations in iron-refractory iron deficiency anemia patients provide new insights into protease activation mechanisms. Hum. Mol. Genet. 2009, 18, 3673–3683. [Google Scholar] [CrossRef]
- Gitlin, J.D. Aceruloplasminemia. Pediatr. Res. 1998, 44, 271–276. [Google Scholar] [CrossRef]
- Heinemann, I.U.; Jahn, M.; Jahn, D. The biochemistry of heme biosynthesis. Arch. Biochem. Biophys. 2008, 474, 238–251. [Google Scholar] [CrossRef]
- Chiabrando, D.; Vinchi, F.; Fiorito, V.; Mercurio, S.; Tolosano, E. Heme in pathophysiology: A matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 2014, 5, 61. [Google Scholar] [CrossRef]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef]
- Larsen, R.; Gozzelino, R.; Jeney, V.; Tokaji, L.; Bozza, F.A.; Japiassú, A.M.; Bonaparte, D.; Cavalcante, M.M.; Ângelo, C.; Ferreira, A.; et al. A Central Role for Free Heme in the Pathogenesis of Severe Sepsis. Sci. Transl. Med. 2010, 2, 51ra71. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; McCulloh, R. Hemopexin and haptoglobin: Allies against heme toxicity from hemoglobin not contenders. Front. Physiol. 2015, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Haggard, J.J.; Croatt, A.J.; Grande, J.P.; Poss, K.D.; Alam, J. The Indispensability of Heme Oxygenase-1 in Protecting against Acute Heme Protein-Induced Toxicity in Vivo. Am. J. Pathol. 2000, 156, 1527–1535. [Google Scholar] [CrossRef]
- Deuel, J.W.; Schaer, C.A.; Boretti, F.S.; Opitz, L.; García-Rubio, I.; Baek, J.; Spahn, D.R.; Buehler, P.W.; Schaer, D.J. Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis. 2016, 7, e2064. [Google Scholar] [CrossRef] [PubMed]
- Dhar, G.J.; Bossenmaier, I.; Cardinal, R.; Petryka, Z.; Watson, C. Transitory renal failure following rapid administration of a relatively large amount of hematin in a patient with acute intermittent porphyria in clinical remission. Acta Medica Scand. 1978, 203, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, N.; Durante, W.; Kroll, M.H.; Schafer, A.I. Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase–stimulatory carbon monoxide. Circulation 1995, 91, 2306–2309. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L.; Johns, D.G.; Kriegsfeld, L.J.; Klein, S.L.; Calvin, D.C.; Demas, G.E.; Schramm, L.P.; Tonegawa, S.; Nelson, R.J.; Snyder, S.H.; et al. Ejaculatory abnormalities in mice with targeted disruption of the gene for heme oxygenase-2. Nat. Med. 1998, 4, 84–87. [Google Scholar] [CrossRef]
- Szlendak, U.; Bykowska, K.; Lipniacka, A. Clinical, Biochemical and Molecular Characteristics of the Main Types of Porphyria. Adv. Clin. Exp. Med. 2016, 25, 361–368. [Google Scholar] [CrossRef]
- Woods, J. Regulation of porphyrin and heme metabolism in the kidney. Semin. Hematol. 1988, 25, 336–348. [Google Scholar]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef]
- Melefors, O.; Goossen, B.; Johansson, H.E.; Stripecke, R.; Gray, N.K.; Hentze, M. Translational control of 5-aminolevulinate synthase mRNA by iron-responsive elements in erythroid cells. J. Biol. Chem. 1993, 268, 5974–5978. [Google Scholar] [CrossRef]
- Sadlon, T.J.; Dell’Oso, T.; Surinya, K.H.; May, B.K. Regulation of erythroid 5-aminolevulinate synthase expression during erythropoiesis. Int. J. Biochem. Cell Biol. 1999, 31, 1153–1167. [Google Scholar] [CrossRef]
- Tian, Q.; Li, T.; Hou, W.; Zheng, J.; Schrum, L.W.; Bonkovsky, H.L. Lon peptidase 1 (LONP1)-dependent breakdown of mitochondrial 5-aminolevulinic acid synthase protein by heme in human liver cells. J. Biol. Chem. 2011, 286, 26424–26430. [Google Scholar] [CrossRef]
- Kubota, Y.; Nomura, K.; Katoh, Y.; Yamashita, R.; Kaneko, K.; Furuyama, K. Novel mechanisms for heme-dependent degradation of ALAS1 protein as a component of negative feedback regulation of heme biosynthesis. J. Biol. Chem. 2016, 291, 20516–20529. [Google Scholar] [CrossRef] [PubMed]
- Rondelli, C.M.; Perfetto, M.; Danoff, A.; Bergonia, H.; Gillis, S.; O’Neill, L.; Jackson, L.; Nicolas, G.; Puy, H.; West, R.; et al. The ubiquitous mitochondrial protein unfoldase CLPX regulates erythroid heme synthesis by control of iron utilization and heme synthesis enzyme activation and turnover. J. Biol. Chem. 2021, 297, 100972. [Google Scholar] [CrossRef]
- Kardon, J.R.; Yien, Y.Y.; Huston, N.C.; Branco, D.S.; Hildick-Smith, G.J.; Rhee, K.Y.; Paw, B.H.; Baker, T.A. Mitochondrial ClpX activates a key enzyme for heme biosynthesis and erythropoiesis. Cell 2015, 161, 858–867. [Google Scholar] [CrossRef]
- Harbin, B.M.; Dailey, H.A. Orientation of ferrochelatase in bovine liver mitochondria. Biochemistry 1985, 24, 366–370. [Google Scholar] [CrossRef]
- Obi, C.D.; Bhuiyan, T.; Dailey, H.A.; Medlock, A.E. Ferrochelatase: Mapping the intersection of iron and porphyrin metabolism in the mitochondria. Front. Cell Dev. Biol. 2022, 10, 894591. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Wittig, J.G.; Ghamari, A.; Maeda, M.; Dailey, T.A.; Bergonia, H.; Kafina, M.D.; Coughlin, E.E.; Minogue, C.E.; Hebert, A.S.; et al. Erythropoietin signaling regulates heme biosynthesis. eLife 2017, 6, e24767. [Google Scholar] [CrossRef]
- Crooks, D.R.; Ghosh, M.C.; Haller, R.G.; Tong, W.H.; Rouault, T.A. Posttranslational stability of the heme biosynthetic enzyme ferrochelatase is dependent on iron availability and intact iron-sulfur cluster assembly machinery. Blood 2010, 115, 860–869. [Google Scholar] [CrossRef]
- Shah, D.I.; Takahashi-Makise, N.; Cooney, J.D.; Li, L.; Schultz, I.J.; Pierce, E.L.; Narla, A.; Seguin, A.; Hattangadi, S.M.; Medlock, A.E.; et al. Mitochondrial Atpif1 regulates haem synthesis in developing erythroblasts. Nature 2012, 491, 608–612. [Google Scholar] [CrossRef]
- Shaw, G.; Cope, J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.; Gwynn, B.; Lambert, A.; Wingert, R.; Traver, D.; et al. Mitoferrin is essential for erythroid iron assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef]
- Yoon, T.; Cowan, J. Frataxin-mediated iron delivery to ferrochelatase in the final step of heme biosynthesis. J. Biol. Chem. 2004, 279, 25943–25946. [Google Scholar] [CrossRef]
- Bencze, K.Z.; Yoon, T.; Millán-Pacheco, C.; Bradley, P.B.; Pastor, N.; Cowan, J.; Stemmler, T.L. Human frataxin: Iron and ferrochelatase binding surface. Chem. Commun. 2007, 1798–1800. [Google Scholar] [CrossRef]
- Schmucker, S.; Puccio, H. Understanding the molecular mechanisms of Friedreich’s ataxia to develop therapeutic approaches. Hum. Mol. Genet. 2010, 19, R103–R110. [Google Scholar] [CrossRef]
- Dietz, J.V.; Willoughby, M.M.; Piel, R.B.; Ross, T.A.; Bohovych, I.; Addis, H.G.; Fox, J.L.; Lanzilotta, W.N.; Dailey, H.A.; Wohlschlegel, J.A.; et al. Mitochondrial contact site and cristae organizing system (MICOS) machinery supports heme biosynthesis by enabling optimal performance of ferrochelatase. Redox Biol. 2021, 46, 102125. [Google Scholar] [CrossRef]
- Medlock, A.E.; Carter, M.; Dailey, T.A.; Dailey, H.A.; Lanzilotta, W.N. Product release rather than chelation determines metal specificity for ferrochelatase. J. Mol. Biol. 2009, 393, 308–319. [Google Scholar] [CrossRef]
- Troadec, M.B.; Warner, D.; Wallace, J.; Thomas, K.; Spangrude, G.J.; Phillips, J.; Khalimonchuk, O.; Paw, B.H.; Ward, D.M.; Kaplan, J. Targeted deletion of the mouse Mitoferrin1 gene: From anemia to protoporphyria. Blood 2011, 117, 5494–5502. [Google Scholar] [CrossRef]
- Chung, J.; Anderson, S.A.; Gwynn, B.; Deck, K.M.; Chen, M.J.; Langer, N.B.; Shaw, G.C.; Huston, N.C.; Boyer, L.F.; Datta, S.; et al. Iron regulatory protein-1 protects against mitoferrin-1-deficient porphyria. J. Biol. Chem. 2014, 289, 7835–7843. [Google Scholar] [CrossRef]
- Phillips, J.; Farrell, C.; Wang, Y.; Singal, A.K.; Anderson, K.; Balwani, M.; Bissell, M.; Bonkovsky, H.; Seay, T.; Paw, B.; et al. Strong correlation of ferrochelatase enzymatic activity with Mitoferrin-1 mRNA in lymphoblasts of patients with protoporphyria. Mol. Genet. Metab. 2019, 128, 391–395. [Google Scholar] [CrossRef]
- Crispin, A.; Guo, C.; Chen, C.; Campagna, D.R.; Schmidt, P.J.; Lichtenstein, D.; Cao, C.; Sendamarai, A.K.; Hildick-Smith, G.J.; Huston, N.C.; et al. Mutations in the iron-sulfur cluster biogenesis protein HSCB cause congenital sideroblastic anemia. J. Clin. Investig. 2020, 130, 5245–5256. [Google Scholar] [CrossRef]
- Medlock, A.E.; Shiferaw, M.T.; Marcero, J.R.; Vashisht, A.A.; Wohlschlegel, J.A.; Phillips, J.D.; Dailey, H.A. Identification of the mitochondrial heme metabolism complex. PLoS ONE 2015, 10, e0135896. [Google Scholar]
- Chen, W.; Dailey, H.A.; Paw, B.H. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood 2010, 116, 628–630. [Google Scholar] [CrossRef]
- Bishop, D.F.; Tchaikovskii, V.; Hoffbrand, A.V.; Fraser, M.E.; Margolis, S. X-linked sideroblastic anemia due to carboxyl-terminal ALAS2 mutations that cause loss of binding to the β-subunit of succinyl-CoA synthetase (SUCLA2). J. Biol. Chem. 2012, 287, 28943–28955. [Google Scholar] [CrossRef]
- Furuyama, K.; Sassa, S. Interaction between succinyl CoA synthetase and the heme-biosynthetic enzyme ALAS-E is disrupted in sideroblastic anemia. J. Clin. Investig. 2000, 105, 757–764. [Google Scholar] [CrossRef]
- Cadenas, B.; Fita-Torró, J.; Bermúdez-Cortés, M.; Hernandez-Rodriguez, I.; Fuster, J.L.; Llinares, M.E.; Galera, A.M.; Romero, J.L.; Pérez-Montero, S.; Tornador, C.; et al. L-Ferritin: One Gene, Five Diseases; from Hereditary Hyperferritinemia to Hypoferritinemia—Report of New Cases. Pharmaceuticals 2019, 12, 17. [Google Scholar] [CrossRef]
- Jabara, H.H.; Boyden, S.E.; Chou, J.; Ramesh, N.; Massaad, M.J.; Benson, H.; Bainter, W.; Fraulino, D.; Rahimov, F.; Sieff, C.; et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat. Genet. 2016, 48, 74–78. [Google Scholar] [CrossRef]
- Fukuda, Y.; Cheong, P.L.; Lynch, J.; Brighton, C.; Frase, S.; Kargas, V.; Rampersaud, E.; Wang, Y.; Sankaran, V.G.; Yu, B.; et al. The severity of hereditary porphyria is modulated by the porphyrin exporter and Lan antigen ABCB6. Nat. Commun. 2016, 7, 1–9. [Google Scholar]
- Dikoglu, E.; Alfaiz, A.; Gorna, M.; Bertola, D.; Chae, J.H.; Cho, T.J.; Derbent, M.; Alanay, Y.; Guran, T.; Kim, O.H.; et al. Mutations in LONP1, a mitochondrial matrix protease, cause CODAS syndrome. Am. J. Med. Genet. Part A 2015, 167, 1501–1509. [Google Scholar] [CrossRef]
- Elder, G.H. Porphyria cutanea tarda. Semin. Liver Dis. 1998, 18, 67–75. [Google Scholar] [CrossRef]
- Badenas, C.; To-Figueras, J.; Phillips, J.; Warby, C.; Munoz, C.; Herrero, C. Identification and characterization of novel uroporphyrinogen decarboxylase gene mutations in a large series of porphyria cutanea tarda patients and relatives. Clin. Genet. 2009, 75, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.; Baker, H.; Vernon-Roberts, B.; Magnus, I.A. Iron Metabolism in Porphyria Cutanea Tarda and in Erythropoietic Protoporphyria. QJM Int. J. Med. 1973, 42, 341–355. [Google Scholar] [CrossRef]
- Alla, V.; Bonkovsky, H.L. Iron in nonhemochromatotic liver disorders. Semin. Liver Dis. 2005, 25, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Bulaj, Z.J.; Phillips, J.D.; Ajioka, R.S.; Franklin, M.R.; Griffen, L.M.; Guinee, D.J.; Edwards, C.Q.; Kushner, J.P. Hemochromatosis genes and other factors contributing to the pathogenesis of porphyria cutanea tarda. Blood 2000, 95, 1565–1571. [Google Scholar] [CrossRef]
- Ryan Caballes, F.; Sendi, H.; Bonkovsky, H.L. Hepatitis C, porphyria cutanea tarda and liver iron: An update. Liver Int. 2012, 32, 880–893. [Google Scholar] [CrossRef]
- Lundvall, O.; Weinfeld, A. Studies of the clinical and metabolic effects of phlebotomy treatment in porphyria cutanea tarda. Acta Med. Scand. 1968, 184, 191–199. [Google Scholar] [CrossRef]
- Felsher, B.F.; Jones, M.L.; Redeker, A.G. Iron and Hepatic Uroporphyrin Synthesis: Relation in Porphyria Cutanea Tarda. JAMA 1973, 226, 663–665. [Google Scholar] [CrossRef]
- Dabrowska, E.; Jabłońska-Kaszewska, I.; Falkiewicz, B. Effect of high fiber vegetable-fruit diet on the activity of liver damage and serum iron level in porphyria cutanea tarda (PCT). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2001, 7, 282–286. [Google Scholar]
- Roberts, A.G.; Whatley, S.D.; Nicklin, S.; Worwood, M.; Pointon, J.J.; Stone, C.; Elder, G.H. The frequency of hemochromatosis-associated alleles is increased in British patients with sporadic porphyria cutanea tarda. Hepatology 1997, 25, 159–161. [Google Scholar] [CrossRef]
- Stuart, K.A.; Busfield, F.; Jazwinska, E.C.; Gibson, P.; Butterworth, L.A.; Cooksley, W.G.; Powell, L.W.; Crawford, D.H. The C282Y mutation in the haemochromatosis gene (HFE) and hepatitis C virus infection are independent cofactors for porphyria cutanea tarda in Australian patients. J. Hepatol. 1998, 28, 404–409. [Google Scholar] [CrossRef]
- Bonkovsky, H.L.; Poh-Fitzpatrick, M.; Pimstone, N.; Obando, J.; Di Bisceglie, A.; Tattrie, C.; Tortorelli, K.; LeClair, P.; Mercurio, M.G.; Lambrecht, R.W. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology 1998, 27, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, M.; Fiorelli, G.; Fargion, S. Iron overload in porphyria cutanea tarda. Haematologica 1999, 84, 248–253. [Google Scholar] [PubMed]
- Tannapfel, A.; Stölzel, U.; Köstler, E.; Melz, S.; Richter, M.; Keim, V.; Schuppan, D.; Wittekind, C. C282Y and H63D mutation of the hemochromatosis gene in German porphyria cutanea tarda patients. Virchows Arch. 2001, 439, 1–5. [Google Scholar] [CrossRef]
- Egger, N.G.; Goeger, D.E.; Payne, D.A.; Miskovsky, E.P.; Weinman, S.A.; Anderson, K.E. Porphyria cutanea tarda: Multiplicity of risk factors including HFE mutations, hepatitis C, and inherited uroporphyrinogen decarboxylase deficiency. Dig. Dis. Sci. 2002, 47, 419–426. [Google Scholar] [CrossRef]
- Phillips, J.D.; Jackson, L.K.; Bunting, M.; Franklin, M.R.; Thomas, K.R.; Levy, J.E.; Andrews, N.C.; Kushner, J.P. A mouse model of familial porphyria cutanea tarda. Proc. Natl. Acad. Sci. USA 2001, 98, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Kushner, J.P.; Barbuto, A.J.; Lee, G.R. An inherited enzymatic defect in porphyria cutanea tarda: Decreased uroporphyrinogen decarboxylase activity. J. Clin. Investig. 1976, 58, 1089–1097. [Google Scholar] [CrossRef]
- Elder, G.H.; Lee, G.B.; Tovey, J.A. Decreased Activity of Hepatic Uroporphyrinogen Decarboxylase in Sporadic Porphyria Cutanea Tarda. N. Eng. J. Med. 1978, 299, 274–278. [Google Scholar] [CrossRef]
- Phillips, J.D.; Bergonia, H.A.; Reilly, C.A.; Franklin, M.R.; Kushner, J.P. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc. Natl. Acad. Sci. USA 2007, 104, 5079–5084. [Google Scholar] [CrossRef]
- Sinclair, R.P.; Gorman, N.; Dalton, T.; Walton, S.H.; Bement, J.W.; Sinclair, F.J.; Smith, G.A.; Nebert, W.D. Uroporphyria produced in mice by iron and 5-aminolaevulinic acid does not occur in Cyp1a2 (-/-) null mutant mice. Biochem. J. 1998, 330, 149–153. [Google Scholar] [CrossRef]
- Phillips, J.D.; Steensma, D.P.; Pulsipher, M.A.; Spangrude, G.J.; Kushner, J.P. Congenital erythropoietic porphyria due to a mutation in GATA1: The first trans-acting mutation causative for a human porphyria. Blood 2007, 109, 2618–2621. [Google Scholar] [CrossRef]
- Di Pierro, E.; Brancaleoni, V.; Granata, F. Advances in understanding the pathogenesis of congenital erythropoietic porphyria. Br. J. Haematol. 2016, 173, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Erwin, A.L.; Desnick, R.J. Congenital erythropoietic porphyria: Recent advances. Mol. Genet. Metab. 2019, 128, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Podlipnik, S.; Guijarro, F.; Combalia, A.; To-Figueras, J.; Badenas, C.; Costa, D.; Rozman, M.; Jorge, S.; Aguilera, P.; Gaya, A. Acquired erythropoietic uroporphyria secondary to myelodysplastic syndrome with chromosome 3 alterations: A case report. Br. J. Dermatol. 2018, 179, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Serra-García, L.; Morgado-Carrasco, D.; Pérez-Valencia, A.I.; Castaño-Díez, S.; Alamon-Reig, F.; Badenas, C.; To-Figueras, J.; Aguilera, P. Acquired Erythropoietic Uroporphyria secondary to Myeloid Malignancy: Case report and literature review. Photodermatol. Photoimmunol. Photomed. 2021, 38, 86–91. [Google Scholar] [CrossRef]
- Egan, D.N.; Yang, Z.; Phillips, J.; Abkowitz, J.L. Inducing iron deficiency improves erythropoiesis and photosensitivity in congenital erythropoietic porphyria. Blood J. Am. Soc. Hematol. 2015, 126, 257–261. [Google Scholar] [CrossRef]
- Katugampola, R.; Anstey, A.; Finlay, A.; Whatley, S.; Woolf, J.; Mason, N.; Deybach, J.; Puy, H.; Ged, C.; de Verneuil, H.; et al. A management algorithm for congenital erythropoietic porphyria derived from a study of 29 cases. Br. J. Dermatol. 2012, 167, 888–900. [Google Scholar] [CrossRef]
- Piomelli, S.; Poh-Fitzpatrick, M.B.; Seaman, C.; Skolnick, L.M.; Berdon, W.E. Complete Suppression of the Symptoms of Congenital Erythropoietic Porphyria by Long-Term Treatment with High-Level Transfusions. N. Eng. J. Med. 1986, 314, 1029–1031. [Google Scholar] [CrossRef]
- Lange, B.; Hofweber, K.; Waldherr, R.; Schärer, K. Congenital erythropoietic porphyria associated with nephrotic syndrome and renal siderosis. Acta Pædiatr. 1995, 84, 1325–1328. [Google Scholar] [CrossRef]
- Mirmiran, A.; Poli, A.; Ged, C.; Schmitt, C.; Lefebvre, T.; Manceau, H.; Daher, R.; Moulouel, B.; Peoc’h, K.; Simonin, S.; et al. Phlebotomy as an efficient long-term treatment of congenital erythropoietic porphyria. Haematologica 2021, 106, 913. [Google Scholar] [CrossRef]
- Blouin, J.M.; Ged, C.; Bernardo-Seisdedos, G.; Cabantous, T.; Pinson, B.; Poli, A.; Puy, H.; Millet, O.; Gouya, L.; Morice-Picard, F.; et al. Identification of novel UROS mutations in a patient with congenital erythropoietic porphyria and efficient treatment by phlebotomy. Mol. Genet. Metab. Rep. 2021, 27, 100722. [Google Scholar] [CrossRef]
- Ged, C.; Mendez, M.; Robert, E.; Lalanne, M.; Lamrissi-Garcia, I.; Costet, P.; Daniel, J.; Dubus, P.; Mazurier, F.; Moreau-Gaudry, F.; et al. A knock-in mouse model of congenital erythropoietic porphyria. Genomics 2006, 87, 84–92. [Google Scholar] [CrossRef][Green Version]
- Millot, S.; Delaby, C.; Moulouel, B.; Lefebvre, T.; Pilard, N.; Ducrot, N.; Ged, C.; Lettéron, P.; De Franceschi, L.; Deybach, J.C.; et al. Hemolytic anemia repressed hepcidin level without hepatocyte iron overload: Lesson from Günther disease model. Haematologica 2017, 102, 260. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Millot, S.; Richard, E.; Blouin, J.M.; Lalanne, M.; Lamrissi-Garcia, I.; Costet, P.; Lyoumi, S.; Gouya, L.; Puy, H.; et al. Genetic background influences hepcidin response to iron imbalance in a mouse model of hemolytic anemia (Congenital erythropoietic porphyria). Biochem. Biophys. Res. Commun. 2019, 520, 297–303. [Google Scholar] [CrossRef] [PubMed]
- To-Figueras, J.; Ducamp, S.; Clayton, J.; Badenas, C.; Delaby, C.; Ged, C.; Lyoumi, S.; Gouya, L.; de Verneuil, H.; Beaumont, C.; et al. ALAS2 acts as a modifier gene in patients with congenital erythropoietic porphyria. Blood 2011, 118, 1443–1451. [Google Scholar] [CrossRef]
- Peoc’h, K.; Nicolas, G.; Schmitt, C.; Mirmiran, A.; Daher, R.; Lefebvre, T.; Gouya, L.; Karim, Z.; Puy, H. Regulation and tissue-specific expression of δ-aminolevulinic acid synthases in non-syndromic sideroblastic anemias and porphyrias. Mol. Genet. Metab. 2019, 128, 190–197. [Google Scholar] [CrossRef]
- Blouin, J.M.; Ged, C.; Lalanne, M.; Lamrissi-Garcia, I.; Morice-Picard, F.; Costet, P.; Daher, R.; Moreau-Gaudry, F.; Bedel, A.; Puy, H.; et al. Iron chelation rescues hemolytic anemia and skin photosensitivity in congenital erythropoietic porphyria. Blood 2020, 136, 2457–2468. [Google Scholar] [CrossRef]
- Di Pierro, E.; Granata, F.; De Canio, M.; Rossi, M.; Ricci, A.; Marcacci, M.; De Luca, G.; Sarno, L.; Barbieri, L.; Ventura, P.; et al. Recognized and Emerging Features of Erythropoietic and X-Linked Protoporphyria. Diagnostics 2022, 12, 151. [Google Scholar] [CrossRef]
- Gouya, L.; Puy, H.; Robreau, A.M.; Bourgeois, M.; Lamoril, J.; Da Silva, V.; Grandchamp, B.; Deybach, J.C. The penetrance of dominant erythropoietic protoporphyria is modulated by expression of wildtype FECH. Nat. Genet. 2002, 30, 27–28. [Google Scholar] [CrossRef]
- Balwani, M. Erythropoietic Protoporphyria and X-Linked Protoporphyria: Pathophysiology, genetics, clinical manifestations, and management. Mol. Genet. Metab. 2019, 128, 298–303. [Google Scholar] [CrossRef]
- Delaby, C.; Lyoumi, S.; Ducamp, S.; Martin-Schmitt, C.; Gouya, L.; Deybach, J.; Beaumont, C.; Puy, H. Excessive erythrocyte PPIX influences the hematologic status and iron metabolism in patients with dominant erythropoietic protoporphyria. Cell. Mol. Biol. 2009, 55, 45–52. [Google Scholar]
- Schneider-Yin, X.; Minder, E.I. Erythropoietic protoporphyria and X-linked dominant protoporphyria. In Handbook of Porphyrin Science (Volume 29) with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine—Volume 29: Porphyrias and Sideroblastic Anemias; World Scientific: Singapore, 2014; pp. 299–328. [Google Scholar]
- Wahlin, S.; Floderus, Y.; Stål, P.; Harper, P. Erythropoietic protoporphyria in Sweden: Demographic, clinical, biochemical and genetic characteristics. J. Intern. Med. 2011, 269, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Graziadei, G.; Duca, L.; Granata, F.; De Luca, G.; De Giovanni, A.; Brancaleoni, V.; Nava, I.; Di Pierro, E. Microcytosis in Erythropoietic Protoporphyria. Front. Physiol. 2022, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Langer, N.B.; Shaw, G.C.; Yang, G.; Li, L.; Kaplan, J.; Paw, B.H.; Bloomer, J.R. Abnormal mitoferrin-1 expression in patients with erythropoietic protoporphyria. Exp. Hematol. 2011, 39, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Bossi, K.; Lee, J.; Schmeltzer, P.; Holburton, E.; Groseclose, G.; Besur, S.; Hwang, S.; Bonkovsky, H.L. Homeostasis of iron and hepcidin in erythropoietic protoporphyria. Eur. J. Clin. Investig. 2015, 45, 1032–1041. [Google Scholar] [CrossRef]
- Barman-Aksoezen, J.; Girelli, D.; Aurizi, C.; Schneider-Yin, X.; Campostrini, N.; Barbieri, L.; Minder, E.I.; Biolcati, G. Disturbed iron metabolism in erythropoietic protoporphyria and association of GDF15 and gender with disease severity. J. Inherit. Metab. Dis. 2017, 40, 433–441. [Google Scholar] [CrossRef]
- Landefeld, C.; Kentouche, K.; Gruhn, B.; Stauch, T.; Rößler, S.; Schuppan, D.; Whatley, S.D.; Beck, J.F.; Stölzel, U. X-linked protoporphyria: Iron supplementation improves protoporphyrin overload, liver damage and anaemia. Br. J. Haematol. 2016, 173, 482–484. [Google Scholar] [CrossRef]
- Holme, S.A.; Worwood, M.; Anstey, A.V.; Elder, G.H.; Badminton, M.N. Erythropoiesis and iron metabolism in dominant erythropoietic protoporphyria. Blood 2007, 110, 4108–4110. [Google Scholar] [CrossRef]
- Rademakers, L.H.P.M.; Koningsberger, J.C.; Sorber, C.W.J.; Faille, H.B.D.L.; Hattum, J.V.; Marx, J.J.M. Accumulation of iron in erythroblasts of patients with erythropoietic protoporphyria. Eur. J. Clin. Investig. 1993, 23, 130–138. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Hollowell, M.L.; Fitzgerald, K.; Butler, J.S.; Fleming, M.D. Mild iron deficiency does not ameliorate the phenotype of a murine erythropoietic protoporphyria model. Am. J. Hematol. 2020, 95, 492–496. [Google Scholar] [CrossRef]
- Lyoumi, S.; Abitbol, M.; Andrieu, V.; Henin, D.; Robert, E.; Schmitt, C.; Gouya, L.; de Verneuil, H.; Deybach, J.C.; Montagutelli, X.; et al. Increased plasma transferrin, altered body iron distribution, and microcytic hypochromic anemia in ferrochelatase-deficient mice. Blood 2007, 109, 811–818. [Google Scholar] [CrossRef]
- Hagiwara, S.; Nishida, N.; Ida, H.; Ueshima, K.; Minami, Y.; Takita, M.; Aoki, T.; Morita, M.; Chishina, H.; Komeda, Y.; et al. Role of phlebotomy in the treatment of liver damage related to erythropoietic porphyria. Sci. Rep. 2022, 12, 6100. [Google Scholar] [CrossRef] [PubMed]
- Kniffen, J. Protoporphyrin removal in intrahepatic porphyrastasis. Gastroenterology 1970, 58, 1027. [Google Scholar]
- Gordeuk, V.R.; Brittenham, G.M.; Hawkins, C.W.; Mukhtar, H.; Bickers, D.R. Iron therapy for hepatic dysfunction in erythropoietic protoporphyria. Ann. Intern. Med. 1986, 105, 27–31. [Google Scholar] [CrossRef]
- Holme, S.A.; Thomas, C.L.; Whatley, S.D.; Bentley, D.P.; Anstey, A.V.; Badminton, M.N. Symptomatic response of erythropoietic protoporphyria to iron supplementation. J. Am. Acad. Dermatol. 2007, 56, 1070–1072. [Google Scholar] [CrossRef]
- Milligan, A.; Graham-Brown, R.; Sarkany, I.; Baker, H. Erythropoietic protoporphyria exacerbated by oral iron therapy. Br. J. Dermatol. 1988, 119, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Baker, H. Erythropoietic protoporphyria provoked by iron therapy. Proc. Roy. Soc. Med. 1971, 64, 610–611. [Google Scholar] [CrossRef]
- McClements, B.; Bingham, A.; Callender, M.; Trimble, E.R. Erythropoietic protoporphyria and iron therapy. Br. J. Dermatol. 1990, 122, 423–424. [Google Scholar] [CrossRef]
- Barman-Aksözen, J.; Minder, E.I.; Schubiger, C.; Biolcati, G.; Schneider-Yin, X. In ferrochelatase-deficient protoporphyria patients, ALAS2 expression is enhanced and erythrocytic protoporphyrin concentration correlates with iron availability. Blood Cells Mol. Dis. 2015, 54, 71–77. [Google Scholar] [CrossRef]
- Barman-Aksözen, J.; Halloy, F.; Iyer, P.S.; Schümperli, D.; Minder, A.E.; Hall, J.; Minder, E.I.; Schneider-Yin, X. Delta-aminolevulinic acid synthase 2 expression in combination with iron as modifiers of disease severity in erythropoietic protoporphyria. Mol. Genet. Metab. 2019, 128, 304–308. [Google Scholar] [CrossRef]
- Inafuku, K.; Takamiyagi, A.; Oshiro, M.; Kinjo, T.; Nakashima, Y.; Nonaka, S. Alteration of mRNA levels of δ-aminolevulinic acid synthase, ferrochelatase and heme oxygenase-1 in griseofulvin induced protoporphyria mice. J. Dermatol. Sci. 1999, 19, 189–198. [Google Scholar] [CrossRef]
- Whitman, J.C.; Paw, B.H.; Chung, J. The role of ClpX in erythropoietic protoporphyria. Hematol. Transfus. Cell Ther. 2018, 40, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Yien, Y.Y.; Ducamp, S.; van der Vorm, L.N.; Kardon, J.R.; Manceau, H.; Kannengiesser, C.; Bergonia, H.A.; Kafina, M.D.; Karim, Z.; Gouya, L.; et al. Mutation in human CLPX elevates levels of δ-aminolevulinate synthase and protoporphyrin IX to promote erythropoietic protoporphyria. Proc. Natl. Acad. Sci. USA 2017, 114, E8045–E8052. [Google Scholar] [CrossRef] [PubMed]
- Ducamp, S.; Luscieti, S.; Ferrer-Cortès, X.; Nicolas, G.; Manceau, H.; Peoc’h, K.; Yien, Y.Y.; Kannengiesser, C.; Gouya, L.; Puy, H.; et al. A mutation in the iron-responsive element of ALAS2 is a modifier of disease severity in a patient suffering from CLPX associated erythropoietic protoporphyria. Haematologica 2021, 106, 2030. [Google Scholar] [CrossRef]
- Cooperman, S.S.; Meyron-Holtz, E.G.; Olivierre-Wilson, H.; Ghosh, M.C.; McConnell, J.P.; Rouault, T.A. Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. Blood 2005, 106, 1084–1091. [Google Scholar] [CrossRef]
- LaVaute, T.; Smith, S.; Cooperman, S.; Iwai, K.; Land, W.; Meyron-Holtz, E.; Drake, S.K.; Miller, G.; Abu-Asab, M.; Tsokos, M.; et al. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat. Genet. 2001, 27, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Cooperman, S.; Lavaute, T.; Tresser, N.; Ghosh, M.; Meyron-Holtz, E.; Land, W.; Ollivierre, H.; Jortner, B.; Switzer III, R.; et al. Severity of neurodegeneration correlates with compromise of iron metabolism in mice with iron regulatory protein deficiencies. Ann. N. Y. Acad. Sci. 2004, 1012, 65–83. [Google Scholar] [CrossRef]
- Costain, G.; Ghosh, M.C.; Maio, N.; Carnevale, A.; Si, Y.C.; Rouault, T.A.; Yoon, G. Absence of iron-responsive element-binding protein 2 causes a novel neurodegenerative syndrome. Brain 2019, 142, 1195–1202. [Google Scholar] [CrossRef]
- Marcacci, M.; Ricci, A.; Cuoghi, C.; Marchini, S.; Pietrangelo, A.; Ventura, P. Challenges in diagnosis and management of acute hepatic porphyrias: From an uncommon pediatric onset to innovative treatments and perspectives. Orphanet J. Rare Dis. 2022, 17, 1–10. [Google Scholar] [CrossRef]
- Ricci, A.; Di Pierro, E.; Marcacci, M.; Ventura, P. Mechanisms of Neuronal Damage in Acute Hepatic Porphyrias. Diagnostics 2021, 11, 2205. [Google Scholar] [CrossRef]
- Ricci, A.; Guida, C.C.; Manzini, P.; Cuoghi, C.; Ventura, P. Kidney Involvement in Acute Hepatic Porphyrias: Pathophysiology and Diagnostic Implications. Diagnostics 2021, 11, 2324. [Google Scholar] [CrossRef]
- Ricci, A.; Sandri, G.; Marcacci, M.; Di Pierro, E.; Granata, F.; Cuoghi, C.; Marchini, S.; Pietrangelo, A.; Ventura, P. Endothelial Dysfunction in Acute Hepatic Porphyrias. Diagnostics 2022, 12, 1303. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.; Bechara, E. 5-Aminolevulinic Acid Induces Lipid Peroxidation in Cardiolipin-Rich Liposomes. Arch. Biochem. Biophys. 1993, 305, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.E.; Ferreira, A.M.; Bechara, E.J. Roles of phosphate and an enoyl radical in ferritin iron mobilization by 5-aminolevulinic acid. Free. Radic. Biol. Med. 2000, 29, 1272–1279. [Google Scholar] [CrossRef]
- Douki, T.; Onuki, J.; Medeiros, M.H.; Bechara, E.J.; Cadet, J.; Di Mascio, P. DNA alkylation by 4, 5-dioxovaleric acid, the final oxidation product of 5-aminolevulinic acid. Chem. Res. Toxicol. 1998, 11, 150–157. [Google Scholar] [CrossRef]
- Di Mascio, P.; Teixeira, P.C.; Onuki, J.; Medeiros, M.H.; Dörnemann, D.; Douki, T.; Cadet, J. DNA damage by 5-aminolevulinic and 4, 5-dioxovaleric acids in the presence of ferritin. Arch. Biochem. Biophys. 2000, 373, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.; Kleinman, C.; Demasi, M.; Bechara, E. 5-Aminolevulinic Acid Induces Iron Release from Ferritin. Arch. Biochem. Biophys. 1995, 316, 607–611. [Google Scholar] [CrossRef]
- Rocha, M.E.M.; Dutra, F.; Bandy, B.; Baldini, R.L.; Gomes, S.L.; Faljoni-Alário, A.; Liria, C.W.; Miranda, M.T.M.; Bechara, E.J.H. Oxidative damage to ferritin by 5-aminolevulinic acid. Arch. Biochem. Biophys. 2003, 409, 349–356. [Google Scholar] [CrossRef]
- Rocha, M.E.M.; Bandy, B.; Costa, C.A.; de Barros, M.P.; Pinto, A.M.; Bechara, E.J. Iron mobilization by succinylacetone methyl ester in rats. A model study for hereditary tyrosinemia and porphyrias characterized by 5-Aminolevulinic acid overload. Free Radic. Res. 2000, 32, 343–353. [Google Scholar] [CrossRef]
- Demasi, M.; Penatti, C.A.; Delucia, R.; Bechara, E.J. The prooxidant effect of 5-aminolevulinic acid in the brain tissue of rats: Implications in neuropsychiatric manifestations in porphyrias. Free Radic. Biol. Med. 1996, 20, 291–299. [Google Scholar] [CrossRef]
- Carvalho, H.; Bechara, E.J.H.; Meneghini, R.; Demasi, M. Haem precursor δ-aminolaevulinic acid induces activation of the cytosolic iron regulatory protein 1. Biochem. J. 1997, 328, 827–832. [Google Scholar] [CrossRef]
- Handschin, C.; Lin, J.; Rhee, J.; Peyer, A.K.; Chin, S.; Wu, P.H.; Meyer, U.A.; Spiegelman, B.M. Nutritional Regulation of Hepatic Heme Biosynthesis and Porphyria through PGC-1α. Cell 2005, 122, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, C.; Montosi, G.; Garuti, C.; Corradini, E.; Sabelli, M.; Canali, S.; Pietrangelo, A. Gluconeogenic signals regulate iron homeostasis via hepcidin in mice. Gastroenterology 2014, 146, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiró, P.A.; Rees, D.C.; Stölzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N. Engl. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef]
- Ventura, P.; Bonkovsky, H.L.; Gouya, L.; Aguilera-Peiró, P.; Montgomery Bissell, D.; Stein, P.E.; Balwani, M.; Anderson, D.K.E.; Parker, C.; Kuter, D.J.; et al. Efficacy and safety of givosiran for acute hepatic porphyria: 24-month interim analysis of the randomized phase 3 ENVISION study. Liver Int. 2022, 42, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Ventura, P. Givosiran for the treatment of acute hepatic porphyria. Expert Rev. Clin. Pharmacol. 2022, 15, 383–393. [Google Scholar] [CrossRef]
- To-Figueras, J.; Lopez, R.M.; Deulofeu, R.; Herrero, C. Preliminary report: Hyperhomocysteinemia in patients with acute intermittent porphyria. Metabolism 2010, 59, 1809–1810. [Google Scholar] [CrossRef]
- Ventura, P.; Corradini, E.; Di Pierro, E.; Marchini, S.; Marcacci, M.; Cuoghi, C.; Buzzetti, E.; Pietrangelo, A. Hyperhomocysteinemia in patients with acute porphyrias: A potentially dangerous metabolic crossroad? Eur. J. Intern. Med. 2020, 79, 101–107. [Google Scholar] [CrossRef]
- Fontanellas, A.; Ávila, M.A.; Arranz, E.; de Salamanca, R.E.; Morales-Conejo, M. Acute intermittent porphyria, givosiran, and homocysteine. J. Inherit. Metab. Dis. 2021, 44, 790. [Google Scholar] [CrossRef]
- To-Figueras, J.; Wijngaard, R.; García-Villoria, J.; Aarsand, A.K.; Aguilera, P.; Deulofeu, R.; Brunet, M.; Gómez-Gómez, À.; Pozo, O.J.; Sandberg, S. Dysregulation of homocysteine homeostasis in acute intermittent porphyria patients receiving heme arginate or givosiran. J. Inherit. Metab. Dis. 2021, 44, 961–971. [Google Scholar] [CrossRef]
- Petrides, P.E.; Klein, M.; Schuhmann, E.; Torkler, H.; Molitor, B.; Loehr, C.; Obermeier, Z.; Beykirch, M.K. Severe homocysteinemia in two givosiran-treated porphyria patients: Is free heme deficiency the culprit? Ann. Hematol. 2021, 100, 1685–1693. [Google Scholar] [CrossRef]
- Vassiliou, D.; Sardh, E. Homocysteine elevation in givosiran treatment: Suggested ALAS1 siRNA effect on cystathionine beta-synthase. J. Intern. Med. 2021, 290, 928–930. [Google Scholar] [CrossRef]
- Ricci, A.; Marcacci, M.; Cuoghi, C.; Pietrangelo, A.; Ventura, P. Hyperhomocysteinemia in patients with acute porphyrias: A possible effect of ALAS1 modulation by siRNAm therapy and its control by vitamin supplementation. Eur. J. Intern. Med. 2021, 92, 121–123. [Google Scholar] [CrossRef]
- Bins, S.; Sardh, E.; Langendonk, J.G. Givosiran Likely Inhibits Cytochrome P450 More Substantially Than Reported. Clin. Pharmacol. Ther. 2022, 112, 24. [Google Scholar] [CrossRef]
- Lazareth, H.; Poli, A.; Bignon, Y.; Mirmiran, A.; Rabant, M.; Schmitt, C.; Puy, H.; Karras, A.; Gouya, L.; Pallet, N.; et al. Renal function decline with small interfering RNA silencing ALAS1 for Acute Intermittent Porphyria. Kidney Int. Rep. 2021, 6, 1904–1911. [Google Scholar] [CrossRef]
- Ducamp, S.; Fleming, M.D. The molecular genetics of sideroblastic anemia. Blood J. Am. Soc. Hematol. 2019, 133, 59–69. [Google Scholar] [CrossRef]
- Abu-Zeinah, G.; DeSancho, M.T. Understanding sideroblastic anemia: An overview of genetics, epidemiology, pathophysiology and current therapeutic options. J. Blood Med. 2020, 11, 305. [Google Scholar] [CrossRef]
- Chiabrando, D.; Bertino, F.; Tolosano, E. Hereditary ataxia: A focus on heme metabolism and fe-s cluster biogenesis. Int. J. Mol. Sci. 2020, 21, 3760. [Google Scholar] [CrossRef]
- Bergmann, A.K.; Campagna, D.R.; McLoughlin, E.M.; Agarwal, S.; Fleming, M.D.; Bottomley, S.S.; Neufeld, E.J. Systematic molecular genetic analysis of congenital sideroblastic anemia: Evidence for genetic heterogeneity and identification of novel mutations. Pediatr. Blood Cancer 2010, 54, 273–278. [Google Scholar] [CrossRef]
- Camaschella, C. Recent advances in the understanding of inherited sideroblastic anaemia. Br. J. Haematol. 2008, 143, 27–38. [Google Scholar] [CrossRef]
- Furuyama, K.; Kaneko, K. Iron metabolism in erythroid cells and patients with congenital sideroblastic anemia. Int. J. Hematol. 2018, 107, 44–54. [Google Scholar] [CrossRef]
- Campanella, A.; Rovelli, E.; Santambrogio, P.; Cozzi, A.; Taroni, F.; Levi, S. Mitochondrial ferritin limits oxidative damage regulating mitochondrial iron availability: Hypothesis for a protective role in Friedreich ataxia. Hum. Mol. Genet. 2009, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Invernizzi, R.; Bergamaschi, G.; Levi, S.; Corsi, B.; Travaglino, E.; Rolandi, V.; Biasiotto, G.; Drysdale, J.; Arosio, P. Mitochondrial ferritin expression in erythroid cells from patients with sideroblastic anemia. Blood J. Am. Soc. Hematol. 2003, 101, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Cooley, T.B. A severe type of Hereditary Anemia with elliptocytosis-interisting sequence of splecectomy. Am. J. Med. Sci. 1945, 209, 561–568. [Google Scholar] [CrossRef]
- Ducamp, S.; Kannengiesser, C.; Touati, M.; Garçon, L.; Guerci-Bresler, A.; Guichard, J.F.; Vermylen, C.; Dochir, J.; Poirel, H.A.; Fouyssac, F.; et al. Sideroblastic anemia: Molecular analysis of the ALAS2 gene in a series of 29 probands and functional studies of 10 missense mutations. Hum. Mutat. 2011, 32, 590–597. [Google Scholar] [CrossRef]
- Bottomley, S.S.; Fleming, M.D. Sideroblastic anemia: Diagnosis and management. Hematol. Clin. 2014, 28, 653–670. [Google Scholar]
- Campagna, D.R.; de Bie, C.I.; Schmitz-Abe, K.; Sweeney, M.; Sendamarai, A.K.; Schmidt, P.J.; Heeney, M.M.; Yntema, H.G.; Kannengiesser, C.; Grandchamp, B.; et al. X-linked sideroblastic anemia due to ALAS2 intron 1 enhancer element GATA-binding site mutations. Am. J. Hematol. 2014, 89, 315–319. [Google Scholar] [CrossRef]
- Kaneko, K.; Furuyama, K.; Fujiwara, T.; Kobayashi, R.; Ishida, H.; Harigae, H.; Shibahara, S. Identification of a novel erythroid-specific enhancer for the ALAS2 gene and its loss-of-function mutation which is associated with congenital sideroblastic anemia. Haematologica 2014, 99, 252. [Google Scholar] [CrossRef]
- Cazzola, M.; May, A.; Bergamaschi, G.; Cerani, P.; Rosti, V.; Bishop, D.F. Familial-skewed X-chromosome inactivation as a predisposing factor for late-onset X-linked sideroblastic anemia in carrier females. Blood J. Am. Soc. Hematol. 2000, 96, 4363–4365. [Google Scholar]
- Aivado, M.; Gattermann, N.; Rong, A.; Giagounidis, A.A.; Prall, W.C.; Czibere, A.; Hildebrandt, B.; Haas, R.; Bottomley, S.S. X-linked sideroblastic anemia associated with a novel ALAS2 mutation and unfortunate skewed X-chromosome inactivation patterns. Blood Cells Mol. Dis. 2006, 37, 40–45. [Google Scholar] [CrossRef]
- Bekri, S.; Kispal, G.; Lange, H.; Fitzsimons, E.; Tolmie, J.; Lill, R.; Bishop, D.F. Human ABC7 transporter: Gene structure and mutation causing X-linked sideroblastic anemia with ataxia with disruption of cytosolic iron-sulfur protein maturation. Blood J. Am. Soc. Hematol. 2000, 96, 3256–3264. [Google Scholar]
- Heeney, M.M.; Berhe, S.; Campagna, D.R.; Oved, J.H.; Kurre, P.; Shaw, P.J.; Teo, J.; Shanap, M.A.; Hassab, H.M.; Glader, B.E.; et al. SLC25A38 congenital sideroblastic anemia: Phenotypes and genotypes of 31 individuals from 24 families, including 11 novel mutations, and a review of the literature. Hum. Mutat. 2021, 42, 1367–1383. [Google Scholar] [CrossRef] [PubMed]
- Guernsey, D.L.; Jiang, H.; Campagna, D.R.; Evans, S.C.; Ferguson, M.; Kellogg, M.D.; Lachance, M.; Matsuoka, M.; Nightingale, M.; Rideout, A.; et al. Mutations in mitochondrial carrier family gene SLC25A38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. Nat. Genet. 2009, 41, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guo, S.; Kang, H.; Zhang, F.; Hu, Y.; Wang, L.; Li, M.; Ru, Y.; Camaschella, C.; Han, B.; et al. Mutation spectrum in Chinese patients affected by congenital sideroblastic anemia and a search for a genotype-phenotype relationship. Haematologica 2013, 98, e158. [Google Scholar] [CrossRef] [PubMed]
- Casas, K.A.; Fischel-Ghodsian, N. Mitochondrial myopathy and sideroblastic anemia. Am. J. Med. Genet. Part A 2004, 125, 201–204. [Google Scholar] [CrossRef]
- Camaschella, C.; Campanella, A.; De Falco, L.; Boschetto, L.; Merlini, R.; Silvestri, L.; Levi, S.; Iolascon, A. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood J. Am. Soc. Hematol. 2007, 110, 1353–1358. [Google Scholar] [CrossRef]
- Liu, G.; Guo, S.; Anderson, G.J.; Camaschella, C.; Han, B.; Nie, G. Heterozygous missense mutations in the GLRX5 gene cause sideroblastic anemia in a Chinese patient. Blood J. Am. Soc. Hematol. 2014, 124, 2750–2751. [Google Scholar] [CrossRef]
- Daher, R.; Mansouri, A.; Martelli, A.; Bayart, S.; Manceau, H.; Callebaut, I.; Moulouel, B.; Gouya, L.; Puy, H.; Kannengiesser, C.; et al. GLRX5 mutations impair heme biosynthetic enzymes ALA synthase 2 and ferrochelatase in Human congenital sideroblastic anemia. Mol. Genet. Metab. 2019, 128, 342–351. [Google Scholar] [CrossRef]
- Ye, H.; Jeong, S.Y.; Ghosh, M.C.; Kovtunovych, G.; Silvestri, L.; Ortillo, D.; Uchida, N.; Tisdale, J.; Camaschella, C.; Rouault, T.A.; et al. Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. J. Clin. Investig. 2010, 120, 1749–1761. [Google Scholar] [CrossRef]
- Schmitz-Abe, K.; Ciesielski, S.J.; Schmidt, P.J.; Campagna, D.R.; Rahimov, F.; Schilke, B.A.; Cuijpers, M.; Rieneck, K.; Lausen, B.; Linenberger, M.L.; et al. Congenital sideroblastic anemia due to mutations in the mitochondrial HSP70 homologue HSPA9. Blood J. Am. Soc. Hematol. 2015, 126, 2734–2738. [Google Scholar] [CrossRef]
- Crispin, A.; Schmidt, P.; Campagna, D.; Cao, C.; Lichtenstein, D.; Sendamarai, A.; Guo, C.; Chen, C.; Hildick-Smith, G.J.; Huston, N.C.; et al. Hscb, a mitochondrial iron-sulfur cluster assembly co-chaperone, is a novel candidate gene for congenital sideroblastic anemia. Blood 2017, 130, 79. [Google Scholar] [CrossRef]
- Wingert, R.A.; Galloway, J.L.; Barut, B.; Foott, H.; Fraenkel, P.; Axe, J.L.; Weber, G.J.; Dooley, K.; Davidson, A.J.; Schmidt, B.; et al. Deficiency of glutaredoxin 5 reveals Fe–S clusters are required for vertebrate haem synthesis. Nature 2005, 436, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Donker, A.E.; Raymakers, R.A.; Vlasveld, L.T.; van Barneveld, T.; Terink, R.; Dors, N.; Brons, P.P.; Knoers, N.V.; Swinkels, D.W. Practice guidelines for the diagnosis and management of microcytic anemias due to genetic disorders of iron metabolism or heme synthesis. Blood J. Am. Soc. Hematol. 2014, 123, 3873–3886. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, R.S.; Campagna, D.R.; Greer, E.L.; Antiochos, B.; McDonald, A.; Chen, J.; Sharp, J.J.; Fujiwara, Y.; Barker, J.E.; Fleming, M.D. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 2005, 37, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Grandchamp, B.; Hetet, G.; Kannengiesser, C.; Oudin, C.; Beaumont, C.; Rodrigues-Ferreira, S.; Amson, R.; Telerman, A.; Nielsen, P.; Kohne, E.; et al. A novel type of congenital hypochromic anemia associated with a nonsense mutation in the STEAP3/TSAP6 gene. Blood J. Am. Soc. Hematol. 2011, 118, 6660–6666. [Google Scholar] [CrossRef]
- Romero-Cortadellas, L.; Hernández, G.; Ferrer-Cortès, X.; Zalba-Jadraque, L.; Fuster, J.L.; Bermúdez-Cortés, M.; Galera-Miñarro, A.M.; Pérez-Montero, S.; Tornador, C.; Sánchez, M. New Cases of Hypochromic Microcytic Anemia Due to Mutations in the SLC11A2 Gene and Functional Characterization of the G75R Mutation. Int. J. Mol. Sci. 2022, 23, 4406. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.D.; Trenor, C.C.; Su, M.A.; Foernzler, D.; Beier, D.R.; Dietrich, W.F.; Andrews, N.C. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat. Genet. 1997, 16, 383–386. [Google Scholar] [CrossRef]
- Iolascon, A.; d’Apolito, M.; Servedio, V.; Cimmino, F.; Piga, A.; Camaschella, C. Microcytic anemia and hepatic iron overload in a child with compound heterozygous mutations in DMT1 (SCL11A2). Blood 2006, 107, 349–354. [Google Scholar] [CrossRef]
- Iolascon, A.; De Falco, L.; Beaumont, C. Molecular basis of inherited microcytic anemia due to defects in iron acquisition or heme synthesis. Haematologica 2009, 94, 395. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, A.; Di Betto, G.; Bergamini, E.; Buzzetti, E.; Corradini, E.; Ventura, P. Iron Metabolism in the Disorders of Heme Biosynthesis. Metabolites 2022, 12, 819. https://doi.org/10.3390/metabo12090819
Ricci A, Di Betto G, Bergamini E, Buzzetti E, Corradini E, Ventura P. Iron Metabolism in the Disorders of Heme Biosynthesis. Metabolites. 2022; 12(9):819. https://doi.org/10.3390/metabo12090819
Chicago/Turabian StyleRicci, Andrea, Giada Di Betto, Elisa Bergamini, Elena Buzzetti, Elena Corradini, and Paolo Ventura. 2022. "Iron Metabolism in the Disorders of Heme Biosynthesis" Metabolites 12, no. 9: 819. https://doi.org/10.3390/metabo12090819
APA StyleRicci, A., Di Betto, G., Bergamini, E., Buzzetti, E., Corradini, E., & Ventura, P. (2022). Iron Metabolism in the Disorders of Heme Biosynthesis. Metabolites, 12(9), 819. https://doi.org/10.3390/metabo12090819






