Relationship between Body Composition and Biochemical Parameters with Antioxidant Status in a Healthy Cohort of Postmenopausal Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sociodemographic Data Collection
2.3. Body Composition and Anthropometry Analysis
2.4. Samples Treatment and Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, L.-Y.; Lv, Z.-D.; Wang, K.; Qian, L.; Song, X.-X.; Li, X.-F.; Shen, H.-X. Estradiol Alleviates Intervertebral Disc Degeneration through Modulating the Antioxidant Enzymes and Inhibiting Autophagy in the Model of Menopause Rats. Oxid. Med. Cell Longev. 2018, 2018, 7890291. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Expósito, M.J.; Sánchez-López, E.; Cueto-Ureña, C.; Dueñas, B.; Carrera-González, P.; Navarro-Cecilia, J.; Mayas, M.D.; Arias de Saavedra, J.M.; Sánchez-Agesta, R.; Martínez-Martos, J.M. Circulating Oxidative Stress Parameters in Pre- and Post-Menopausal Healthy Women and in Women Suffering from Breast Cancer Treated or Not with Neoadjuvant Chemotherapy. Exp. Gerontol. 2014, 58, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Chandankhede, M.; Gupta, M.; Pakhmode, S. Assessment of Psychological Status and Oxidative Stress in Postmenopausal Women: A Cross-Sectional Study. J. Menopausal. Med. 2021, 27, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.M.; Waśkiewicz, A.; Zujko, M.E.; Szcześniewska, D.; Pająk, A.; Stepaniak, U.; Drygas, W. Dietary Polyphenol Intake, but Not the Dietary Total Antioxidant Capacity, Is Inversely Related to Cardiovascular Disease in Postmenopausal Polish Women: Results of WOBASZ and WOBASZ II Studies. Oxid. Med. Cell Longev. 2017, 2017, 5982809. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.L.; Mendonça, A.M.; Giolo, J.S.; Costa, J.G.; Mariano, I.M.; de Souza, T.C.F.; Batista, J.P.; Rodrigues, M.L.; de Souza, A.V.; Caixeta, D.C.; et al. The Effects of Isoflavone Supplementation plus Combined Exercise on Salivary Markers of Oxidative Stress in Postmenopausal Women. J. Clin. Biochem. Nutr. 2020, 66, 43–48. [Google Scholar] [CrossRef]
- Montoya-Estrada, A.; Velázquez-Yescas, K.G.; Veruete-Bedolla, D.B.; Ruiz-Herrera, J.D.; Villarreal-Barranca, A.; Romo-Yañez, J.; Ortiz-Luna, G.F.; Arellano-Eguiluz, A.; Solis-Paredes, M.; Flores-Pliego, A.; et al. Parameters of Oxidative Stress in Reproductive and Postmenopausal Mexican Women. Int. J. Environ. Res. Public Health 2020, 17, 1492. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Siassi, F.; Koohdani, F.; Qorbani, M.; Mozaffari, H.; Aslani, Z.; Soleymani, M.; Entezarian, M.; Sotoudeh, G. Dietary Total Antioxidant Capacity Is Inversely Associated with Depression, Anxiety and Some Oxidative Stress Biomarkers in Postmenopausal Women: A Cross-Sectional Study. Ann. Gen. Psychiatry 2019, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, M.; Lee, S.-G.; Davis, C.G.; Kenny, A.; Koo, S.I.; Chun, O.K. Plasma Total Antioxidant Capacity Is Associated with Dietary Intake and Plasma Level of Antioxidants in Postmenopausal Women. J. Nutr. Biochem. 2012, 23, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Carillon, J.; Rouanet, J.-M.; Cristol, J.-P.; Brion, R. Superoxide Dismutase Administration, a Potential Therapy against Oxidative Stress Related Diseases: Several Routes of Supplementation and Proposal of an Original Mechanism of Action. Pharm. Res. 2013, 30, 2718–2728. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Baltacıoğlu, E.; Akalın, F.A.; Alver, A.; Balaban, F.; Ünsal, M.; Karabulut, E. Total Antioxidant Capacity and Superoxide Dismutase Activity Levels in Serum and Gingival Crevicular Fluid in Post-Menopausal Women with Chronic Periodontitis. J. Clin. Periodontol. 2006, 33, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Borrás, C.; Ferrando, M.; Inglés, M.; Gambini, J.; Lopez-Grueso, R.; Edo, R.; Mas-Bargues, C.; Pellicer, A.; Viña, J. Estrogen Replacement Therapy Induces Antioxidant and Longevity-Related Genes in Women after Medically Induced Menopause. Oxid. Med. Cell Longev. 2021, 2021, 8101615. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, X.; Gao, Q.; Lv, C.; Xu, J.; Jin, H.; Zhu, H. Quercetin Increases the Antioxidant Capacity of the Ovary in Menopausal Rats and in Ovarian Granulosa Cell Culture in Vitro. J. Ovarian Res. 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Sternfeld, B.; Huang, M.; Han, W.; Karvonen-Gutierrez, C.; Ruppert, K.; Cauley, J.A.; Finkelstein, J.S.; Jiang, S.-F.; Karlamangla, A.S. Changes in Body Composition and Weight during the Menopause Transition. JCI Insight 2019, 4, e124865. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Fan, S.H.; Say, Y.H. Plasma Total Antioxidant Capacity (TAC) in Obese Malaysian Subjects. Malays. J. Nutr. 2012, 18, 345–354. [Google Scholar] [PubMed]
- Habdous, M.; Herbeth, B.; Vincent-Viry, M.; Lamont, J.V.; Fitzgerald, P.S.; Visvikis, S.; Siest, G. Serum Total Antioxidant Status, Erythrocyte Superoxide Dismutase and Whole-Blood Glutathione Peroxidase Activities in the Stanislas Cohort: Influencing Factors and Reference Intervals. Clin. Chem. Lab. Med. 2003, 41, 209–215. [Google Scholar] [CrossRef]
- Victorino, V.J.; Panis, C.; Campos, F.C.; Cayres, R.C.; Colado-Simão, A.N.; Oliveira, S.R.; Herrera, A.C.S.A.; Cecchini, A.L.; Cecchini, R. Decreased Oxidant Profile and Increased Antioxidant Capacity in Naturally Postmenopausal Women. Age 2013, 35, 1411–1421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed]
- Fryar, C.D.; Kruszon-Moran, D.; Gu, Q.; Ogden, C.L. Mean Body Weight, Height, Waist Circumference, and Body Mass Index Among Adults: United States, 1999–2000 Through 2015–2016. Natl. Health Stat. Rep. 2018, 122, 1–16. [Google Scholar]
- Dimala, C.A.; Ngu, R.C.; Kadia, B.M.; Tianyi, F.-L.; Choukem, S.P. Markers of Adiposity in HIV/AIDS Patients: Agreement between Waist Circumference, Waist-to-Hip Ratio, Waist-to-Height Ratio and Body Mass Index. PLoS ONE 2018, 13, e0194653. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, M.A.; Castrejón-Delgado, L.; Zacarías-Flores, M.; Arronte-Rosales, A.; Mendoza-Núñez, V.M. Quality of Life among Post-Menopausal Women Due to Oxidative Stress Boosted by Dysthymia and Anxiety. BMC Women’s Health 2017, 17, 1. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Petelin, A.; Tedeschi, P.; Maietti, A.; Jurdana, M.; Brandolini, V.; Pražnikar, Z.J. Total Serum Antioxidant Capacity in Healthy Normal Weight and Asymptomatic Overweight Adults. Exp. Clin. Endocrinol. Diabetes 2017, 125, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Hamedi-Shahraki, S.; Amirkhizi, F. Vitamin D Status and Systemic Redox Biomarkers in Adults with Obesity. Clin. Nutr. ESPEN 2021, 45, 292–298. [Google Scholar] [CrossRef]
- Amani, R.; Parohan, M.; Jomehzadeh, N.; Haghighizadeh, M.H. Dietary and Biochemical Characteristics Associated with Normal-Weight Obesity. Int. J. Vitam. Nutr. Res. 2019, 89, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Léger, C.L.; Carbonneau, M.A.; Michel, F.; Mas, E.; Monnier, L.; Cristol, J.P.; Descomps, B. A Thromboxane Effect of a Hydroxytyrosol-Rich Olive Oil Wastewater Extract in Patients with Uncomplicated Type I Diabetes. Eur. J. Clin. Nutr. 2005, 59, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Greabu, M.; Didilescu, A.; Puiu, L.; Miricescu, D.; Totan, A. Salivary Antioxidant Biomarkers in Non-Ferrous Metals Mine Workers--a Pilot Study. J. Oral. Pathol. Med. 2012, 41, 490–493. [Google Scholar] [CrossRef]
- Dani, C.; Martelli, E.; Bertini, G.; Pezzati, M.; Filippi, L.; Rossetti, M.; Rizzuti, G.; Rubaltelli, F.F. Plasma Bilirubin Level and Oxidative Stress in Preterm Infants. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F119–F123. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, G.J.; Mumby, S.; Martin, G.S.; Bernard, G.R.; Gutteridge, J.M.C.; Evans, T.W. Albumin Influences Total Plasma Antioxidant Capacity Favorably in Patients with Acute Lung Injury. Crit. Care Med. 2004, 32, 755–759. [Google Scholar] [CrossRef]
- Aycicek, A.; Erel, O.; Kocyigit, A.; Selek, S.; Demirkol, M.R. Breast Milk Provides Better Antioxidant Power than Does Formula. Nutrition 2006, 22, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gajardo, R.; Matamala, J.M.; Gutiérrez, R.; Lozano, P.; Cortés-Fuentes, I.A.; Sotomayor, C.G.; Bustamante, G.; Pasten, J.A.; Vargas, G.; Guerrero, R.; et al. Relationship between Infarct Size and Serum Uric Acid Levels during the Acute Phase of Stroke. PLoS ONE 2019, 14, e0219402. [Google Scholar] [CrossRef]
- Ndrepepa, G. Uric Acid and Cardiovascular Disease. Clin. Chim. Acta 2018, 484, 150–163. [Google Scholar] [CrossRef]
- Lussignoli, S.; Fraccaroli, M.; Andrioli, G.; Brocco, G.; Bellavite, P. A Microplate-Based Colorimetric Assay of the Total Peroxyl Radical Trapping Capability of Human Plasma. Anal. Biochem. 1999, 269, 38–44. [Google Scholar] [CrossRef]
- Sofic, E.; Rustembegovic, A.; Kroyer, G.; Cao, G. Serum Antioxidant Capacity in Neurological, Psychiatric, Renal Diseases and Cardiomyopathy. J. Neural. Transm. 2002, 109, 711–719. [Google Scholar] [CrossRef]
- Psotová, J.; Zahálková, J.; Hrbác, J.; Simánek, V.; Bartek, J. Determination of Total Antioxidant Capacity in Plasma by Cyclic Voltammetry. Two Case Reports. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech Repub. 2001, 145, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Kaliaperumal, R.; Venkatachalam, R.; Nagarajan, P.; Sabapathy, S.K. Association of Serum Magnesium with Oxidative Stress in the Pathogenesis of Diabetic Cataract. Biol. Trace Elem. Res. 2021, 199, 2869–2873. [Google Scholar] [CrossRef] [PubMed]
- Chehaibi, K.; Trabelsi, I.; Mahdouani, K.; Slimane, M.N. Correlation of Oxidative Stress Parameters and Inflammatory Markers in Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 2585–2593. [Google Scholar] [CrossRef]
- Gawlik, K.; Naskalski, J.W.; Fedak, D.; Pawlica-Gosiewska, D.; Grudzień, U.; Dumnicka, P.; Małecki, M.T.; Solnica, B. Markers of Antioxidant Defense in Patients with Type 2 Diabetes. Oxid Med. Cell Longev. 2016, 2016, 2352361. [Google Scholar] [CrossRef] [PubMed]
- Gnanapragasam, A.; Ebenezar, K.K.; Sathish, V.; Govindaraju, P.; Devaki, T. Protective Effect of Centella Asiatica on Antioxidant Tissue Defense System against Adriamycin Induced Cardiomyopathy in Rats. Life Sci. 2004, 76, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.; Ajith, T.A.; Jose, N.; Janardhanan, K.K. Antimutagenic Activity of Methanolic Extract of Ganoderma Lucidum and Its Effect on Hepatic Damage Caused by Benzo[a]Pyrene. J. Ethnopharmacol. 2006, 107, 297–303. [Google Scholar] [CrossRef]
- Almasmoum, H.; Refaat, B.; Ghaith, M.M.; Almaimani, R.A.; Idris, S.; Ahmad, J.; Abdelghany, A.H.; BaSalamah, M.A.; El-Boshy, M. Protective Effect of Vitamin D3 against Lead Induced Hepatotoxicity, Oxidative Stress, Immunosuppressive and Calcium Homeostasis Disorders in Rat. Environ. Toxicol. Pharmacol. 2019, 72, 103246. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.Y.; Hu, M.M.; Xin, Y.F.; Gang, C. Resveratrol Alleviates Vascular Inflammatory Injury by Inhibiting Inflammasome Activation in Rats with Hypercholesterolemia and Vitamin D2 Treatment. Inflamm. Res. 2015, 64, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Paprocki, J.; Sutkowy, P.; Piechocki, J.; Woźniak, A. Association between Vitamin D Supplements, Oxidative Stress Biomarkers, and Hyperbaric Therapy in Patients with Sudden Sensorineural Hearing Loss. Oxid. Med. Cell Longev. 2021, 2021, 8895323. [Google Scholar] [CrossRef]
- Liao, F.-L.; Peng, D.-H.; Chen, W.; Hu, H.-N.; Tang, P.; Liu, Y.-Y.; Luo, Y.; Yao, T. Evaluation of Serum Hepatic Enzyme Activities in Different COVID-19 Phenotypes. J. Med. Virol. 2021, 93, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Chen, Y.; Chong, T.H.; Peng, J.; Mak, T.K.; Wang, C.; Yang, J. Effect of Bariatric Surgery on Serum Enzyme Status in Obese Patients. Obes. Surg. 2020, 30, 2700–2707. [Google Scholar] [CrossRef]
- Abou-Seif, M.A.; Youssef, A.-A. Evaluation of Some Biochemical Changes in Diabetic Patients. Clin. Chim. Acta 2004, 346, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowska, K.G.; Lozinska, L.; Woliński, J.; Pierzynowski, S. The Inverse Relationship between Blood Amylase and Insulin Levels in Pigs during Development, Bariatric Surgery, and Intravenous Infusion of Amylase. PLoS ONE 2018, 13, e0198672. [Google Scholar] [CrossRef]
- Reddy, V.S.; Gouroju, S.; Suchitra, M.M.; Suresh, V.; Sachan, A.; Srinivasa Rao, P.V.L.N.; Bitla, A.R. Antioxidant Defense in Overt and Subclinical Hypothyroidism. Horm. Metab. Res. 2013, 45, 754–758. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Rowiński, R.; Kornatowski, M.; Dąbrowski, A.; Kędziora-Kornatowska, K.; Strachecka, A. Relation of Moderate Physical Activity to Blood Markers of Oxidative Stress and Antioxidant Defense in the Elderly. Oxid. Med. Cell Longev. 2019, 2019, 5123628. [Google Scholar] [CrossRef]
- Gilca, M.; Piriu, G.; Gaman, L.; Delia, C.; Iosif, L.; Atanasiu, V.; Stoian, I. A Study of Antioxidant Activity in Patients with Schizophrenia Taking Atypical Antipsychotics. Psychopharmacology 2014, 231, 4703–4710. [Google Scholar] [CrossRef]

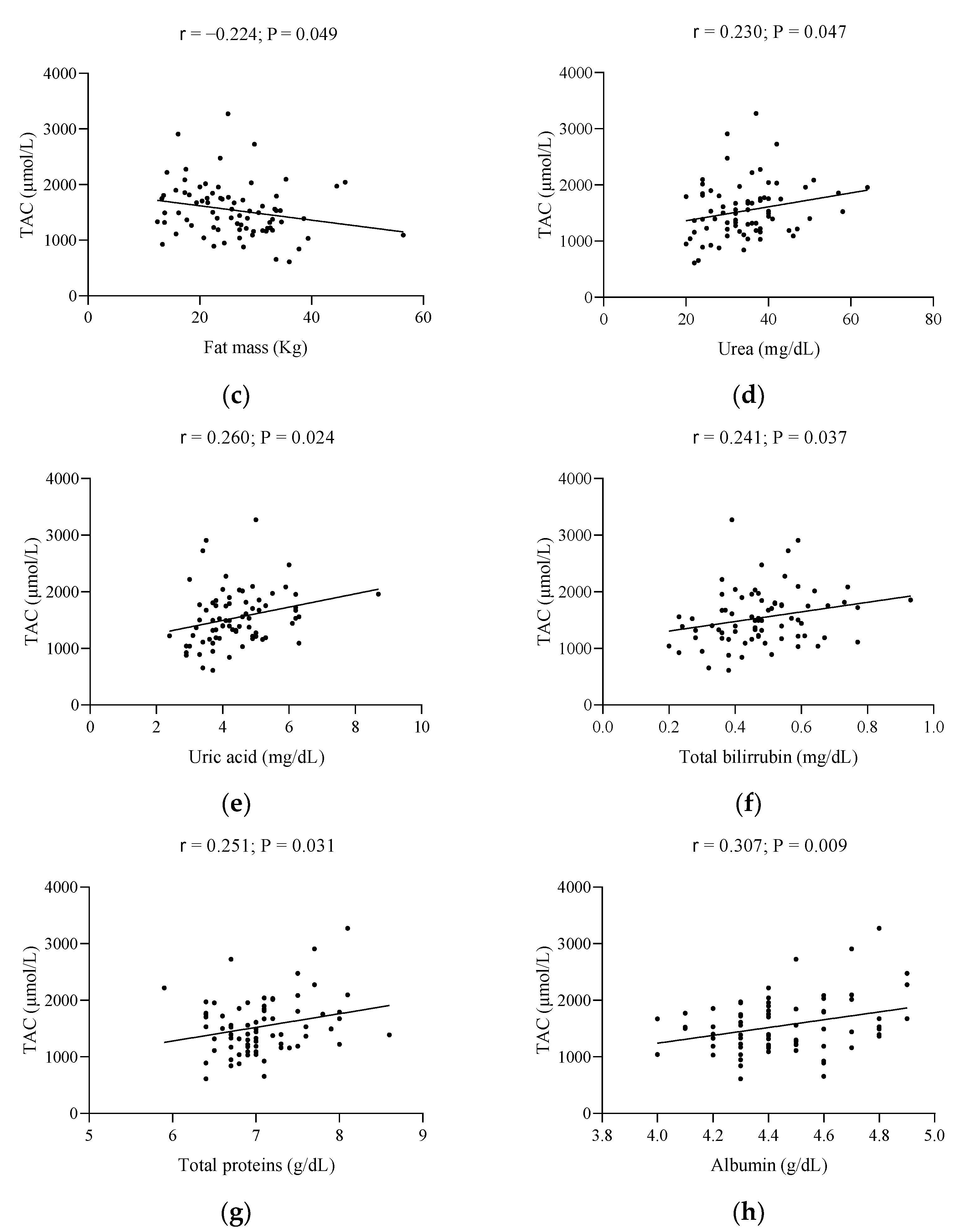
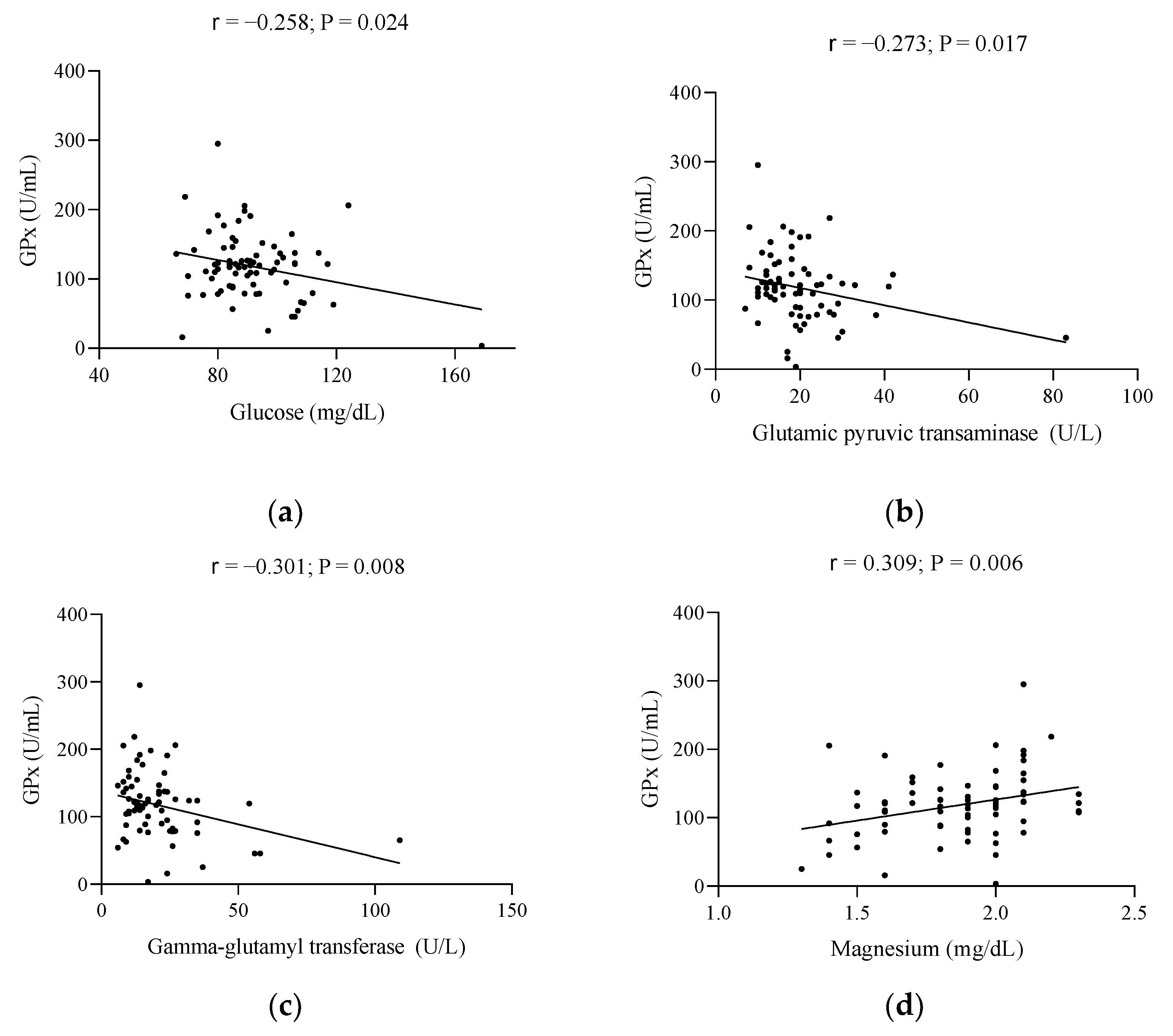
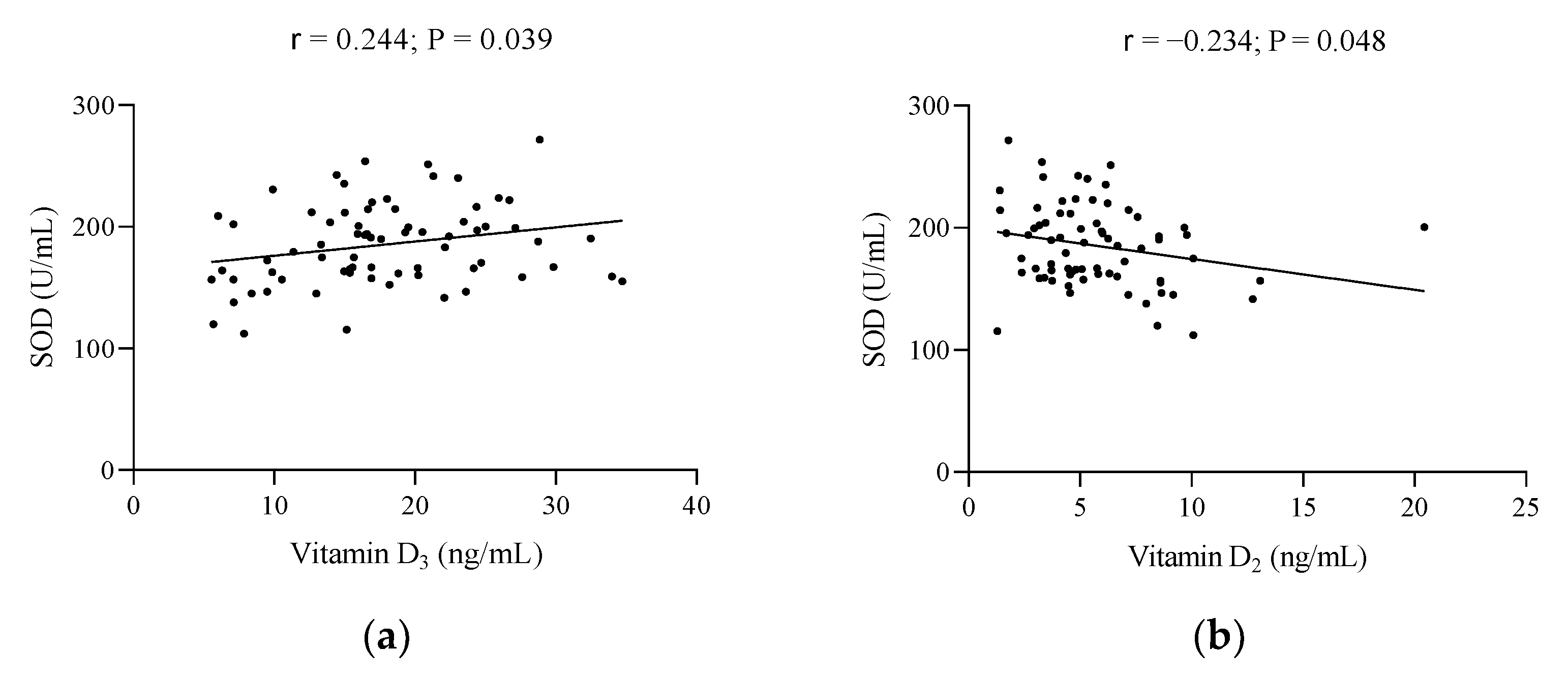
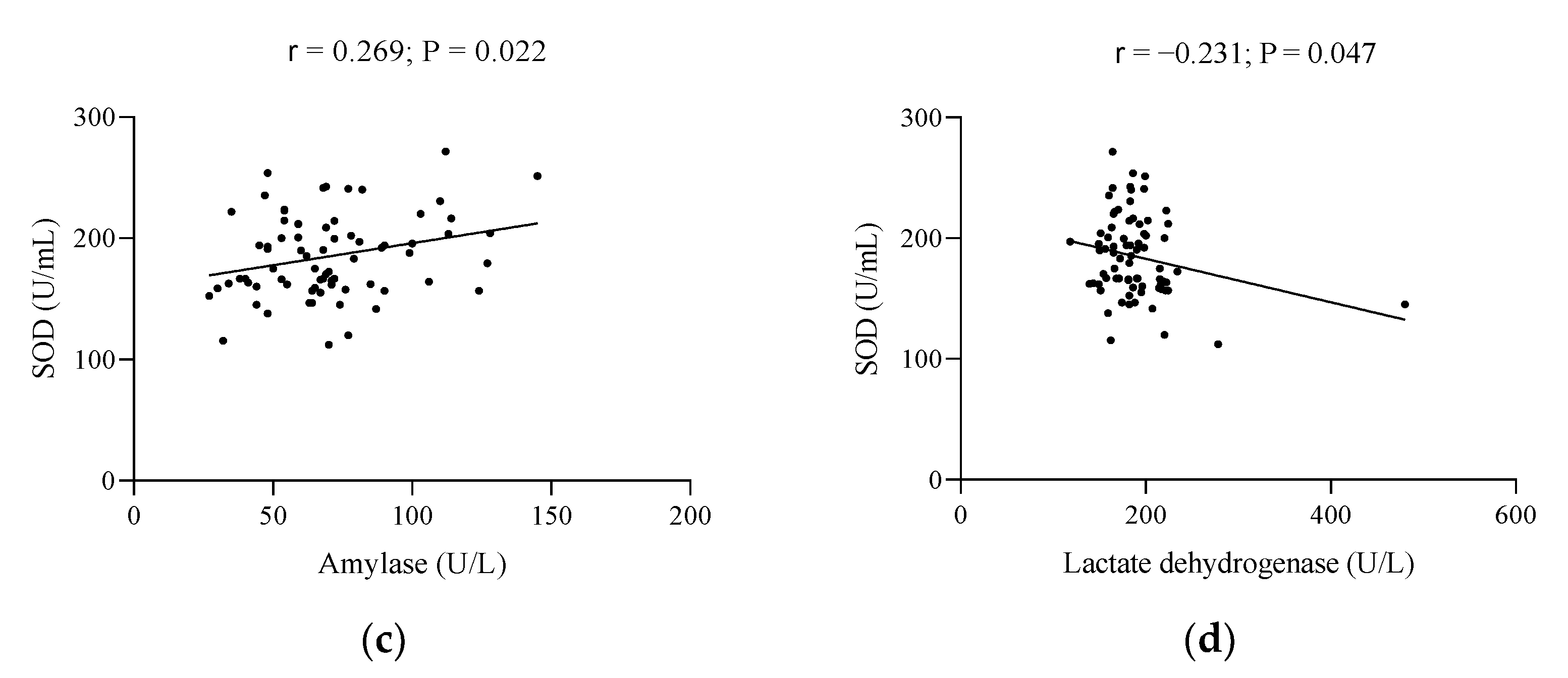
| Characteristics | Total Population (n = 78) | Below Reference | Early Menopause (n = 39) | Late Menopause (n = 39) | Reference Values |
|---|---|---|---|---|---|
| Mean ± SD | % | Mean ± SD | Mean ± SD | Range | |
| Body Composition | |||||
| Weight (kg) | 68.7 ± 13.2 | - | 70.3 ± 13.9 | 66.9 ± 12.3 | - |
| Height (m) | 159.3 ± 6.23 | - | 161.5 ± 6.20 | 156.8 ± 5.30 * | - |
| BMI (kg/m2) | 27.0 ± 4.60 | 14.1 | 26.9 ± 4.80 | 27.2 ± 4.40 | 22.0–27.0 |
| WP (cm) | 89.0 ± 12.6 | 50.0 | 87.8 ± 12.4 | 90.5 ± 13.0 | < 90.0 |
| HP (cm) | 105.8 ± 10.5 | 65.4 | 105.7 ± 9.50 | 105.9 ± 11.6 | < 110.0 |
| Waist/hip ratio | 0.83 ± 0.08 | 12.8 | 0.80 ± 0.10 | 0.80 ± 0.10 | < 0.80 |
| FM (%) | 37.6 ± 5.92 | 1.30 | 37.4 ± 5.50 | 37.8 ± 6.40 | 23.0–31.0 |
| FM (kg) | 26.3 ± 8.48 | - | 26.8 ± 8.50 | 25.7 ± 8.60 | - |
| FFM (%) | 62.4 ± 5.92 | 100.0 | 43.5 ± 6.30 | 40.2 ± 8.30 * | > 69.0 |
| Biochemical parameters | |||||
| Glucose (mg/dL) | 92.2 ± 15.9 | 3.90 | 87.4 ± 12.4 | 97.4 ± 17.9 * | 70.0–110.0 |
| Creatinine (mg/dL) | 0.69 ± 0.13 | 2.60 | 0.67 ± 0.10 | 0.73 ± 0.20 | 0.50–0.90 |
| Urea (mg/dL) | 34.5 ± 9.08 | 0.00 | 32.2 ± 8.00 | 37.2 ± 9.60 * | 10.0–50.0 |
| Uric acid (mg/dL) | 4.40 ± 1.07 | 0.00 | 4.10 ± 0.90 | 4.70 ± 1.20 * | 2.40–5.70 |
| Total bilirubin (mg/dL) | 0.47 ± 0.14 | 0.00 | 0.40 ± 0.10 | 0.50 ± 0.10 * | 0.10–1.20 |
| Total proteins (g/dL) | 7.08 ± 0.52 | 14.7 | 7.20 ± 0.50 | 7.00 ± 0.50 | 6.60–8.70 |
| Albumin (g/dL) | 4.44 ± 0.21 | 0.00 | 4.50 ± 0.20 | 4.40 ± 0.20 * | 3.50–5.20 |
| Prealbumin (mg/dL) | 25.2 ± 5.07 | 11.1 | 25.6 ± 4.50 | 24.6 ± 5.80 | 20.0–40.0 |
| Transferrin (mg/dL) | 280.2 ± 45.9 | 3.20 | 279.0 ± 43.1 | 281.8 ± 50.0 | 200.0–360.0 |
| CRP (mg/L) | 1.04 ± 6.95 | 0.00 | 1.70 ± 9.30 | 0.20 ± 0.20 | 0.02–5.00 |
| Hcy (µmol/L) | 11.7 ± 4.76 | 73.3 | 11.6 ± 4.45 | 11.8 ± 5.17 | < 13.0 |
| GOT (U/L) | 22.3 ± 6.47 | 97.4 | 22.1 ± 4.80 | 22.5 ± 8.00 | < 37.0 |
| GPT (U/L) | 19.7 ± 10.5 | 96.1 | 19.0 ± 7.20 | 20.6 ± 13.4 | < 41.0 |
| GGT (U/L) | 20.0 ± 14.8 | 19.7 | 19.9 ± 17.3 | 20.1 ± 11.5 | 11.0–50.0 |
| Amylase (U/L) | 69.8 ± 25.5 | 9.50 | 66.0 ± 23.6 | 74.1 ± 27.1 | 40.0–140.0 |
| LDH (U/L) | 186.4 ± 46.3 | 1.30 | 183.9 ± 53.2 | 189.3 ± 37.3 | 110.0–295.0 |
| TG (mg/dL) | 108.2 ± 67.9 | 3.90 | 108.2 ± 82.0 | 108.2 ± 48.4 | 50.0–200.0 |
| HDL (mg/dL) | 66.6 ± 15.6 | 1.30 | 66.9 ± 12.1 | 66.4 ± 19.0 | 40.0–60.0 |
| LDL (mg/dL) | 128.0 ± 31.3 | 3.90 | 126.4 ± 30.3 | 130.0 ± 32.8 | 70.0–190.0 |
| TC (mg/dL) | 220.5 ± 34.4 | 0.00 | 219.1 ± 33.7 | 222.1 ± 35.6 | 110.0–200.0 |
| Osteocalcin (ng/mL) | 15.3 ± 9.82 | 48.0 | 12.8 ± 8.60 | 18.3 ± 10.5 * | 15.0–46.0 |
| PTH (pg/mL) | 56.2 ± 23.8 | 0.00 | 54.9 ± 27.0 | 57.8 ± 19.4 | 20.0–70.0 |
| Leptin (ng/mL) | 13.9 ± 4.83 | 0.00 | 14.0 ± 5.10 | 13.8 ± 4.6 | 3.60–11.1 |
| Folic acid (ng/mL) | 11.2 ± 4.09 | 0.00 | 10.8 ± 4.29 | 11.6 ± 3.85 | 2.70–17.0 |
| Vitamin B12 (pg/mL) | 527.1 ± 271.9 | 1.40 | 527.5 ± 220.9 | 526.5 ± 330.7 | 190.0–900.0 |
| 25–OH–D (ng/mL) | 23.5 ± 7.40 | 79.2 | 23.7 ± 7.80 | 23.3 ± 7.10 | 30.0–100.0 |
| 25–OH–D3 (ng/mL) | 17.7 ± 7.06 | 62.5 | 17.7 ± 7.10 | 17.8 ± 7.10 | > 20 |
| 25–OH–D2 (ng/mL) | 5.74 ± 3.11 | 93.1 | 6.00 ± 3.30 | 5.50 ± 2.90 | > 10 |
| Ca (mg/dL) | 9.21 ± 0.44 | 6.50 | 9.20 ± 0.40 | 9.30 ± 0.50 | 8.60–10.2 |
| P (mg/dL) | 3.49 ± 0.50 | 3.90 | 3.40 ± 0.50 | 3.60 ± 0.40 | 2.70–4.50 |
| Mg (mg/dL) | 1.87 ± 0.25 | 23.1 | 1.90 ± 0.20 | 1.90 ± 0.30 | 1.70–2.20 |
| Fe (µg/dL) | 92.6 ± 30.7 | 13.0 | 88.3 ± 32.8 | 97.5 ± 27.8 | 60.0–170.0 |
| Cu (µg/dL) | 101.4 ± 23.0 | 27.0 | 107.3 ± 23.0 | 92.9 ± 20.8 * | 85.0–180.0 |
| Characteristics | Total Population (n = 78) | Below Reference | Early Menopause (n = 39) | Late Menopause (n = 39) | Reference Values |
|---|---|---|---|---|---|
| Mean ± SD | % | Mean ± SD | Mean ± SD | Range | |
| Antioxidant Parameters | |||||
| TAC (µmol/L) | 1539.3 ± 483.1 | 51.9 | 1534 ± 594.8 | 1545.7 ± 308.1 | 1500.0 |
| GPX (U/mL) | 118.2 ± 47.7 | 50.6 | 117.3 ± 40.9 | 119.4 ± 55.4 | 120.0 |
| SOD (U/mL) | 184.4 ± 34.2 | 31.2 | 184.5 ± 34.5 | 184.2 ± 34.4 | 164.0–240.0 |
| SOD/GPx ratio | 2.42 ± 4.97 | 0.00 | 1.90 ± 1.45 | 3.03 ± 7.20 | – |
| GAP (µmol/L) | 823.9 ± 473.3 | – | 845.6 ± 593.2 | 799.8 ± 293.9 | – |
| Characteristics | Model 0 | Model 1 | ||||
|---|---|---|---|---|---|---|
| ß | R2 | P | ß | R2 | P | |
| SOD/GPx Ratio | ||||||
| Glucose (mg/dL) | 0.425 | 0.309 | 0.001 | 0.582 | 0.312 | 0.001 |
| GAP | ||||||
| Weight (kg) | −0.310 | 0.096 | 0.008 | −0.309 | 0.096 | 0.250 |
| BMI (kg/m2) | −0.274 | 0.075 | 0.020 | −0.278 | 0.080 | 0.012 |
| HP | −0.274 | 0.075 | 0.020 | −0.271 | 0.076 | 0.854 |
| FM | −0.272 | 0.075 | 0.021 | −0.270 | 0.075 | 0.994 |
| FFM | −0.285 | 0.081 | 0.015 | −0.283 | 0.081 | 0.078 |
| Total proteins (g/dL) | 0.235 | 0.055 | 0.048 | 0.246 | 0.062 | 0.095 |
| Albumin (g/dL) | 0.267 | 0.071 | 0.023 | 0.295 | 0.085 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Lorente, H.; Herrera-Quintana, L.; Molina-López, J.; Gamarra-Morales, Y.; López-González, B.; Planells, E. Relationship between Body Composition and Biochemical Parameters with Antioxidant Status in a Healthy Cohort of Postmenopausal Women. Metabolites 2022, 12, 746. https://doi.org/10.3390/metabo12080746
Vázquez-Lorente H, Herrera-Quintana L, Molina-López J, Gamarra-Morales Y, López-González B, Planells E. Relationship between Body Composition and Biochemical Parameters with Antioxidant Status in a Healthy Cohort of Postmenopausal Women. Metabolites. 2022; 12(8):746. https://doi.org/10.3390/metabo12080746
Chicago/Turabian StyleVázquez-Lorente, Héctor, Lourdes Herrera-Quintana, Jorge Molina-López, Yenifer Gamarra-Morales, Beatriz López-González, and Elena Planells. 2022. "Relationship between Body Composition and Biochemical Parameters with Antioxidant Status in a Healthy Cohort of Postmenopausal Women" Metabolites 12, no. 8: 746. https://doi.org/10.3390/metabo12080746
APA StyleVázquez-Lorente, H., Herrera-Quintana, L., Molina-López, J., Gamarra-Morales, Y., López-González, B., & Planells, E. (2022). Relationship between Body Composition and Biochemical Parameters with Antioxidant Status in a Healthy Cohort of Postmenopausal Women. Metabolites, 12(8), 746. https://doi.org/10.3390/metabo12080746








