Analysis of Different Methods of Extracting NSAIDs in Biological Fluid Samples for LC-MS/MS Assays: Scoping Review
Abstract
1. Introduction
2. Extraction Techniques Found in the Literature for Tests in Liquid Chromatography and Mass Spectrometry
2.1. Methods That Use Solvents
- I.
- Liquid–Liquid Extraction (LLE)
- II.
- Parallel artificial liquid membrane extraction (PALME)
- III.
- Magnetic solvent bar liquid-phase microextraction (MSB-LPME)
2.2. Methods That Use Sorbents
- I.
- Solid-phase microextraction in-tube online (IN-TUBE SPE)
2.3. Scoping Review
- What is the methodology and/or techniques most used for the extraction of non-steroidal anti-inflammatory drugs in bioanalytical assays using high-performance LC-MS/MS?
3. Material and Methods
- I.
- The selection of studies was carried out by two reviewers independently so that in the first step (pre-selection), titles and abstracts were read and, in the second step, the full texts, to filter only those that were compatible with the eligibility criterion. The following inclusion criteria were applied:
- II.
- Studies with NSAID;
- III.
- Studies with LC-MS/MS;
- IV.
- Studies in English;
- V.
- Studies that presented similar analytical methodology;
- VI.
- Studies covering extraction methods targeting well-established classical techniques and their miniaturization.
- I.
- Studies with drugs other than NSAID;
- II.
- Studies with gas, ion-exchange, and affinity chromatography;
- III.
- Studies carried out with animals;
- IV.
- Literature reviews;
- V.
- Not published in English;
- VI.
- Studies that did not allow access to the full content.
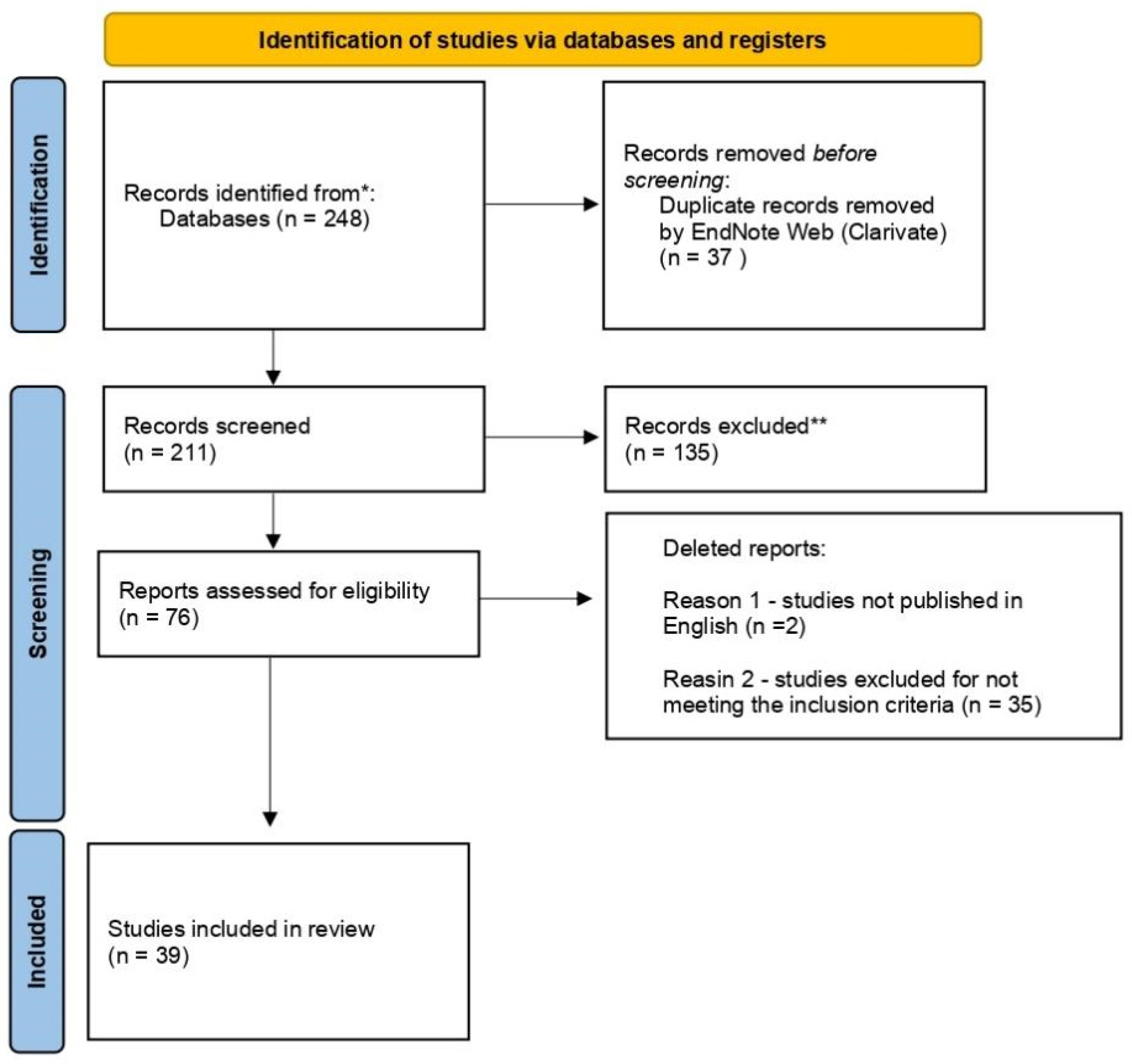
4. Results
4.1. Selection of Studies
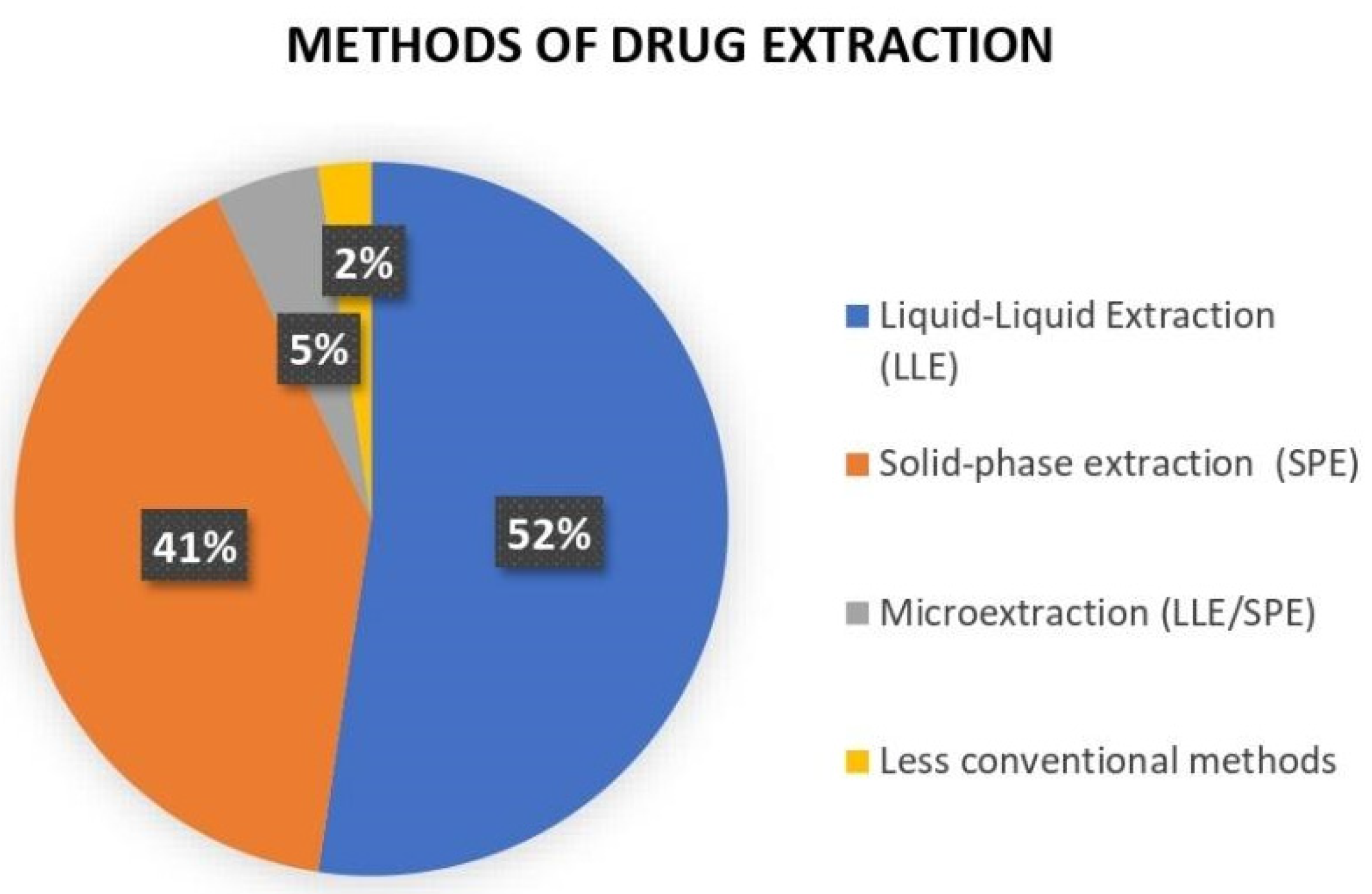
4.2. Study Characteristics
4.3. Summary of Included Studies
4.4. Limitations of the Scoping Review Process
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rollason, V.; Samer, C.F.; Daali, Y.; Desmeules, J.A. Prediction by pharmacogenetics of safety and efficacy of non-steroidal anti- inflammatory drugs: A review. Curr. Drug Metab. 2014, 15, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Abbott, P.V. Drugs for pain management in dentistry. Aust. Dent. J. 2005, 50, S14–S22. [Google Scholar] [CrossRef] [PubMed]
- Suhr, A.C.; Bruegel, M.; Maier, B.; Holdt, L.M.; Kleinhempel, A.; Teupser, D.; Grimm, S.H.; Vogeser, M. Ferromagnetic particles as a rapid and robust sample preparation for the absolute quantification of seven eicosanoids in human plasma by UHPLC–MS/MS. J. Chromatogr. B 2016, 1022, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Armando, A.M.; Quehenberger, O.; Yan, C.; Dennis, E.A. Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples. J. Chromatogr. A 2014, 1359, 60–69. [Google Scholar] [CrossRef]
- Laaks, J.; Jochmann, M.A.; Schmidt, T.C. Solvent-free microextraction techniques in gas chromatography. Anal. Bioanal. Chem. 2012, 402, 565–571. [Google Scholar] [CrossRef]
- He, J.; Huang, M.; Wang, D.; Zhang, Z.; Li, G. Magnetic separation techniques in sample preparation for biological analysis: A review. J. Pharm. Biomed. Anal. 2014, 101, 84–101. [Google Scholar] [CrossRef]
- Leung, K.S.-Y.; Fong, B.M.-W. LC-MS/MS in the routine clinical laboratory: Has its time come? Anal. Bioanal. Chem. 2014, 406, 2289–2301. [Google Scholar] [CrossRef]
- Huang, Z.; Lee, H.K. Materials-based approaches to minimizing solvent usage in analytical sample preparation. TrAC Trends Anal. Chem. 2012, 39, 228–244. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Milani Hosseini, M.-R.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Gopinath, S.; Kumar, R.S.; Shankar, M.B.; Danabal, P. Development and validation of a sensitive and high-throughput LC-MS/MS method for the simultaneous determination of esomeprazole and naproxen in human plasma. Biomed. Chromatogr. 2013, 27, 894–899. [Google Scholar] [CrossRef]
- Altun, Z.; Abdel-Rehim, M. Study of the factors affecting the performance of microextraction by packed sorbent (MEPS) using liquid scintillation counter and liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2008, 630, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.S.; Engelhardt, T.; Cameron, G.A.; Hawwa, A.F.; Helms, P.J.; McLay, J.S. Development of an enantiomer selective microsampling assay for the quantification of ketorolac suitable for paediatric pharmacokinetic studies. Biopharm. Drug Dispos. 2013, 34, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Bolani, B.; Oliveira, G.M.; Dionísio, T.J.; Faria, F.A.C.; Fernandes, M.H.R.; Santos, C.F.; Calvo, A.M. Pharmacogenetic and Pharmacokinetic Assays from Saliva Samples Can Guarantee Personalized Drug Prescription. Braz. Dent. J. 2021, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Barrientos-Astigarraga, R.E.; Vannuchi, Y.B.; Sucupira, M.; Moreno, R.A.; Muscará, M.N.; De Nucci, G. Quantification of nimesulide in human plasma by high-performance liquid chromatography/tandem mass spectrometry. Application to bioequivalence studies. J. Mass Spectrom. 2001, 36, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Ask, K.S.; Øiestad, E.L.; Pedersen-Bjergaard, S.; Gjelstad, A. Dried blood spots and parallel artificial liquid membrane extraction—A simple combination of microsampling and microextraction. Anal. Chim. Acta 2018, 1009, 56–64. [Google Scholar] [CrossRef]
- Roldán-Pijuán, M.; Pedersen-Bjergaard, S.; Gjelstad, A. Parallel artificial liquid membrane extraction of acidic drugs from human plasma. Anal. Bioanal. Chem. 2015, 407, 2811–2819. [Google Scholar] [CrossRef]
- Li, X.; Huang, A.; Liao, X.; Chen, J.; Xiao, Y. Restricted access supramolecular solvent based magnetic solvent bar liquid-phase microextraction for determination of non-steroidal anti-inflammatory drugs in human serum coupled with high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1634, 461700. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Razavi, N.; Yazdinejad, S.R. Separation and determination of amitriptyline and nortriptyline by dispersive liquid-liquid microextraction combined with gas chromatography flame ionization detection. Talanta 2008, 75, 1293–1299. [Google Scholar] [CrossRef]
- Huck, C.W.; Bonn, G.K. Recent developments in polymer-based sorbents for solid-phase extraction. J. Chromatogr. A 2000, 885, 51–72. [Google Scholar] [CrossRef]
- Bräutigam, L.; Nefflen, J.U.; Geisslinger, G. Determination of etoricoxib in human plasma by liquid chromatography-tandem mass spectrometry with electrospray ionisation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 788, 309–315. [Google Scholar] [CrossRef]
- Dongari, N.; Sauter, E.R.; Tande, B.M.; Kubátová, A. Determination of Celecoxib in human plasma using liquid chromatography with high resolution time of flight-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 955–956, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hoke, S.H., 2nd; Pinkston, J.D.; Bailey, R.E.; Tanguay, S.L.; Eichhold, T.H. Comparison of packed-column supercritical fluid chromatography--tandem mass spectrometry with liquid chromatography—Tandem mass spectrometry for bioanalytical determination of (R)- and (S)-ketoprofen in human plasma following automated 96-well solid-pha. Anal. Chem. 2000, 72, 4235–4241. [Google Scholar] [CrossRef] [PubMed]
- Nakov, N.; Petkovska, R.; Ugrinova, L.; Kavrakovski, Z.; Dimitrovska, A.; Svinarov, D. Critical development by design of a rugged HPLC-MS/MS method for direct determination of ibuprofen enantiomers in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 992, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.P.; Sharma, P.; Sanyal, M.; Singhal, P.; Shrivastav, P.S. Challenges in the simultaneous quantitation of sumatriptan and naproxen in human plasma: Application to a bioequivalence study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 902, 122–131. [Google Scholar] [CrossRef]
- Sultan, M.; Stecher, G.; Stöggl, W.M.; Bakry, R.; Zaborski, P.; Huck, C.W.; El Kousy, N.M.; Bonn, G.K. Sample pretreatment and determination of non steroidal anti-inflammatory drugs (NSAIDs) in pharmaceutical formulations and biological samples (blood, plasma, erythrocytes) by HPLC-UV-MS and micro-HPLC. Curr. Med. Chem. 2005, 12, 573–588. [Google Scholar] [CrossRef]
- Toffoli, A.; Lancas, F. Recentes avanços da microextração em fase sólida no tubo (in-tube SPME) e sua aplicação em análises ambientais e alimentícias. Sci. Chromatogr. 2015, 7, 297–315. [Google Scholar] [CrossRef][Green Version]
- Kataoka, H.; Ishizaki, A.; Nonaka, Y.; Saito, K. Developments and applications of capillary microextraction techniques: A review. Anal. Chim. Acta 2009, 655, 8–29. [Google Scholar] [CrossRef]
- Yu, Q.W.; Wang, X.; Ma, Q.; Yuan, B.-F.; He, H.-B.; Feng, Y.-Q. Automated analysis of non-steroidal anti-inflammatory drugs in human plasma and water samples by in-tube solid-phase microextraction coupled to liquid chromatography-mass spectrometry based on a poly(4-vinylpyridine-co-ethylene dimethacrylate) monolith. Anal. Methods 2012, 4, 1538–1545. [Google Scholar] [CrossRef]
- Cordeiro, L.; Soares, C.B. Revisão de escopo: Potencialidades para a síntese de metodologias utilizadas em pesquisa primária qualitativa. Bol. do Inst. Saúde BIS 2019, 20, 37–43. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. Susanne Hempel PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Banda, J.; Lakshmanan, R.; Katepalli, R.B.; Reddy Venati, U.K.; Koppula, R.; Shiva Prasad, V.V.S. Determination of mesalazine, a low bioavailability olsalazine metabolite in human plasma by UHPLC-MS/MS: Application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1008, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n72. [Google Scholar]
- Bharwad, K.D.; Shah, P.A.; Shrivastav, P.S.; Sharma, V.S.; Singhal, P. Quantification of fenoprofen in human plasma using UHPLC-tandem mass spectrometry for pharmacokinetic study in healthy subjects. Biomed. Chromatogr. 2020, 34, e4708. [Google Scholar] [CrossRef] [PubMed]
- Bonato, P.S.; Del Lama, M.P.F.M.; de Carvalho, R. Enantioselective determination of ibuprofen in plasma by high-performance liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 796, 413–420. [Google Scholar] [CrossRef]
- Brêtas, J.M.; César, I.C.; Brêtas, C.M.; Teixeira, L.d.S.; Bellorio, K.B.; Mundim, I.M.; Pianetti, G.A. Development and validation of an LC-ESI-MS/MS method for the simultaneous quantification of naproxen and sumatriptan in human plasma: Application to a pharmacokinetic study. Anal. Bioanal. Chem. 2016, 408, 3981–3992. [Google Scholar] [CrossRef]
- Calvo, A.M.; Santos, G.M.; Dionísio, T.J.; Marques, M.P.; Brozoski, D.T.; Lanchote, V.L.; Fernandes, M.H.R.; Faria, F.A.C.; Santos, C.F. Quantification of piroxicam and 5′-hydroxypiroxicam in human plasma and saliva using liquid chromatography-tandem mass spectrometry following oral administration. J. Pharm. Biomed. Anal. 2016, 120, 212–220. [Google Scholar] [CrossRef]
- Dionísio, T.J.; Oliveira, G.M.; Morettin, M.; Faria, F.C.; Santos, C.F.; Calvo, A.M. Simultaneous separation of naproxen and 6-O-desmethylnaproxen metabolite in saliva samples by liquid chromatography-tandem mass spectrometry: Pharmacokinetic study of naproxen alone and associated with esomeprazole. PLoS ONE 2020, 15, e0236297. [Google Scholar] [CrossRef]
- Dubey, N.K.; Haq, K.U.; Fozdar, B.I. Overcoming the Charged Ion Competition in Triple Quad Chemical Ionization Ion Source for Estimation of Celecoxib in Biological Matrix and its Application to Bioequivalence Study. Drug Res. 2019, 69, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Halder, D.; Dan, S.; Sarkar, P.; Das, D.; Chandra Halder, U.; Kumar Pal, T. LC-MS/MS determination of 4-hydroxynimesulide, an active metabolite of nimesulide and application to bioequivalence study in Indian subjects. Eur. J. Mass Spectrom. 2019, 25, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Seo, J.-H.; Kim, Y.-W.; Jeong, S.-Y.; Lee, K.-T. Determination of zaltoprofen in human plasma by liquid chromatography with electrospray tandem mass spectrometry: Application to a pharmacokinetic study. Rapid Commun. Mass Spectrom. 2006, 20, 2675–2680. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kang, I.-M.; Lee, H.-W.; Seo, J.-H.; Ryu, J.-H.; Choi, S.-J.; Lee, M.-J.; Jeong, S.-Y.; Cho, Y.-W.; Lee, K.-T. Development and validation of a high performance liquid chromatography-tandem mass spectrometry for the determination of etodolac in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 863, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-I.; Choi, C.-I.; Byeon, J.-Y.; Lee, J.-E.; Park, S.-Y.; Kim, Y.-H.; Kim, S.-H.; Lee, Y.-J.; Jang, C.-G.; Lee, S.-Y. Simultaneous determination of flurbiprofen and its hydroxy metabolite in human plasma by liquid chromatography-tandem mass spectrometry for clinical application. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 971, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, M.; Dhaneshwar, S.; Bhavsar, R. A high performance liquid chromatography-tandem mass spectrometric method for the determination of mefenamic acid in human plasma: Application to pharmacokinetic study. Biomed. Chromatogr. 2012, 26, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Nakov, N.; Bogdanovska, L.; Acevska, J.; Tonic-Ribarska, J.; Petkovska, R.; Dimitrovska, A.; Kasabova, L.; Svinarov, D. High-Throughput HPLC-MS/MS Method for Quantification of Ibuprofen Enantiomers in Human Plasma: Focus on Investigation of Metabolite Interference. J. Chromatogr. Sci. 2016, 54, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Rathod, R.; Padh, H. Quantification of 4-methylaminoantipyrine, the active metabolite of dipyrone, in human plasma. Bioanalysis 2009, 1, 293–298. [Google Scholar] [CrossRef]
- Park, M.-S.; Shim, W.-S.; Yim, S.-V.; Lee, K.-T. Development of simple and rapid LC-MS/MS method for determination of celecoxib in human plasma and its application to bioequivalence study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 902, 137–141. [Google Scholar] [CrossRef]
- Patel, B.N.; Sharma, N.; Sanyal, M.; Prasad, A.; Shrivastav, P.S. High-throughput LC-MS/MS assay for 6-methoxy-2-naphthylacetic acid, an active metabolite of nabumetone in human plasma and its application to bioequivalence study. Biomed. Chromatogr. 2008, 22, 1213–1224. [Google Scholar] [CrossRef]
- Patel, D.S.; Sharma, N.; Patel, M.C.; Patel, B.N.; Shrivastav, P.S.; Sanyal, M. Sensitive and selective determination of diflunisal in human plasma by LC-MS. J. Chromatogr. Sci. 2013, 51, 872–882. [Google Scholar] [CrossRef]
- Scott, R.J.; Palmer, J.; Lewis, I.A.; Pleasance, S. Determination of a “GW cocktail” of cytochrome P450 probe substrates and their metabolites in plasma and urine using automated solid phase extraction and fast gradient liquid chromatography tandem mass spectrometry. Rapid Commun. Mass Spectrom. 1999, 13, 2305–2319. [Google Scholar] [CrossRef]
- Shinde, D.D.; Kim, K.-B.; Oh, K.-S.; Abdalla, N.; Liu, K.-H.; Bae, S.K.; Shon, J.-H.; Kim, H.-S.; Kim, D.-H.; Shin, J.G. LC-MS/MS for the simultaneous analysis of arachidonic acid and 32 related metabolites in human plasma: Basal plasma concentrations and aspirin-induced changes of eicosanoids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 911, 113–121. [Google Scholar] [CrossRef]
- Shirako, J.; Kawasaki, M.; Komine, K.; Kunisue, Y.; Terada, M.; Sasaki, C.; Irie, W.; Murakami, C.; Tonooka, K.; Tomobe, K. Simultaneous determination for oxicam non-steroidal anti-inflammatory drugs in human serum by liquid chromatography-tandem mass spectrometry. Forensic Sci. Int. 2013, 227, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Suenami, K.; Lim, L.W.; Takeuchi, T.; Sasajima, Y.; Sato, K.; Takekoshi, Y.; Kanno, S. Rapid and simultaneous determination of nonsteroidal anti-inflammatory drugs in human plasma by LC-MS with solid-phase extraction. Anal. Bioanal. Chem. 2006, 384, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xue, K.-L.; Jiao, X.-Y.; Chen, Q.; Xu, L.; Zheng, H.; Ding, Y.-F. Simultaneous determination of nimesulide and its four possible metabolites in human plasma by LC-MS/MS and its application in a study of pharmacokinetics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1027, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.J.; Jones, C.E.; Dodds, H.M.; Hogan, N.S.; Johnson, A.G. Plasma indomethacin assay using high-performance liquid chromatography-electrospray-tandem mass spectrometry: Application to therapeutic drug monitoring and pharmacokinetic studies. Ther. Drug Monit. 1998, 20, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Werner, U.; Werner, D.; Pahl, A.; Mundkowski, R.; Gillich, M.; Brune, K. Investigation of the pharmacokinetics of celecoxib by liquid chromatography-mass spectrometry. Biomed. Chromatogr. 2002, 16, 56–60. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Fast, D.M.; Breau, A.P. Development and validation of an automated SPE-LC-MS/MS assay for valdecoxib and its hydroxylated metabolite in human plasma. J. Pharm. Biomed. Anal. 2003, 33, 61–72. [Google Scholar] [CrossRef]
- Tehranirokh, M.; Van den Bronk, M.; Smith, P.; Dai, Z.; Ragunathan, K.; Muscalu, A.; Mills, S.; Breadmore, M.C.; Shellie, R.A. Automated liquid-liquid extraction of organic compounds from aqueous samples using a multifunction autosampler syringe. J. Chromatogr. A 2021, 1642, 462032. [Google Scholar] [CrossRef]
- Bitas, D.; Kabir, A.; Locatelli, M.; Samanidou, V. Food Sample Preparation for the Determination of Sulfonamides by High-Performance Liquid Chromatography: State-of-the-Art. Separations 2018, 5, 31. [Google Scholar] [CrossRef]
- Khatibi, S.A.; Hamidi, S.; Siahi-Shadbad, M.R. Application of Liquid-Liquid Extraction for the Determination of Antibiotics in the Foodstuff: Recent Trends and Developments. Crit. Rev. Anal. Chem. 2022, 52, 327–342. [Google Scholar] [CrossRef]
- Junza, A.; Dorival-García, N.; Zafra-Gómez, A.; Barrón, D.; Ballesteros, O.; Barbosa, J.; Navalón, A. Multiclass method for the determination of quinolones and β-lactams, in raw cow milk using dispersive liquid–liquid microextraction and ultra high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1356, 10–22. [Google Scholar] [CrossRef]
- Kechagia, M.; Samanidou, V. Trends in Microextraction-Based Methods for the Determination of Sulfonamides in Milk. Separations 2017, 4, 23. [Google Scholar] [CrossRef]
- Alampanos, V.; Samanidou, V.; Papadoyannis, I. Trends in sample preparation for the hplc determination of penicillins in biofluids. J. Appl. Bioanal. 2019, 5, 9–17. [Google Scholar] [CrossRef]
- Quintana, J.B.; Rodríguez, I. Strategies for the microextraction of polar organic contaminants in water samples. Anal. Bioanal. Chem. 2006, 384, 1447–1461. [Google Scholar] [CrossRef] [PubMed]
- Gjelstad, A.; Rasmussen, K.E.; Parmer, M.P.; Pedersen-Bjergaard, S. Parallel artificial liquid membrane extraction: Micro-scale liquid-liquid-liquid extraction in the 96-well format. Bioanalysis 2013, 5, 1377–1385. [Google Scholar] [CrossRef]
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1043, 44–62. [Google Scholar] [CrossRef]
- Ranjbar Banforuzi, S.; Hadjmohammadi, M.R. Two-phase hollow fiber-liquid microextraction based on reverse micelle for the determination of quercetin in human plasma and vegetables samples. Talanta 2017, 173, 14–21. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Rubio, S.; Pérez-Bendito, D. Potential of supramolecular solvents for the extraction of contaminants in liquid foods. J. Chromatogr. A 2009, 1216, 530–539. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Manbohi, A.; Heydar, K.T. Electrochemically controlled in-tube solid phase microextraction of naproxen from urine samples using an experimental design. Analyst 2015, 140, 497–505. [Google Scholar] [CrossRef]
- Queiroz, M.E.C.; Melo, L.P. Selective capillary coating materials for in-tube solid-phase microextraction coupled to liquid chromatography to determine drugs and biomarkers in biological samples: A review. Anal. Chim. Acta 2014, 826, 1–11. [Google Scholar] [CrossRef]
| Author/Year/Country | Type of Study | Analyte | Equipment | Ionization | Sample Method Preparation | Matrix |
|---|---|---|---|---|---|---|
| Ask, 2018 Norway, [15] | Microsampling Assay | Amitriptyline, quetiapine, ketoprofen, fenoprofen, flurbiprofen and ibuprofen | UHPLC-MS/MS Thermo Scientific LTQ XL Linear Ion Trap (Thermo Scientific, Califónia, USA) | Electrospray (ESI) | Liquid–Liquid Extraction (LLE) Parallel Artificial Liquid Membrane Extraction (PALME) | Whole Blood |
| Banda, 2016 India, [32] | Bioanalytical Assay | Olsalazine Sodium | UHPLC (Shimadzu, Kyoto, Japan) MS/MS Triple quadrupole API-6500 (MDS Sciex, Ontário, Canada) | Turbo Ion spray | LLE | Human Plasma |
| Barrientos-Astigarraga, 2001 Brazil, [14] | Bioequivalence Study | Nimesulide | LCMS/MS Micromass Quattro II (Waters Corporation/Micromass Uk Ltd., Manchester, UK) | ESI | LLE | Human Plasma |
| Bharwad, 2020 India, [33] | Pharmacokinetic Study | Fenoprofen | UHPLC Waters Acquity (Waters Corporation, Massachusetts, USA) MS/MS Quattro Premier XE™ (Waters Micro Mass Technologies, Massachusetts, USA) | ESI | Solid-Phase Extraction (SPE)—Orochem DVB-LP | Human Plasma |
| Bolani, 2021 Brazil, [13] | Bioanalytical Assay | Piroxicam | LCMS/MS Triple quadrupole Quattro Micro (Waters Corporation/Micromass Uk Ltd., Manchester, UK) | ESI | LLE | Saliva |
| Bonato, 2003 Brazil, [34] | Enantioselective Analysis | Ibuprofen | HPLC (Shimadzu, Kyoto, Japan) MS/MS Triple quadrupole Quattro Micro (Waters Corporation/Micromass Uk Ltd., Manchester, UK) | ESI | LLE | Human Plasma |
| Brautigam, 2003 Germany, [20] | Bioanalytical Assay | Etoricoxib | LC Degasser Jasco DG 1580-53 (Gross-Umstadt, Germany) MS/MS Triple quadrupole API 3000 (Applied Biosystems, Langen, Germany) | ESI | SPE—Oasis HLB | Human Plasma |
| Brêtas, 2016 Brazil, [35] | Bioanalytical Assay | Naproxen and sumatriptan | LC-ESI-MS/MS Waters System (Waters Corporation, Massachusetts, USA) MS/MS Quattro LC—triple quadrupole (Waters Corporation, Massachusetts, USA) | ESI | LLE | Human Plasma |
| Calvo, 2016 Brazil, [36] | Bioanalytical Assay | Piroxicam and 5′-hidroxypiroxicam | LCMS/MS Triple quadrupole Quatto Micro (Micromass UK Ltd., Manchester, UK) | ESI | LLE | Human Plasma and Saliva |
| Díonisio, 2020 Brazil, [37] | Bioanalytical Assay | Naproxen | LCMS/MS quadrupole 8040 (Shimadzu, Kyoto, Japan) | ESI | LLE | Saliva |
| Dongari, 2014 USA, [21] | Bioanalytical Assay | Celecoxib | LC–ESI–TOF–MS HPLC Agilent 1100 Series with a Agilent G1969 TOF/MS System (Agilent, California, EUA) | ESI | SPE—Bond Elute C 18 | Human Plasma |
| Dubey, 2019 India, [38] | Bioanalytical Assay | Celecoxib | LC-10 (Shimadzu, Kyoto, Japan) MS/MS API 3200 (MDS Sciex, Ontario, Canada) | Turbo Ion spray | LLE | Human Plasma |
| Eichhold, 2000 USA, [22] | Bioanalytical Assay | (R)- and (S)-Ketoprofen | HPLC modular Gilson (Gilson Inc., Wisconsin, USA) MS/MS PerkinElmer API III + (MDS Sciex, Ontario, Canada) | ESI | SPE—Oasis HLB | Human Plasma |
| Gopinath, 2013 India, [10] | Bioanalytical Assay | Naproxen and Esomeprazole | LCMS/MS Agilent Technologies series 1200 triple quadrupole Agilent 6460 (Agilent Technologies, Germany) | ESI | SPE—Oasis HLB | Human Plasma |
| Halder, 2019 India, [39] | Bioequivalence Study | Nimesulide and 4-hidroxynimesulide | LCMS/MS API 2000 MS/MS Tandem triple quadrupole (MDS Sciex, Ontario, Canada) | ESI | LLE | Human Plasma |
| Hoke, 2000 USA, [22] | Bioanalytical Assay | Cetoprofen | LCMS/MS PerkinElmer API III + (MDS Sciex, Ontario, Canada) IMPROVED FLUIDITY LIQUID CHROMATOGRAPHY (pcSFC-MS/MS) Gilson (Gilson Inc., Wisconsin, USA) | Turbo Ion spray/ESI | SPE—Oasis HLB | Human Plasma |
| Lee, 2006 Korea, [40] | Pharmacokinetic Study | Zaltoprofen | HPLC Waters 2795 MS/MS Triple quadrupole Waters Micromass Quattro Premier (Waters Corporation/Micromass UL Ltd., Watford, UK) | ESI | LLE | Human Plasma |
| Lee, 2008, [41] Korea | Bioanalytical Assay | Etodolac | HPLC Waters 2795 MS/MS Triple quadrupole Waters Micromass Quattro Premier (Waters Corporation/Micromass Uk Ltd., Watford, UK) | ESI | LLE | Human Plasma |
| Lee, 2014 Korea, [42] | Bioanalytical Assay | Flurbiprofen | HPLC Agilent 1200 series (Agilent Technologies Inc., California, USA) MS/MS API 3200 (MDS Sciex, Ontario, Canada) | ESI | LLE | Human Plasma |
| Li, 2020 China, [17] | Bioanalytical Assay | Cetoprofen, naproxen, indomethacin, and diclofenac | HPLC 20A (Shimadzu, Kyoto, Japan) MS/MS Triple quadrupole 4000 QTrap (AB Sciex, Washington, USA) | ESI | Liquid-phase microextraction based on supramolecular magnetic solvent HFIP-alkanol with solvent bar (MSB-LPME based on HFIP-alkanol SUPRAS) | Human Serum |
| Mahadik, 2012 India, [43] | Bioanalytical Assay | Mefenamic Acid | LCMS/MS PerkinElmer API-3000 (MDS Sciex, EUA) coupled to high performance liquid chromatography (Shimadzu, Kyoto, Japão) | Atmospheric Pressure Chemical Ionization (APCI) | LLE | Human Plasma |
| Mohammed, 2013, United Kingdom, [14] | Microsampling Assay | Ketorolac | HPLC-MS/MS TSQ Quantum Discovery Max triple quadripole (Thermo Scientific, USA) | ESI | LLE | Human Plasma |
| Nakov, 2015 Macedonia, [23] | Bioanalytical Assay | Ibuprofen | HPLC-MS/MS TSQ Quantum Discovery Max triple quadripole (Thermo Scientific, USA) | ESI | LLE/SPE | Human Plasma |
| Nakov, 2016 Macedonia, [44] | Bioanalytical Assay | Ibuprofen | HPLC-MS/MS TSQ Quantum Discovery Max triple quadripole (Thermo Scientific, USA) | ESI | LLE | Human Plasma |
| Ojha, 2009 India, [45] | Bioanalytical Method Validation | 4-methylaminoantipyrine—dipyrone active metabolite | LC—Atmospheric pressure ionization (Ion Spray) MS Simple Quadrupole (PerkinElmer MDS Sciex, USA) | APCI | LLE | Human Plasma |
| Park, 2012, [46] Korea | Bioanalytical Assay | Celecoxib | HPLC Agilent 1100 (Agilent, USA) MS/MS Triple quadrupole API-2000 (MDS Sciex, Ontario, Canada) | ESI | LLE | Human Plasma |
| Patel, 2008 India, [47] | Bioanalytical Assay | 6-methoxy-2-naphthylacetic acid - nabumetone active metabolite | LCMS/MS Triple quadrupole API-3000 (Shimadzu, Kyoto, Japan) | Turbo Ion spray | SPE—Oasis HLB Cartridges | Human Plasma |
| Patel, 2012 India, [24] | Bioanalytical Assay | Sumatriptan and naproxen | UPLC Waters Acquity System and a triple quadrupole Waters Quattro Premier XE (Waters Corporation, Massachusetts, USA) | ESI | SPE —Phenomenex Strata-X Cartridges | Human Plasma |
| Patel, 2013 India, [48] | Bioanalytical Assay | Diflunisal—salicylic acid difluorophenyl derivative | LCMS/MS Triple quadrupole API-3000 (Shimadzu, Kyoto, Japan) | ESI | SPE—Oasis HLB Cartridges | Human Plasma |
| Scott, 1999 United Kingdom, [49] | Bioanalytical Assay | Green ford-ware cocktail (Diclofenac) | LCMS/MS 200 series triple quadrupole API-365 (PerkinElmer MDS Sciex, Onario, Canada) | Turbo Ion spray/ESI | 96-extraction-well HLB SPE block—automated extraction | Human Plasma and Urine |
| Shinde, 2012 Korea, [50] | Bioanalytical Assay | Aspirine | HPLC Agilent 1200 series (Applied Biosystems, California, USA) MS/MS QTrap 5500 (Applied Biosystems, California, USA) | ESI | SPE—Discovery DSC-C8 cartridges | Human Plasma |
| Shirako, 2013, Japan, [51] | Bioanalytical Assay | Ampiroxicam, tenoxicam, piroxicam, meloxicam, and lornoxicam | LCMS/MS API-4000 (AB Sciex, Massachusetts, USA) | ESI | MAX-SPE—Oasis cartridges column | Human Plasma |
| Suenami, 2006 Japan, [52] | Bioanalytical Assay | Acetaminofen, aspirine, loxoprofen, cetoprofen, acemetacin, oxaprozin, fenoprofen, flurbiprofen, indomethacin, diclofenac, ibuprofen, henylbutazone, flufenamic acid, mefenamic acid, tolfenamic acid, and naproxen | HPLC Alliance 2690 coupled to MS/MS Quadrupole Micromass ZMD (Waters Corporation, Massachusetts, USA) | ESI | SPE—Oasis HLB cartridges | Human Plasma |
| Sultan, 2005 Egypt, [25] | Bioanalytical Assay | Salicin, salicylic acid, tenoxicam, ketorolac, piroxicam, tolmetin, naproxen, flurbiprofen, diclofenac, and ibuprofen | HPLC 616 model (Waters Corporation, Massachusetts, USA) coupled to a MS/MS Finnigan-MAT TSQ triple quadrupole (Thermo Finnigan, California, USA) | APCI | LLE/SPE—copolymer-based cartridges (poli(N-vinilimidazol-co-divinilbenzeno)) | Human Plasma and Whole Blood |
| Sun, 2016 China, [53] | Bioanalytical Assay | Nimesulide | LC (Shimadzu, Kyoto, Japan) coupled to a MS/MS QTrap5500 (Applied Biosystems, California, USA) | ESI | LLE | Human Plasma |
| Taylor, 1998 Australia, [54] | Bioanalytical Assay | Indometacin | HPLC (Waters Corporation, Massachusetts, USA) coupled to a MS/MS quadruple API III (PerkinElmer MDS Sciex, Ontario, Canada) | ESI | SPE | Human Plasma |
| Werner, 2002 Germany, [55] | Bioanalytical Assay | Celecoxib | HPLC (Jasco, Groß-Umstadt, Germany) MS/MS Trap Finnigan MAT LCQ (Thermoquest, Egelsbach, Germany) | APCI | LLE | Human Plasma |
| Yu, 2012 China, [28] | Bioanalytical Assay | Cetoprofen, fenbufen, and ibuprofen | LC-MS-2010EV HPLC-ESI/MS (Shimadzu, Kioto, Japan) | ESI | In-tube solid-phase microextraction (IN-TUBE SPE) | Human Plasma and Environmental Water |
| Zhang, 2003 USA, [56] | Bioanalytical Assay | Valdecoxib | HPLC Agilent 1050 (Agilent, California, USA) MS/MS Quadrupole PerkinElmer Sciex API-III-Plus (Ontario, Canada) | ESI | Autmated system SPE RapidTrace™—Bond Elut cartridges | Human Plasma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siqueira Sandrin, V.S.; Oliveira, G.M.; Weckwerth, G.M.; Polanco, N.L.D.H.; Faria, F.A.C.; Santos, C.F.; Calvo, A.M. Analysis of Different Methods of Extracting NSAIDs in Biological Fluid Samples for LC-MS/MS Assays: Scoping Review. Metabolites 2022, 12, 751. https://doi.org/10.3390/metabo12080751
Siqueira Sandrin VS, Oliveira GM, Weckwerth GM, Polanco NLDH, Faria FAC, Santos CF, Calvo AM. Analysis of Different Methods of Extracting NSAIDs in Biological Fluid Samples for LC-MS/MS Assays: Scoping Review. Metabolites. 2022; 12(8):751. https://doi.org/10.3390/metabo12080751
Chicago/Turabian StyleSiqueira Sandrin, Viviane Silva, Gabriela Moraes Oliveira, Giovana Maria Weckwerth, Nelson Leonel Del Hierro Polanco, Flávio Augusto Cardoso Faria, Carlos Ferreira Santos, and Adriana Maria Calvo. 2022. "Analysis of Different Methods of Extracting NSAIDs in Biological Fluid Samples for LC-MS/MS Assays: Scoping Review" Metabolites 12, no. 8: 751. https://doi.org/10.3390/metabo12080751
APA StyleSiqueira Sandrin, V. S., Oliveira, G. M., Weckwerth, G. M., Polanco, N. L. D. H., Faria, F. A. C., Santos, C. F., & Calvo, A. M. (2022). Analysis of Different Methods of Extracting NSAIDs in Biological Fluid Samples for LC-MS/MS Assays: Scoping Review. Metabolites, 12(8), 751. https://doi.org/10.3390/metabo12080751






