Metabolic Profiling of Heliotropium crispum Aerial Parts Using HPLC and FTIR and In Vivo Evaluation of Its Anti-Ulcer Activity Using an Ethanol Induced Acute Gastric Ulcer Model
Abstract
1. Introduction
2. Results
2.1. Metabolic Profiling of H. crispum Aerial Parts
2.1.1. Fourier-Transform Infrared Spectroscopy (FTIR)
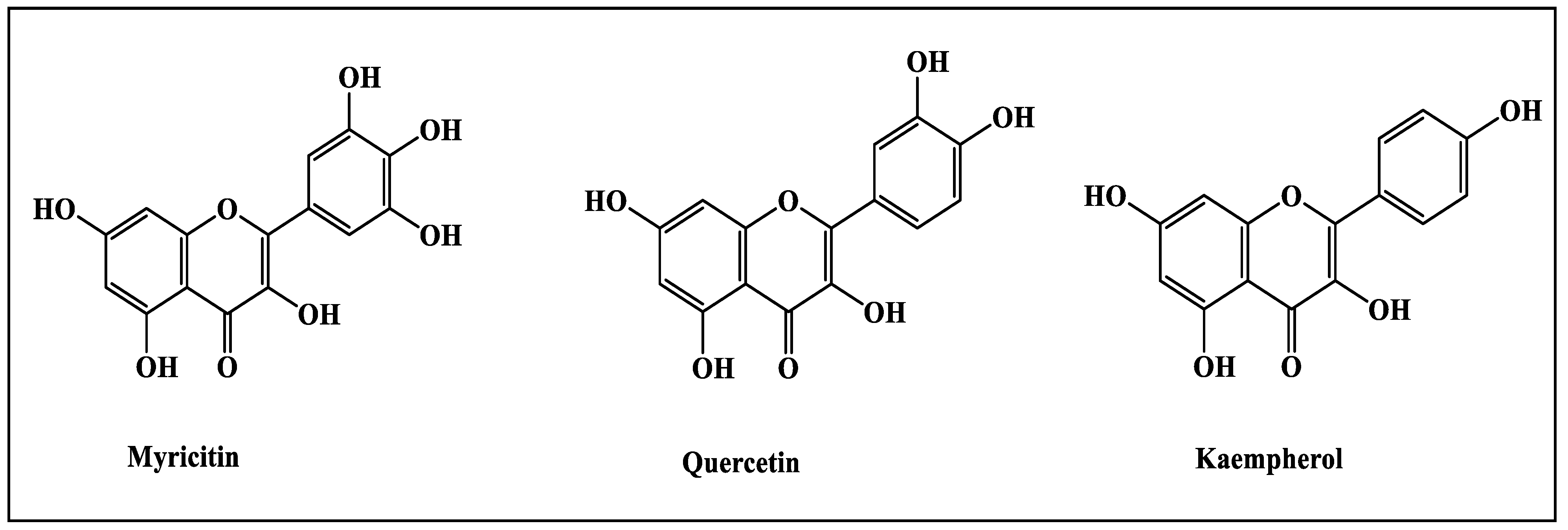
2.1.2. High Performance Liquid Chromatography (HPLC)
2.2. In Vivo Determination of H. crispum Anti-Ulcer Activity Using Ethanol-Induced Acute Gastric Ulcer Model
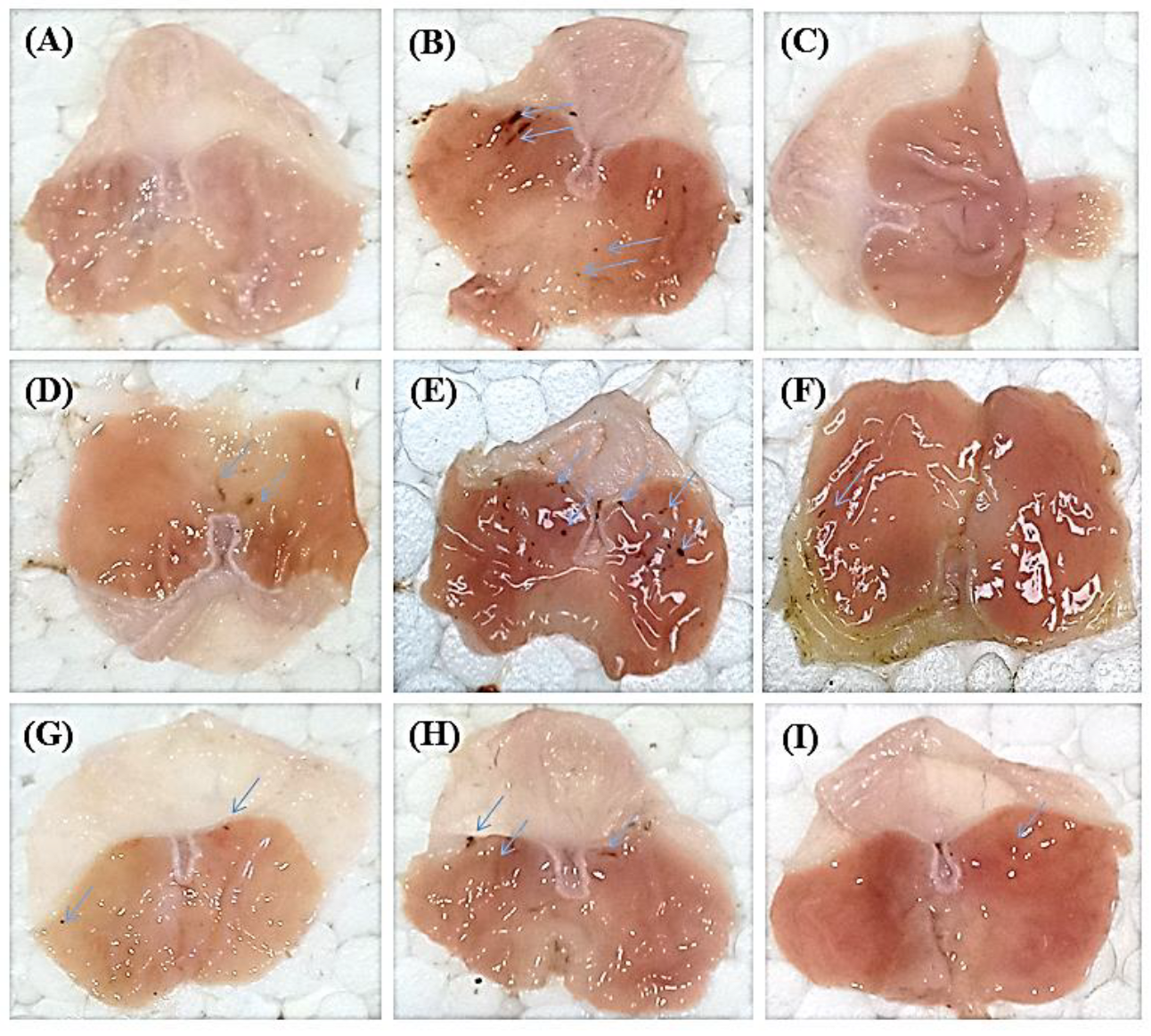
2.2.1. Macroscopic Analysis of Gastric Mucosa
2.2.2. Determination of Ulcer Score, Ulcer Index and Percentage of Ulcer Protection
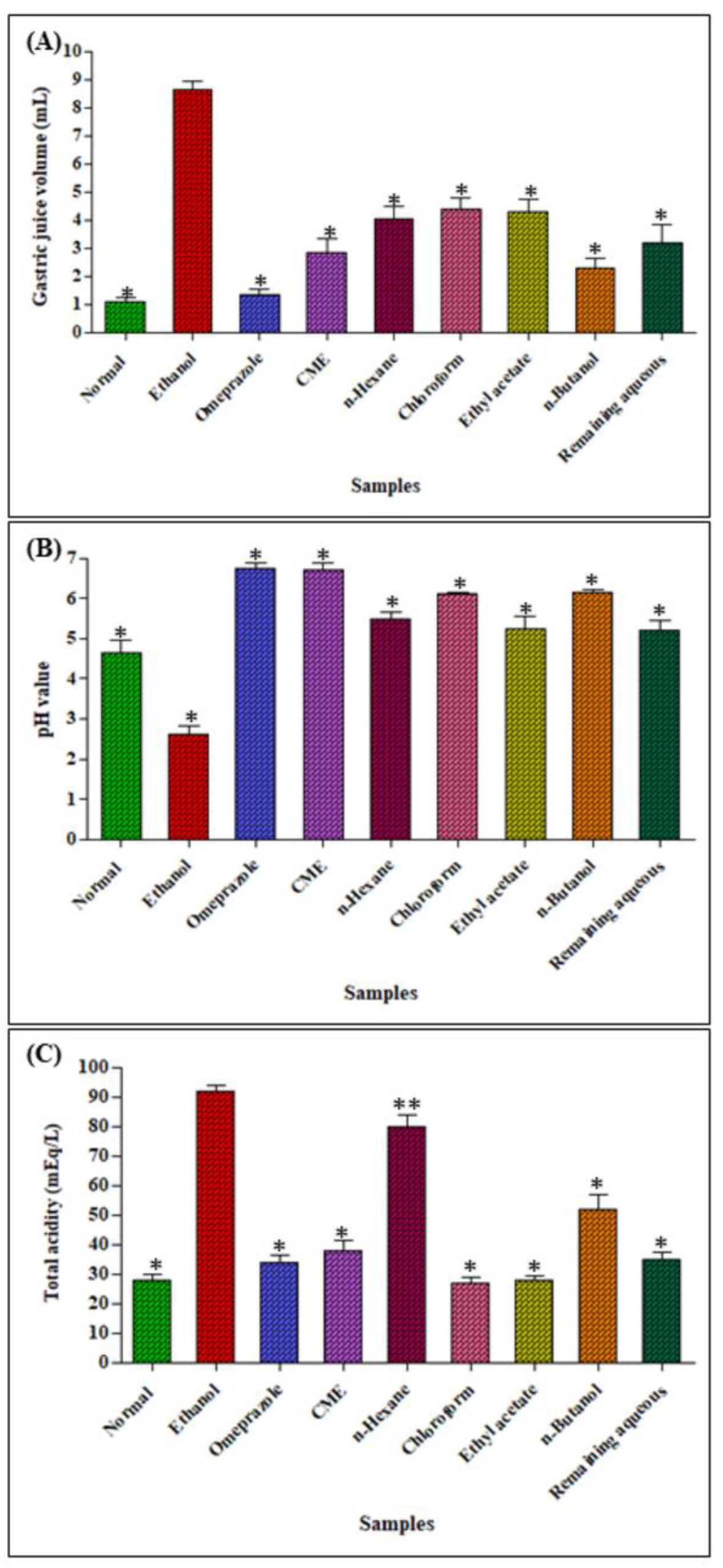
2.2.3. Determination of Gastric Juice Volume, pH and Total Acidity
2.2.4. Effect on Total Protein and Total Mucus Content
2.2.5. Histological Examination of Stomach Wall
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Drugs and Chemicals
4.3. Preparation of H. crispum Methanol Extract and Successive Fractionation
4.4. Metabolic Profiling of H. crispum Aerial Parts
4.4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
4.4.2. High Performance Liquid Chromatography (HPLC)
4.5. In Vivo Determination of H. crispum Anti-Ulcer Activity Using an Ethanol-Induced Acute Gastric Ulcer Model
4.5.1. Experimental Animals
4.5.2. Experimental Protocol
4.5.3. Gross and Histological Evaluation of Stomach Tissues
Determination of Ulcer Scoring
Determination of Ulcer Index
Determination of Percentage of Ulcer Protection
4.5.4. Determination of Gastric Juice Parameters
Estimation of Total Acidity
4.5.5. Determination of Gastric Mucous
4.5.6. Histopathological Examination of the Gastric Tissues
4.5.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shoaib, A.; Tarique, M.; Khushtar, M.; Siddiqui, H. Antiulcerogenic activity of hydromethanolic extract of Andrographis paniculata in indomethacin and indomethacin plus pylorus ligation induced gastric ulcer in rats. Asian J. Biomed. Pharm. Sci. 2014, 4, 8–15. [Google Scholar] [CrossRef]
- Patidar, D.K. Anti-ulcer activity of aqueous extract of Murraya koenigii in albino rats. Int. J. Pharma Bio. Sci. 2011, 2, 524–529. [Google Scholar]
- Klein, L.C.; Gandolfi, R.B.; Santin, J.R.; Lemos, M.; Cechinel Filho, V.; de Andrade, S.F. Antiulcerogenic activity of extract, fractions, and some compounds obtained from Polygala cyparissias St. Hillaire & Moquin (Polygalaceae). Naunyn-Schmied. Arch. Pharmacol. 2010, 381, 121–126. [Google Scholar] [CrossRef]
- De Barros, M.P.; Lemos, M.; Maistro, E.L.; Leite, M.F.; Sousa, J.P.B.; Bastos, J.K.; de Andrade, S.F. Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Propolis. J. Ethnopharmacol. 2008, 120, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Mincis, M.; Chebli, J.; Khouri, S.; Mincis, R. Ethanol and the gastrointestinal tract. Arch. Gastroenerol. 1995, 32, 131–139. [Google Scholar]
- Musa, A.; Shady, N.H.; Ahmed, S.R.; Alnusaire, T.S.; Sayed, A.M.; Alowaiesh, B.F.; Sabouni, I.; Al-Sanea, M.M.; Mostafa, E.M.; Youssif, K.A. Antiulcer potential of Olea europea L. cv. arbequina leaf extract supported by metabolic profiling and molecular docking. Antioxidants 2021, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Paguigan, N.D.; Castillo, D.H.B.; Chichioco-Hernandez, C.L. Anti-ulcer activity of leguminosae plants. Arch. Gastroenerol. 2014, 51, 64–67. [Google Scholar] [CrossRef]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Ahmed, M.M.; Aleisa, A.M.; Alkhamees, O. Possible biochemical effects following inhibition of ethanol-induced gastric mucosa damage by Gymnema sylvestre in male Wistar albino rats. Pharm. Biol. 2012, 50, 1542–1550. [Google Scholar] [CrossRef]
- Shenoy, A.M.; Shastry, S.C.; Shetiya, P. Anti ulcer activity of Heliotrpium indicum leaves extract. Int. J. Pharma. Sci. Res. 2011, 2, 1288–1292. [Google Scholar]
- Coté, G.A.; Howden, C.W. Potential adverse effects of proton pump inhibitors. Curr. Gastroenterol. Rep. 2008, 10, 208–214. [Google Scholar] [CrossRef]
- Pension, J.; Wormsley, K. Adverse reactions and interactions with H2-receptor antagonists. Med. Toxicol. 1986, 1, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Eid, S.Y.; Alshammari, E.; Ashour, M.L.; Wink, M.; El-Readi, M.Z. Chrysanthemum indicum and Chrysanthemum morifolium: Chemical composition of their essential oils and their potential use as natural preservatives with antimicrobial and antioxidant activities. Foods 2020, 9, 1460. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Khémiri, I.; Bitri, L. Effectiveness of Opuntia ficus indica L. inermis seed oil in the protection and the healing of experimentally induced gastric mucosa ulcer. Oxidative Med. Cell. Long. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Korcan, S.E.F.; Cigerci, I.H.; Dilek, M.; Kargioglu, M.; Cenkci, S.; Konuk, M. Antimicrobial activity of an endemic species, Thermopsis turcica, Turkey. Kuwait J. Sci. Eng. 2009, 36, 101–112. [Google Scholar]
- Arshad, A.; Ahemad, S.; Saleem, H.; Saleem, M.; Zengin, G.; Abdallah, H.H.; Tousif, M.I.; Ahemad, N.; Fawzi Mahomoodally, M. RP-UHPLC-MS chemical profiling, biological and in silico docking studies to unravel the therapeutic potential of Heliotropium crispum Desf. as a Novel Source of Neuroprotective Bioactive Compounds. Biomolecules 2021, 11, 53. [Google Scholar] [CrossRef]
- Karick, C. Pharmacopoeial Standards of Herbal Plants, Vol I.; Indian Book Centre: Delhi, India, 1994; pp. 10–58. [Google Scholar]
- Ahmed, S.; Hasan, M.M. A review on globally used antiurolithiatic monoherbal formulations belonging to Boraginaceae, Brassicaceae, Malvaceae and Poaceae families. World J. Pharm. Pharm. Sci. 2017, 6, 48–61. [Google Scholar] [CrossRef][Green Version]
- Altyar, A.E.; Munir, A.; Ishtiaq, S.; Rizwan, M.; Abbas, K.; Kensara, O.; Elhady, S.S.; Rizg, W.Y.; Youssef, F.S.; Ashour, M.L. Malva parviflora leaves and fruits mucilage as natural sources of anti-inflammatory, antitussive and gastro-protective agents: A comparative study using rat models and Gas chromatography. Pharmaceuticals 2022, 15, 427. [Google Scholar] [CrossRef]
- Magierowski, M.; Magierowska, K.; Kwiecien, S.; Brzozowski, T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules 2015, 20, 9099–9123. [Google Scholar] [CrossRef]
- Lam, E.K.; Tai, E.K.; Koo, M.W.; Wong, H.P.; Wu, W.K.; Yu, L.; So, W.H.; Woo, P.C.; Cho, C. Enhancement of gastric mucosal integrity by Lactobacillus rhamnosus GG. Life Sci. 2007, 80, 2128–2136. [Google Scholar] [CrossRef]
- Salama, S.M.; Gwaram, N.S.; AlRashdi, A.S.; Khalifa, S.A.; Abdulla, M.A.; Ali, H.M.; El-Seedi, H.R. A zinc morpholine complex prevents HCl/ethanol-induced gastric ulcers in a rat model. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bienia, A.; Sodolski, W.; Luchowska, E. The effect of chronic alcohol abuse on gastric and duodenal mucosa. Ann. Univ. Mariae Curie-Sklodowska Sectio D Med. 2002, 57, 570–582. [Google Scholar]
- Katzung, B.G. Basic and Clinical Pharmacology; Mc Graw Hill: New York, NY, USA, 2012. [Google Scholar]
- Gupta, J.; Kumar, D.; Gupta, A. Evaluation of gastric anti–ulcer activity of methanolic extract of Cayratia trifolia in experimental animals. Asian Pac. J. Trop. Dis. 2012, 2, 99–102. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Alkofahi, A.; Atta, A. Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J. Ethnopharmacol. 1999, 67, 341–345. [Google Scholar] [CrossRef]
- Moncada, D. Production, structure, and function of gastrointestinal mucins. Infect. Gastrointest. Tract. 2002, 1, 57–79. [Google Scholar]
- Sharifi-Rad, M.; Fokou, P.V.T.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef]
- Serafim, C.; Araruna, M.E.; Júnior, E.A.; Diniz, M.; Hiruma-Lima, C.; Batista, L. A review of the role of flavonoids in peptic ulcer (2010–2020). Molecules 2020, 25, 5431. [Google Scholar] [CrossRef]
- El-Din, M.I.G.; Youssef, F.S.; Said, R.S.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. Chemical constituents and gastro-protective potential of Pachira glabra leaves against ethanol-induced gastric ulcer in experimental rat model. Inflammopharmacology 2021, 29, 317–332. [Google Scholar] [CrossRef]
- El-Din, M.I.G.; Youssef, F.S.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. New γ-pyrone glycoside from Pachira glabra and assessment of its gastroprotective activity using an alcohol-induced gastric ulcer model in rats. Food Funct. 2020, 11, 1958–1965. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ishihara, M.; Segami, T.; Ito, M. Anti-ulcer effects of antioxidants, quercetin, α-tocopherol, nifedipine and tetracycline in rats. Jap. J. Pharmacol. 1998, 78, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Izzo, A.A. The plant kingdom as a source of anti-ulcer remedies. Phytother. Res. 2000, 14, 581–591. [Google Scholar] [CrossRef]
- El-Dien, R.T.M.; Maher, S.A.; Abdelmohsen, U.R.; AboulMagd, A.M.; Fouad, M.A.; Kamel, M.S. Antiulcer secondary metabolites from Elaeocarpus grandis, family Elaeocarpaceae, supported by in silico studies. RSC Adv. 2020, 10, 34788–34799. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Siddiqui, M.A.; Khan, M.M.; Ajmal, M.; Ahsan, R.; Rahaman, M.A.; Ahmad, M.A.; Arshad, M.; Khushtar, M. Current pharmacological trends on myricetin. Drug Res. 2020, 70, 448–454. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Ichimura, A.; Sato, S.; Fujii, T.; Oishi, S.; Sakai, H.; Takeshima, H. The natural flavonoid myricetin inhibits gastric H+, K+-ATPase. Eur. J. Pharmacol. 2018, 820, 217–221. [Google Scholar] [CrossRef]
- Park, H.-s.; Seo, C.-S.; Baek, E.B.; Rho, J.-h.; Won, Y.-S.; Kwun, H.-j. Gastroprotective effect of myricetin on ethanol-induced acute gastric injury in rats. Evid. Based Complement. Alt. Med. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Nabil, M.; El Raey, M.A.; Abdo, W.; Abdelfattah, M.A.; El-Shazly, A.M.; Sobeh, M.; Mahmoud, M.F. Gastro-protective effects of Albizia anthelmintica leaf extract on indomethacin-induced gastric ulcer in wistar rats: In silico and in vivo Studies. Antioxidants 2021, 10, 176. [Google Scholar] [CrossRef]
- Li, Q.; Hu, X.; Xuan, Y.; Ying, J.; Fei, Y.; Rong, J.; Zhang, Y.; Zhang, J.; Liu, C.; Liu, Z. Kaempferol protects ethanol-induced gastric ulcers in mice via pro-inflammatory cytokines and NO. Acta Biochim. Biophys. Sin. 2018, 50, 246–253. [Google Scholar] [CrossRef]
- Beber, A.P.; de Souza, P.; Boeing, T.; Somensi, L.B.; Mariano, L.N.B.; Cury, B.J.; Burci, L.M.; da Silva, C.B.; Simionatto, E.; de Andrade, S.F. Constituents of leaves from Bauhinia curvula Benth. exert gastroprotective activity in rodents: Role of quercitrin and kaempferol. Inflammopharmacology 2018, 26, 539–550. [Google Scholar] [CrossRef]
- Dhivya, K. Screening of phytoconstituents, UV-VIS Spectrum and FTIR analysis of Micrococca mercurialis (L.) Benth. Int. J. Herbal Med. 2017, 5, 40–44. [Google Scholar] [CrossRef]
- Hollander, D.; Tarnawski, A.; Krause, W.J.; Gergely, H. Protective effect of sucralfate against alcohol-induced gastric mucosal injury in the rat: Macroscopic, histologic, ultrastructural, and functional time sequence analysis. Gastroenterology 1985, 88, 366–374. [Google Scholar] [CrossRef]
- Adane, H.; Atnafie, S.A.; Kifle, Z.D.; Ambikar, D. Evaluation of in vivo antiulcer activity of hydro-methanol extract and solvent fractions of the stem bark of Ficus thonningii (Moraceae) on rodent models. BioMed Res. Int. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S. Hand Book of Experimental Pharmacology, 3rd ed.; Vallabh Prakashan: Delhi, India, 2006. [Google Scholar]
- Sharma, A.L.; Bhot, M.A.; Chandra, N. Gastroprotective effect of aqueous extract and mucilage from Bryophyllum pinnatum (Lam.) Kurz. Ancient Sci. Life 2014, 33, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Nwinyi, F.; Kwanashie, H. Comparative effects of Sorghum bicolor leaf base extract on tissues isolated from some body systems of experimental animals. J. Med. Plants Res. 2013, 7, 3041–3051. [Google Scholar]
- Corne, S. A method for the quantitative estimation of gastric barrier mucus. J. Physiol. 1974, 242, 116–117. [Google Scholar]
- Al Batran, R.; Al-Bayaty, F.; Jamil Al-Obaidi, M.M.; Abdualkader, A.M.; Hadi, H.A.; Ali, H.M.; Abdulla, M.A. In Vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS ONE 2013, 8, e64751. [Google Scholar] [CrossRef]
- Al-Wajeeh, N.S.; Hajerezaie, M.; Noor, S.M.; Halabi, M.F.; Al-Henhena, N.; Azizan, A.H.S.; Kamran, S.; Hassandarvish, P.; Shwter, A.N.; Ali, H.M. The gastro protective effects of Cibotium barometz hair on ethanol-induced gastric ulcer in Sprague-Dawley rats. BMC Vet. Res. 2016, 13, 27. [Google Scholar] [CrossRef]

| Peak Value | Functional Group | Functional Group Name | Vibrations |
|---|---|---|---|
| 3296.5 | Ac-O-H | Carboxylic acids | |
| N-H | Primary amides | ||
| R-C=O-NR2 | Secondary amides | ||
| O-H | Polymer alcohols and phenols | ||
| 2918.0 | H-OH | Hydroxyl group | |
| -NH | Secondary free or bonded NH strand | ||
| 1596.3 | (i).Secondary NH bond | Secondary aromatic amine | Deformed bond |
| (ii).N=N | Azo compound | ||
| (iii).C=N | Imine compounds | Bent bond | |
| (iv).R-NH2 | Primary aliphatic amine | Deformed | |
| (v).RC(=O)NR/R// | Primary amide | Deformed or bent bond | |
| 1410.0 | R2-CHOH | Tertiary alcohols | Deformed form |
| 1241.8 | R-C6H5-R | Alkyl aryl group | |
| 2R-C6H5- | Diaryl group | ||
| 1013.5 | C-F | Floro group(halo alkane) | |
| C-OH | Primary alcohol | ||
| 568.9 | C-Br | Bromo group (halo alkane) | |
| 490.8 | C-I | Iodo group (halo alkane) |
| Fraction | Retention Time | Compound |
|---|---|---|
| CME | 4.933 | Myricetin |
| Chloroform | 5.240 | Myricetin |
| 8.862 | Quercetin | |
| Ethyl acetate | 5.433 | Myricetin |
| 11.381 | Kampherol | |
| n-Butanol | 5.387 | Myricetin |
| Remaining Aqueous | No peaks found | No compound detected |
| Groups | Ulcer Number | Incidence of Ulcer | Ulcer Score | Ulcer Index | % Inhibition |
|---|---|---|---|---|---|
| Normal | 0 ± 0 a | 0 a | 0 ± 0 a | 0 a | 0 a |
| Ethanol | 11.33 ± 1.909 | 100 | 13.833 ± 2.27 a | 125.17 a | 0 a |
| Omeprazole | 0.167 ± 0.167 a | 16.667 a | 0.166 ± 0.17 a | 17 a | 84.410 a |
| CME | 0.167 ± 0.167 a | 16.667 a | 0.500 ± 0.22 a | 17.33 a | 86.150 a |
| n-Hexane | 0.833 ± 0.307 a | 66.67 a | 10.667 ± 0.667 a | 69.33 a | 44.600 a |
| Chloroform | 0.167 ± 0.167 a | 16.667 a | 0.1667 ± 0.1667 a | 17 a | 86.418 a |
| Ethyl acetate | 0.170 ± 0.166 a | 16.667 a | 0.167 ± 0.166 a | 17 a | 86.418 a |
| n-Butanol | 1.500 ± 0.340 a | 83.333 a | 1.500 ± 0.223 a | 86.17 a | 31.025 a |
| Remaining aqueous | 0.830 ± 0.307 a | 66.667 a | 1.000 ± 0.258 a | 68.5 a | 45.272 a |
| Groups | Total Protein Content | Gastric Mucin Content |
|---|---|---|
| Normal | 258.42 ± 6.65 c | 507.83 ± 8.36 c |
| Ethanol | 89.33 ± 7.43 c | 428.67 ± 9.12 |
| Omeprazole | 217.00 ± 15.24 c | 481.83 ± 4.79 b |
| CME | 248.50 ±13.63 c | 481.83 ± 14.15 b |
| n-Hexane | 160.00 ± 10.88 c | 444.17 ± 15.60 ns |
| Chloroform | 252.33 ± 3.56 c | 498.67 ± 7.83 c |
| Ethyl acetate | 234.83 ± 7.93 c | 460.00 ± 13.05 ns |
| n-Butanol | 198.50 ± 5.36 c | 503.50 ± 4.09 a |
| Remaining aqueous | 184.50 ± 5.50 c | 480.33 ± 12.18 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, S.F.; Ishtiaq, S.; Lashkar, M.O.; Youssef, F.S.; Ashour, M.L.; Elhady, S.S. Metabolic Profiling of Heliotropium crispum Aerial Parts Using HPLC and FTIR and In Vivo Evaluation of Its Anti-Ulcer Activity Using an Ethanol Induced Acute Gastric Ulcer Model. Metabolites 2022, 12, 750. https://doi.org/10.3390/metabo12080750
Fatima SF, Ishtiaq S, Lashkar MO, Youssef FS, Ashour ML, Elhady SS. Metabolic Profiling of Heliotropium crispum Aerial Parts Using HPLC and FTIR and In Vivo Evaluation of Its Anti-Ulcer Activity Using an Ethanol Induced Acute Gastric Ulcer Model. Metabolites. 2022; 12(8):750. https://doi.org/10.3390/metabo12080750
Chicago/Turabian StyleFatima, Syeda Farheen, Saiqa Ishtiaq, Manar O. Lashkar, Fadia S. Youssef, Mohamed L. Ashour, and Sameh S. Elhady. 2022. "Metabolic Profiling of Heliotropium crispum Aerial Parts Using HPLC and FTIR and In Vivo Evaluation of Its Anti-Ulcer Activity Using an Ethanol Induced Acute Gastric Ulcer Model" Metabolites 12, no. 8: 750. https://doi.org/10.3390/metabo12080750
APA StyleFatima, S. F., Ishtiaq, S., Lashkar, M. O., Youssef, F. S., Ashour, M. L., & Elhady, S. S. (2022). Metabolic Profiling of Heliotropium crispum Aerial Parts Using HPLC and FTIR and In Vivo Evaluation of Its Anti-Ulcer Activity Using an Ethanol Induced Acute Gastric Ulcer Model. Metabolites, 12(8), 750. https://doi.org/10.3390/metabo12080750










