Association of Nasopharyngeal and Serum Glutathione Metabolism with Bronchiolitis Severity and Asthma Risk: A Prospective Multicenter Cohort Study
Abstract
1. Introduction
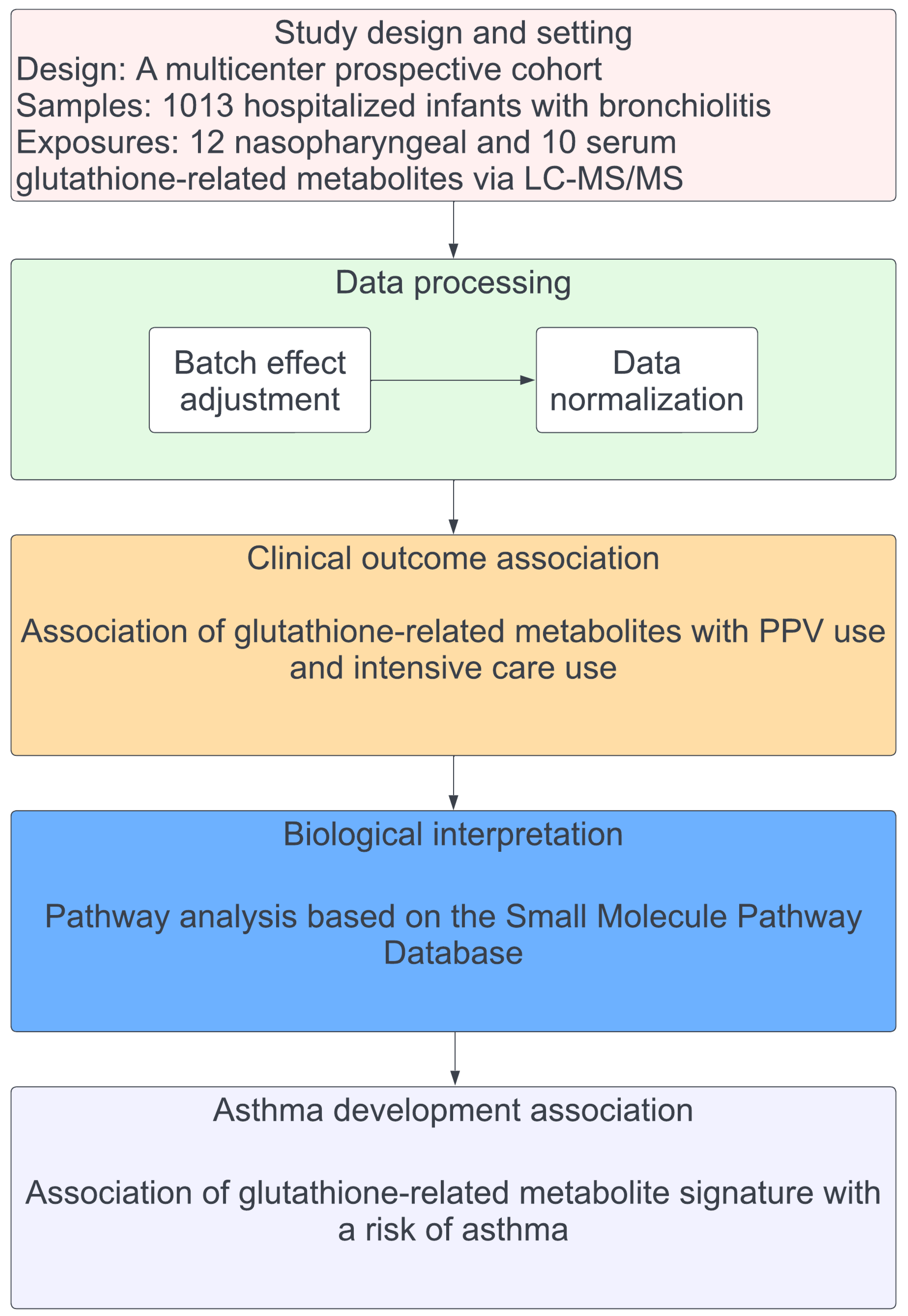
2. Results
2.1. Patient Characteristics
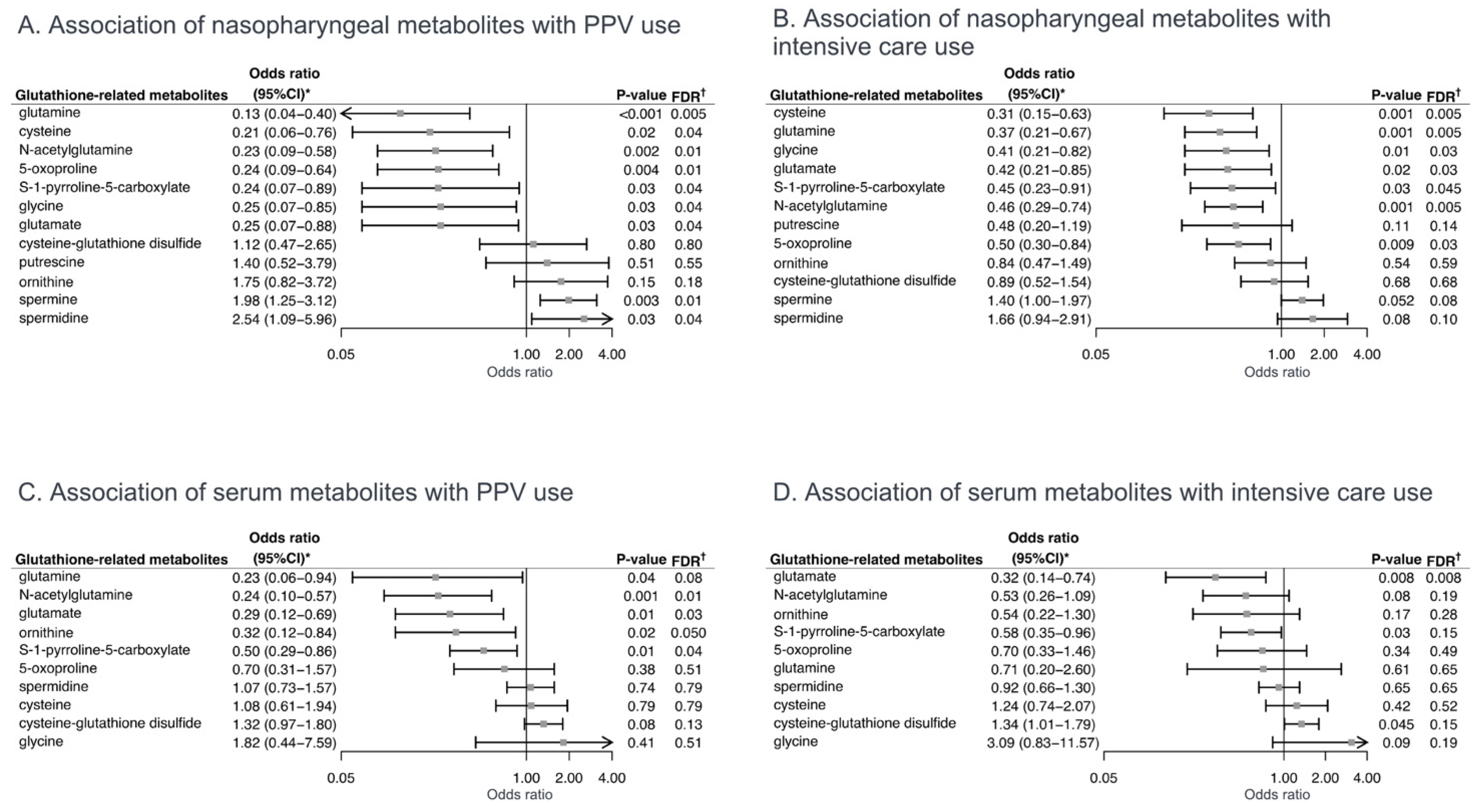
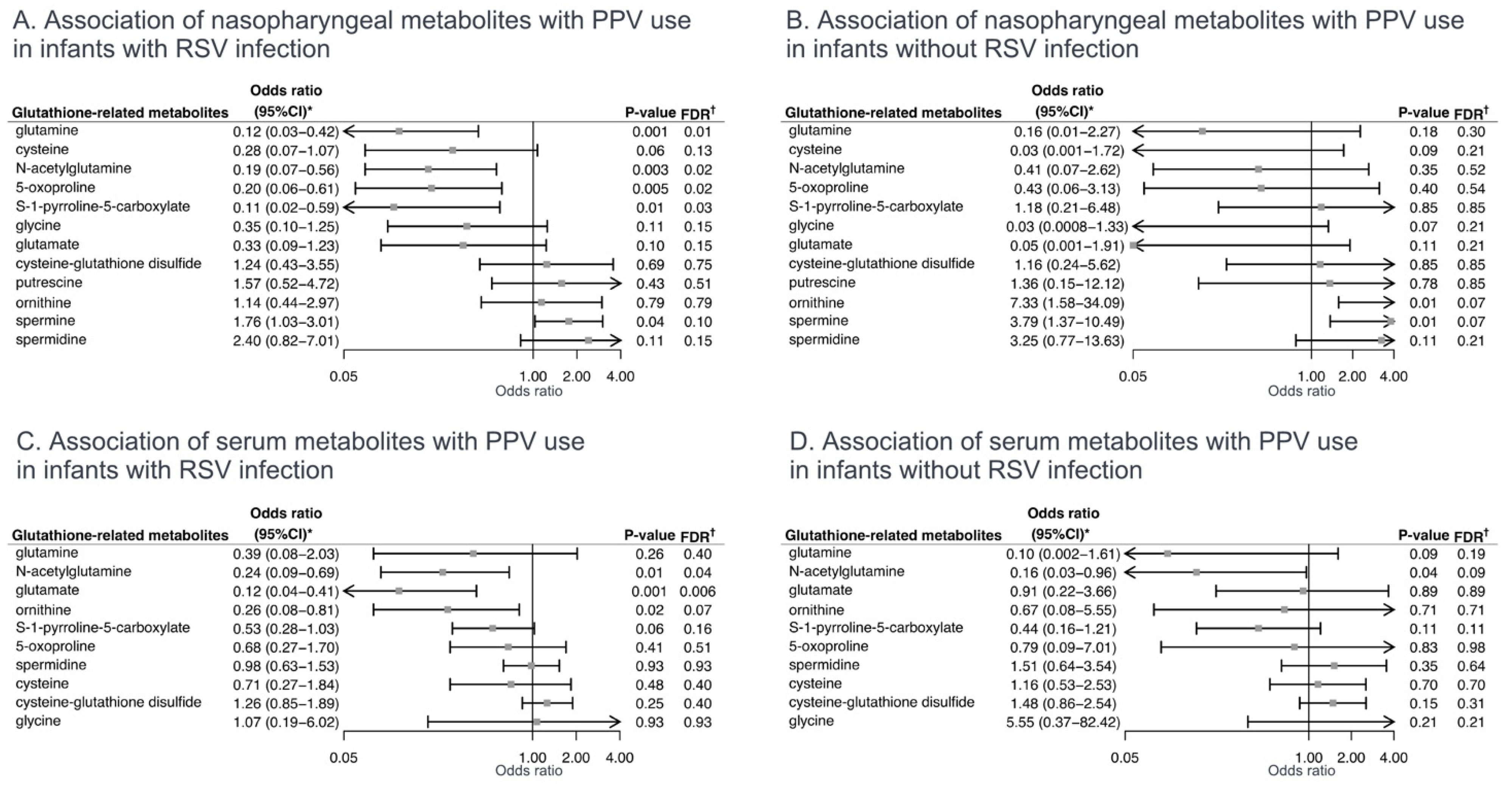
2.2. Associations of Glutathione-Related Metabolites with Severity Outcomes
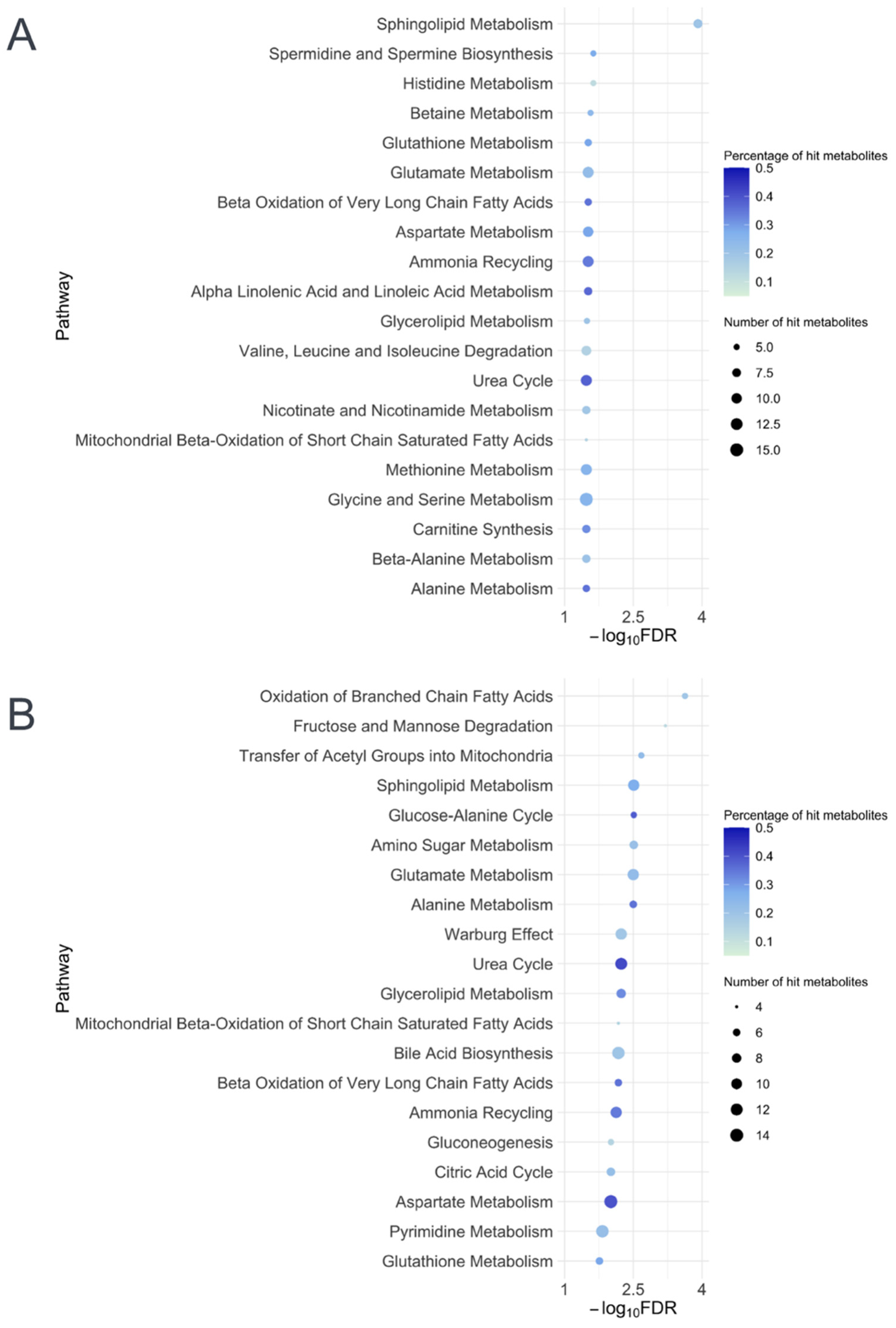
2.3. Metabolite Set Enrichment Analysis
2.4. Association of Severity-Related Glutathione Metabolite Signatures with Asthma
3. Discussion
3.1. Results in Context
3.2. Potential Mechanisms
3.3. Limitations
4. Materials and Methods
4.1. Study Design, Setting, and Participants
4.2. Data Collection and Metabolome Profiling
4.3. Outcome Measures
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujiogi, M.; Goto, T.; Yasunaga, H.; Fujishiro, J.; Mansbach, J.M.; Camargo, C.A.; Hasegawa, K. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics 2019, 144, e20192614. [Google Scholar] [CrossRef] [PubMed]
- Mansbach, J.M.; Piedra, P.A.; Stevenson, M.D.; Sullivan, A.F.; Forgey, T.F.; Clark, S.; Espinola, J.A.; Camargo, C.A.; MARC-30 Investigators. Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics 2012, 130, e492–e500. [Google Scholar] [CrossRef] [PubMed]
- Törmänen, S.; Lauhkonen, E.; Riikonen, R.; Koponen, P.; Huhtala, H.; Helminen, M.; Korppi, M.; Nuolivirta, K. Risk factors for asthma after infant bronchiolitis. Allergy 2018, 73, 916–922. [Google Scholar] [CrossRef]
- Dumas, O.; Hasegawa, K.; Mansbach, J.M.; Sullivan, A.F.; Piedra, P.A.; Camargo, C.A. Severe bronchiolitis profiles and risk of recurrent wheeze by age 3 years. J. Allergy Clin. Immunol. 2019, 143, 1371–1379.e7. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Rocca, A.; Poletti, G.; Fabi, M.; Lanari, M. Rhinovirus infection in children with acute bronchiolitis and its impact on recurrent wheezing and asthma development. Microorganisms 2020, 8, 1620. [Google Scholar] [CrossRef]
- Balekian, D.S.; Linnemann, R.W.; Hasegawa, K.; Thadhani, R.; Camargo, C.A. Cohort study of severe bronchiolitis during infancy and risk of asthma by age 5 years. J. Allergy Clin. Immunol. Pract. 2017, 5, 92–96. [Google Scholar] [CrossRef]
- Sigurs, N.; Bjarnason, R.; Sigurbergsson, F.; Kjellman, B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care. Med. 2000, 161, 1501–1507. [Google Scholar] [CrossRef]
- Kusel, M.M.H.; de Klerk, N.H.; Kebadze, T.; Vohma, V.; Holt, P.G.; Johnston, S.L.; Sly, P.D. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 2007, 119, 1105–1110. [Google Scholar] [CrossRef]
- Bergroth, E.; Aakula, M.; Elenius, V.; Remes, S.; Piippo-Savolainen, E.; Korppi, M.; Piedra, P.A.; Bochkov, Y.A.; Gern, J.E.; Camargo, C.A.; et al. Rhinovirus type in severe bronchiolitis and the development of asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 588–595.e4. [Google Scholar] [CrossRef]
- Wang, G.; Han, D.; Jiang, Z.; Li, M.; Yang, S.; Liu, L. Association between early bronchiolitis and the development of childhood asthma: A meta-analysis. BMJ Open 2021, 11, e043956. [Google Scholar] [CrossRef]
- Liu, L.; Pan, Y.; Zhu, Y.; Song, Y.; Su, X.; Yang, L.; Li, M. Association between rhinovirus wheezing illness and the development of childhood asthma: A meta-analysis. BMJ Open 2017, 7, e013034. [Google Scholar] [CrossRef] [PubMed]
- Makrinioti, H.; Hasegawa, K.; Lakoumentas, J.; Xepapadaki, P.; Tsolia, M.; Castro-Rodriguez, J.A.; Feleszko, W.; Jartti, T.; Johnston, S.L.; Bush, A.; et al. The role of respiratory syncytial virus- and rhinovirus-induced bronchiolitis in recurrent wheeze and asthma-A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2022, 33, e13741. [Google Scholar] [CrossRef] [PubMed]
- Régnier, S.A.; Huels, J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: Systematic review and meta-analysis. Pediatr. Infect. Dis. J. 2013, 32, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.N.; Wu, P.; Gebretsadik, T.; Griffin, M.R.; Dupont, W.D.; Mitchel, E.F.; Hartert, T.V. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J. Allergy Clin. Immunol. 2009, 123, 1055–1061, 1061.e1. [Google Scholar] [CrossRef]
- Mansbach, J.M.; Hasegawa, K.; Geller, R.J.; Espinola, J.A.; Sullivan, A.F.; Camargo, C.A.; MARC-35 Investigators. Bronchiolitis severity is related to recurrent wheezing by age 3 years in a prospective, multicenter cohort. Pediatr. Res. 2020, 87, 428–430. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Dundaroz, R.; Erenberk, U.; Turel, O.; Demir, A.D.; Ozkaya, E.; Erel, O. Oxidative and antioxidative status of children with acute bronchiolitis. J. Pediatr. 2013, 89, 407–411. [Google Scholar] [CrossRef][Green Version]
- Michaeloudes, C.; Chang, P.J.; Petrou, M.; Chung, K.F. Transforming growth factor-β and nuclear factor E2–related factor 2 regulate antioxidant responses in airway smooth muscle cells: Role in asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 894–903. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Liu, T.; Castro, S.M.; Garofalo, R.P.; Casola, A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol. 2009, 41, 348–357. [Google Scholar] [CrossRef]
- Moreno-Solís, G.; Dela Torre-Aguilar, M.J.; Torres-Borrego, J.; Llorente-Cantarero, F.J.; Fernández-Gutiérrez, F.; Gil-Campos, M.; Túnez-Fiñana, I.; Pérez-Navero, J.L. Oxidative stress and inflamatory plasma biomarkers in respiratory syncytial virus bronchiolitis. Clin. Respir. J. 2017, 11, 839–846. [Google Scholar] [CrossRef]
- Stewart, C.J.; Mansbach, J.M.; Wong, M.C.; Ajami, N.J.; Petrosino, J.F.; Camargo, C.A.; Hasegawa, K. Associations of nasopharyngeal metabolome and microbiome with severity among infants with bronchiolitis. A multiomic analysis. Am. J. Respir. Crit. Care Med. 2017, 196, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Mansbach, J.M.; Ajami, N.J.; Espinola, J.A.; Henke, D.M.; Petrosino, J.F.; Piedra, P.A.; Shaw, C.A.; Sullivan, A.F.; Camargo, C.A.; et al. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur. Respir. J. 2016, 48, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Mansbach, J.M.; Ajami, N.J.; Petrosino, J.F.; Zhu, Z.; Liang, L.; Camargo, C.A.; Hasegawa, K. Serum metabolome is associated with the nasopharyngeal microbiota and disease severity among infants with bronchiolitis. J. Infect. Dis. 2019, 219, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Mansbach, J.M.; Geller, R.J.; Hasegawa, K.; Espinola, J.A.; Stevenson, M.D.; Sullivan, A.F.; Camargo, C.A. Association of serum albumin with apnea in infants with bronchiolitis: A secondary analysis of data from the MARC-35 study. JAMA Netw. Open 2019, 2, e197100. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Piedra, P.A.; Bauer, C.S.; Celedón, J.C.; Mansbach, J.M.; Spergel, J.M.; Espinola, J.A.; Camargo, C.A.; MARC-35 Investigators. Nasopharyngeal CCL5 in infants with severe bronchiolitis and risk of recurrent wheezing: A multi-center prospective cohort study. Clin. Exp. Allergy 2018, 48, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; Ingham, T.; Wickens, K.; Thadhani, R.; Silvers, K.M.; Epton, M.J.; Town, G.I.; Pattemore, P.K.; Espinola, J.A.; Crane, J.; et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 2011, 127, e180–e187. [Google Scholar] [CrossRef]
- Raita, Y.; Camargo, C.A.; Bochkov, Y.A.; Celedón, J.C.; Gern, J.E.; Mansbach, J.M.; Rhee, E.P.; Freishtat, R.J.; Hasegawa, K. Integrated-omics endotyping of infants with rhinovirus bronchiolitis and risk of childhood asthma. J. Allergy Clin. Immunol. 2021, 147, 2108–2117. [Google Scholar] [CrossRef]
- Raita, Y.; Pérez-Losada, M.; Freishtat, R.J.; Harmon, B.; Mansbach, J.M.; Piedra, P.A.; Zhu, Z.; Camargo, C.A.; Hasegawa, K. Integrated omics endotyping of infants with respiratory syncytial virus bronchiolitis and risk of childhood asthma. Nat. Commun. 2021, 12, 3601. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Zhu, Z.; Camargo, C.A.; Raita, Y.; Fujiogi, M.; Liang, L.; Rhee, E.P.; Woodruff, P.G.; Hasegawa, K. Metabolome subtyping of severe bronchiolitis in infancy and risk of childhood asthma. J. Allergy Clin. Immunol. 2022, 149, 102–112. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. MSEA: A web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 2010, 38, W71–W77. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.; Knox, C.; Lim, E.; Jewison, T.; Law, V.; Hau, D.D.; Liu, P.; Gautam, B.; Ly, S.; Guo, A.C.; et al. SMPDB: The small molecule pathway database. Nucleic Acids Res 2010, 38, D480–D487. [Google Scholar] [CrossRef] [PubMed]
- Dickerhof, N.; Pearson, J.F.; Hoskin, T.S.; Berry, L.J.; Turner, R.; Sly, P.D.; Kettle, A.J.; Arest, C.F. Oxidative stress in early cystic fibrosis lung disease is exacerbated by airway glutathione deficiency. Free Radic. Biol. Med. 2017, 113, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, S.; Paliogiannis, P.; Sotgiu, E.; Mellino, S.; Zinellu, E.; Fois, A.G.; Pirina, P.; Carru, C.; Mangoni, A.A.; Zinellu, A. Systematic review and meta-analysis of the blood glutathione redox state in chronic obstructive pulmonary disease. Antioxidants 2020, 9, 1146. [Google Scholar] [CrossRef]
- Fujiogi, M.; Camargo, C.A.; Raita, Y.; Bochkov, Y.A.; Gern, J.E.; Mansbach, J.M.; Piedra, P.A.; Hasegawa, K. Respiratory viruses are associated with serum metabolome among infants hospitalized for bronchiolitis: A multicenter study. Pediatr. Allergy Immunol. 2020, 31, 755–766. [Google Scholar] [CrossRef]
- Fujiogi, M.; Camargo, C.A.; Raita, Y.; Bochkov, Y.A.; Gern, J.E.; Mansbach, J.M.; Piedra, P.A.; Hasegawa, K. Association of rhinovirus species with nasopharyngeal metabolome in bronchiolitis infants: A multicenter study. Allergy 2020, 75, 2379–2383. [Google Scholar] [CrossRef]
- Stewart, C.J.; Mansbach, J.M.; Piedra, P.A.; Toivonen, L.; Camargo, C.A.; Hasegawa, K. Association of respiratory viruses with serum metabolome in infants with severe bronchiolitis. Pediatr. Allergy Immunol. 2019, 30, 848–851. [Google Scholar] [CrossRef]
- Teoh, S.T.; Leimanis-Laurens, M.L.; Comstock, S.S.; Winters, J.W.; Vandenbosch, N.L.; Prokop, J.W.; Bachmann, A.S.; Lunt, S.Y.; Rajasekaran, S. Combined plasma and urinary metabolomics uncover metabolic perturbations associated with severe respiratory syncytial viral infection and future development of asthma in infant patients. Metabolites 2022, 12, 178. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Jantzi, P.D.; Esham, D.L.; Spratt, H.; Kurosky, A.; Casola, A.; Garofalo, R.P. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 2011, 183, 1550–1560. [Google Scholar] [CrossRef]
- Hosakote, Y.M.; Komaravelli, N.; Mautemps, N.; Liu, T.; Garofalo, R.P.; Casola, A. Antioxidant mimetics modulate oxidative stress and cellular signaling in airway epithelial cells infected with respiratory syncytial virus. Am. J. Physiol. 2012, 303, L991–L1000. [Google Scholar] [CrossRef]
- Rahman, I.; Mulier, B.; Gilmour, P.S.; Watchorn, T.; Donaldson, K.; Jeffery, P.K.; MacNee, W. Oxidant-mediated lung epithelial cell tolerance: The role of intracellular glutathione and nuclear factor-kappaB. Biochem. Pharmacol. 2001, 62, 787–794. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Aspects Med. 2022, 85, 101026. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Hisada, T.; Utsugi, M.; Ishizuka, T.; Shimizu, Y.; Ono, A.; Murata, Y.; Hamuro, J.; Mori, M.; Dobashi, K. Glutathione redox regulates airway hyperresponsiveness and airway inflammation in mice. Am. J. Respir. Cell Mol. Biol. 2007, 37, 322–329. [Google Scholar] [CrossRef]
- Poole, A.; Urbanek, C.; Eng, C.; Schageman, J.; Jacobson, S.; O’Connor, B.P.; Galanter, J.M.; Gignoux, C.R.; Roth, L.A.; Kumar, R.; et al. Dissecting childhood asthma with nasal transcriptomics distinguishes subphenotypes of disease. J. Allergy Clin. Immunol. 2014, 133, 670–678.e12. [Google Scholar] [CrossRef]
- Pires, K.M.P.; Melo, A.C.; Lanzetti, M.; Casquilho, N.V.; Zin, W.A.; Porto, L.C.; Valença, S.S. Low tidal volume mechanical ventilation and oxidative stress in healthy mouse lungs. J. Bras. Pneumol. 2012, 38, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Mansbach, J.M.; Bochkov, Y.A.; Gern, J.E.; Piedra, P.A.; Bauer, C.S.; Teach, S.J.; Wu, S.; Sullivan, A.F.; Camargo, C.A. Association of rhinovirus C bronchiolitis and immunoglobulin E sensitization during infancy with development of recurrent wheeze. JAMA Pediatr. 2019, 173, 544–552. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Fujiogi, M.; Camargo, C.A.; Raita, Y.; Zhu, Z.; Celedón, J.C.; Mansbach, J.M.; Spergel, J.M.; Hasegawa, K. Integrated associations of nasopharyngeal and serum metabolome with bronchiolitis severity and asthma: A multicenter prospective cohort study. Pediatr. Allergy Immunol. 2021, 32, 905–916. [Google Scholar] [CrossRef]
- Mansbach, J.M.; Piedra, P.A.; Teach, S.J.; Sullivan, A.F.; Forgey, T.; Clark, S.; Espinola, J.A.; Camargo, C.A.; MARC-30 Investigators. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch. Pediatr. Adolesc. Med. 2012, 166, 700–706. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Jartti, T.; Mansbach, J.M.; Laham, F.R.; Jewell, A.M.; Espinola, J.A.; Piedra, P.A.; Camargo, C.A. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: Multicenter cohort studies in the United States and Finland. J. Infect. Dis. 2015, 211, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Ball, T.M.; Castro-Rodriguez, J.A.; Griffith, K.A.; Holberg, C.J.; Martinez, F.D.; Wright, A.L. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N. Engl. J. Med. 2000, 343, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Griese, M.; Kappler, M.; Eismann, C.; Ballmann, M.; Junge, S.; Rietschel, E.; van Koningsbruggen-Rietschel, S.; Staab, D.; Rolinck-Werninghaus, C.; Mellies, U.; et al. Inhalation treatment with glutathione in patients with cystic fibrosis. A randomized clinical trial. Am. J. Respir. Crit. Care Med. 2013, 188, 83–89. [Google Scholar] [CrossRef]
- Emergency Medicine Network. Available online: http://www.emnet-usa.org/ (accessed on 4 May 2022).
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- Dehaven, C.D.; Evans, A.M.; Dai, H.; Lawton, K.A. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminform. 2010, 2, 9. [Google Scholar] [CrossRef]
- Van Elden, L.J.R.; van Loon, A.M.; van der Beek, A.; Hendriksen, K.A.W.; Hoepelman, A.I.M.; van Kraaij, M.G.J.; Schipper, P.; Nijhuis, M. Applicability of a real-time quantitative PCR assay for diagnosis of respiratory syncytial virus infection in immunocompromised adults. J. Clin. Microbiol. 2003, 41, 4378–4381. [Google Scholar] [CrossRef]
- Kuypers, J.; Wright, N.; Ferrenberg, J.; Huang, M.-L.; Cent, A.; Corey, L.; Morrow, R. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J. Clin. Microbiol. 2006, 44, 2382–2388. [Google Scholar] [CrossRef]


| Patient Characteristics | Nasopharyngeal Sample (n = 1013) | Serum Sample (n = 140) |
|---|---|---|
| Demographics | ||
| Age (month), median (IQR) | 3 (2–6) | 3 (1–6) |
| Female sex | 406 (40) | 53 (38) |
| Race/ethnicity | ||
| Non-Hispanic white | 428 (42) | 54 (39) |
| Non-Hispanic black | 239 (24) | 29 (21) |
| Hispanic | 308 (30) | 53 (38) |
| Other or unknown | 38 (4) | 4 (3) |
| C-section delivery | 347 (35) | 52 (37) |
| Prematurity (32–36.9 weeks) | 186 (18) | 34 (24) |
| History of eczema | 149 (15) | 20 (14) |
| Ever attended daycare | 233 (23) | 24 (17) |
| Cigarette smoke exposure at home | 155 (15) | 15 (11) |
| Maternal smoking during pregnancy | 147 (15) | 17 (12) |
| Parent history of eczema | 198 (20) | 32 (23) |
| Previous breathing problems (count) | ||
| 0 | 808 (80) | 106 (76) |
| 1 | 159 (16) | 24 (17) |
| 2 | 46 (5) | 10 (7) |
| Previous ICU admission | 17 (2) | 5 (4) |
| Clinical presentation at index hospitalization | ||
| Weight (kg), median (IQR) | 6.1 (4.7–7.7) | 6.0 (4.4–7.8) |
| Respiratory rate (per min), median (IQR) | 48 (40–60) | 48 (40–60) |
| Oxygen saturation at presentation | ||
| <90% | 91 (9) | 17 (12) |
| 90–93% | 155 (16) | 26 (19) |
| ≥94% | 746 (75) | 94 (69) |
| Respiratory virus | ||
| RSV | 818 (81) | 97 (69) |
| Rhinovirus | 213 (21) | 55 (39) |
| Other pathogens * | 237 (23) | 37 (26) |
| Laboratory data | ||
| Any IgE sensitization † | 203 (20) | 28 (20) |
| Clinical outcomes | ||
| Positive pressure ventilation use ‡ | 55 (5) | 38 (27) |
| Intensive care use § | 163 (16) | 70 (50) |
| Length of hospital stay (days), median (IQR) | 2 (1–3) | 3 (2–6) |
| Asthma at age 6 years || | 328 (32) | 68 (49) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyo, M.; Zhu, Z.; Nanishi, M.; Shibata, R.; Ooka, T.; Freishtat, R.J.; Mansbach, J.M.; Camargo, C.A., Jr.; Hasegawa, K. Association of Nasopharyngeal and Serum Glutathione Metabolism with Bronchiolitis Severity and Asthma Risk: A Prospective Multicenter Cohort Study. Metabolites 2022, 12, 674. https://doi.org/10.3390/metabo12080674
Kyo M, Zhu Z, Nanishi M, Shibata R, Ooka T, Freishtat RJ, Mansbach JM, Camargo CA Jr., Hasegawa K. Association of Nasopharyngeal and Serum Glutathione Metabolism with Bronchiolitis Severity and Asthma Risk: A Prospective Multicenter Cohort Study. Metabolites. 2022; 12(8):674. https://doi.org/10.3390/metabo12080674
Chicago/Turabian StyleKyo, Michihito, Zhaozhong Zhu, Makiko Nanishi, Ryohei Shibata, Tadao Ooka, Robert J. Freishtat, Jonathan M. Mansbach, Carlos A. Camargo, Jr., and Kohei Hasegawa. 2022. "Association of Nasopharyngeal and Serum Glutathione Metabolism with Bronchiolitis Severity and Asthma Risk: A Prospective Multicenter Cohort Study" Metabolites 12, no. 8: 674. https://doi.org/10.3390/metabo12080674
APA StyleKyo, M., Zhu, Z., Nanishi, M., Shibata, R., Ooka, T., Freishtat, R. J., Mansbach, J. M., Camargo, C. A., Jr., & Hasegawa, K. (2022). Association of Nasopharyngeal and Serum Glutathione Metabolism with Bronchiolitis Severity and Asthma Risk: A Prospective Multicenter Cohort Study. Metabolites, 12(8), 674. https://doi.org/10.3390/metabo12080674







