Serum Oxylipin Profiles Identify Potential Biomarkers in Patients with Acute Aortic Dissection
Abstract
1. Introduction
2. Results
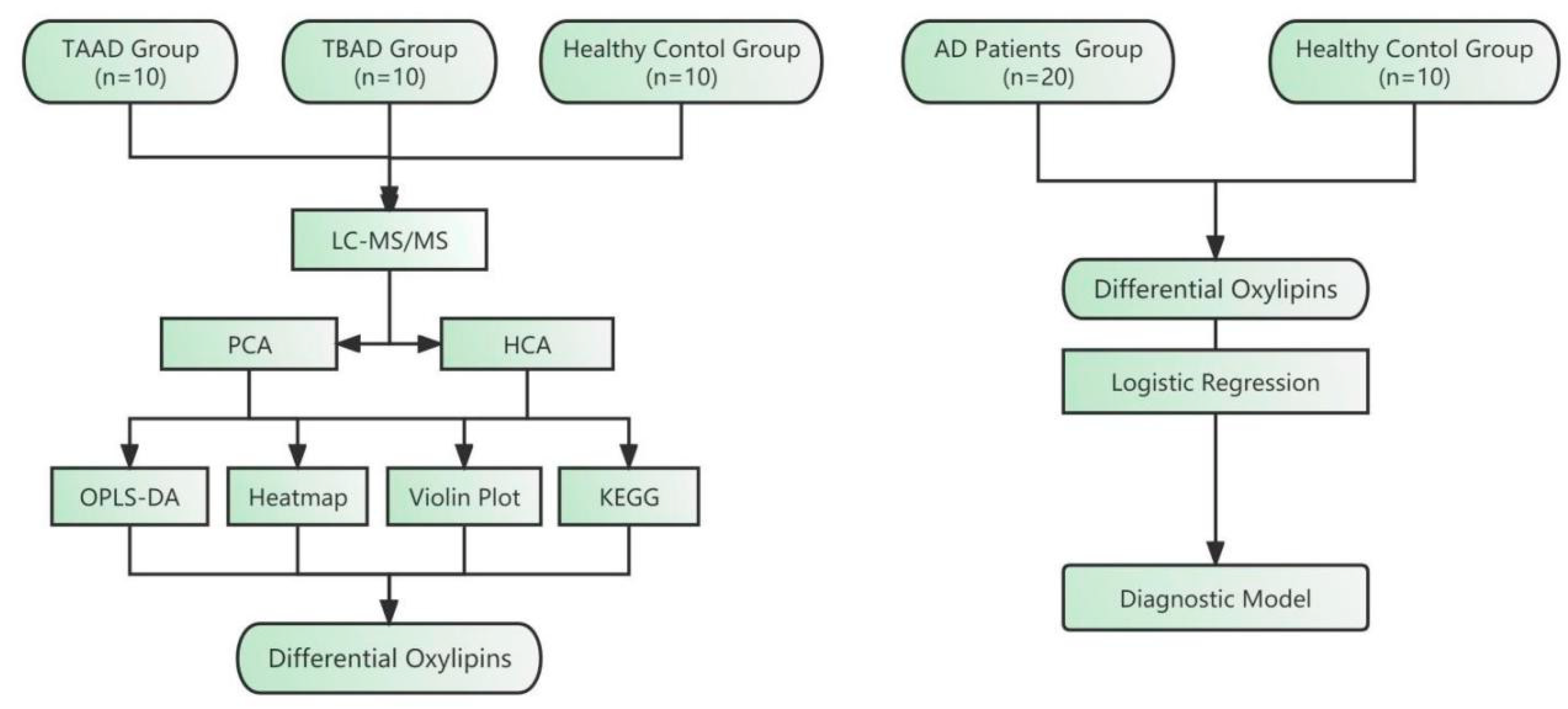
2.1. Clinical Characteristics of the Studied Population
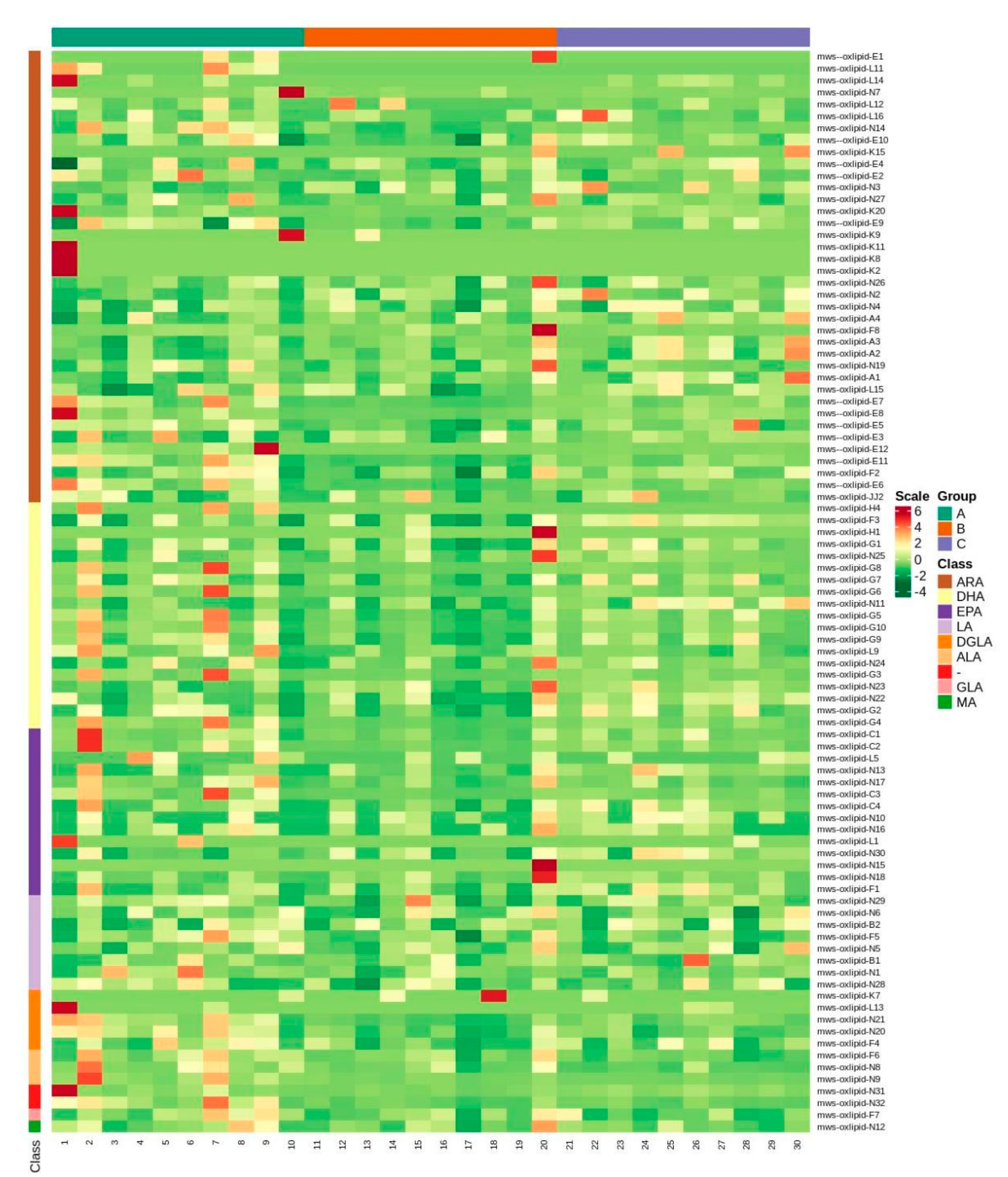
2.2. Data Preprocessing and PCA Analysis of Oxylipins
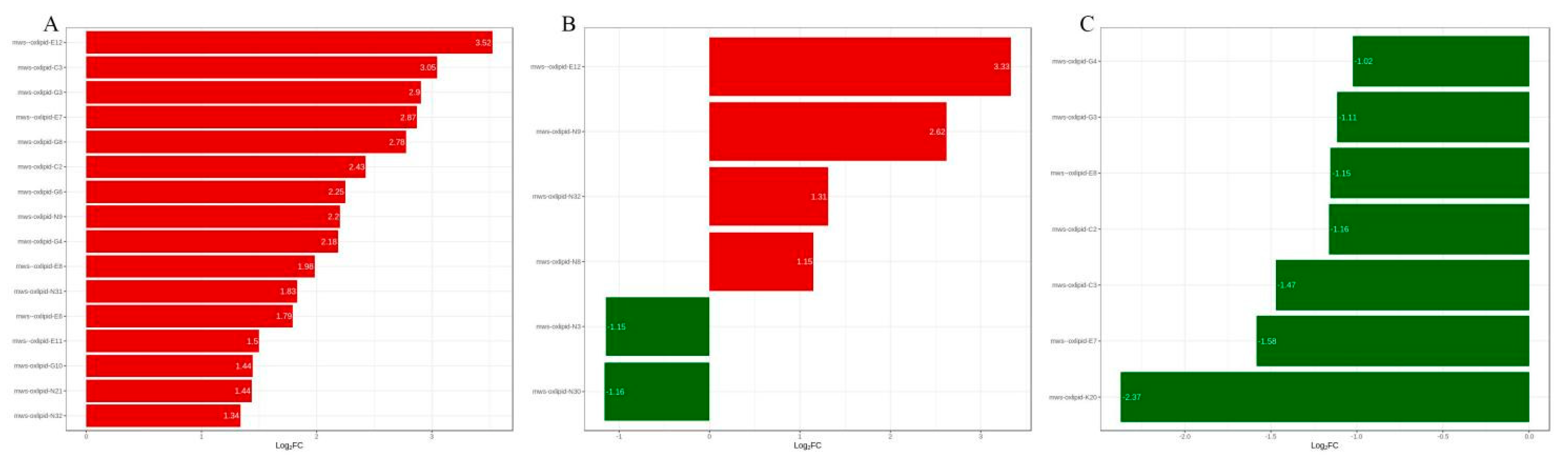
2.3. Differential Metabolite Screening
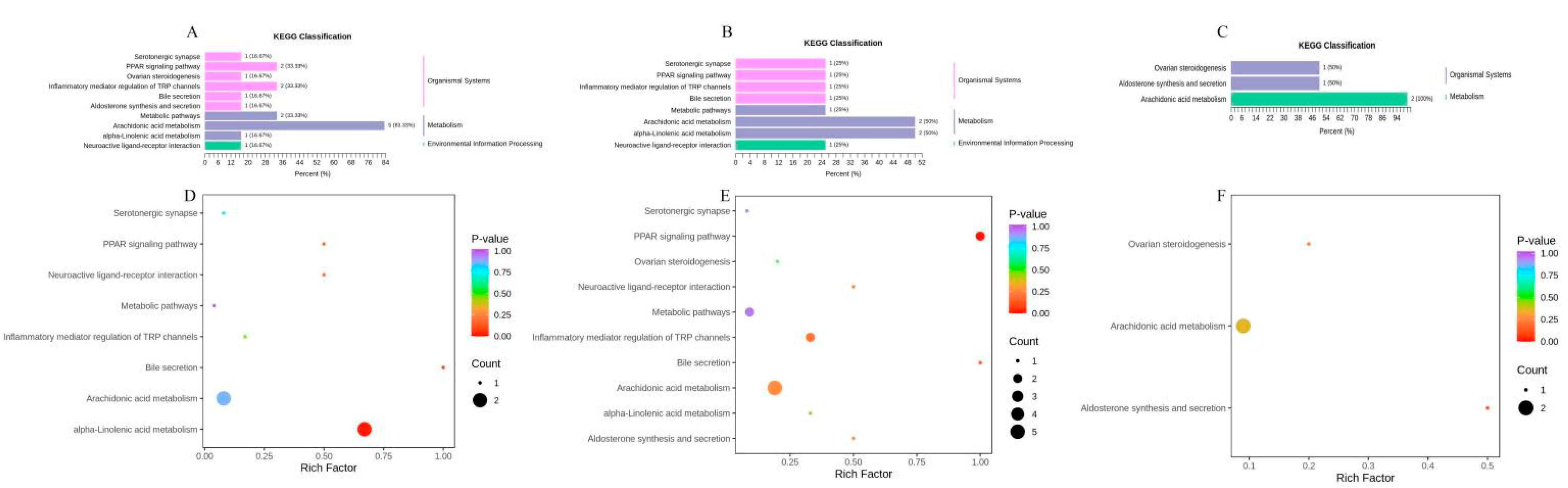
2.4. KEGG Enrichment of Oxylipins
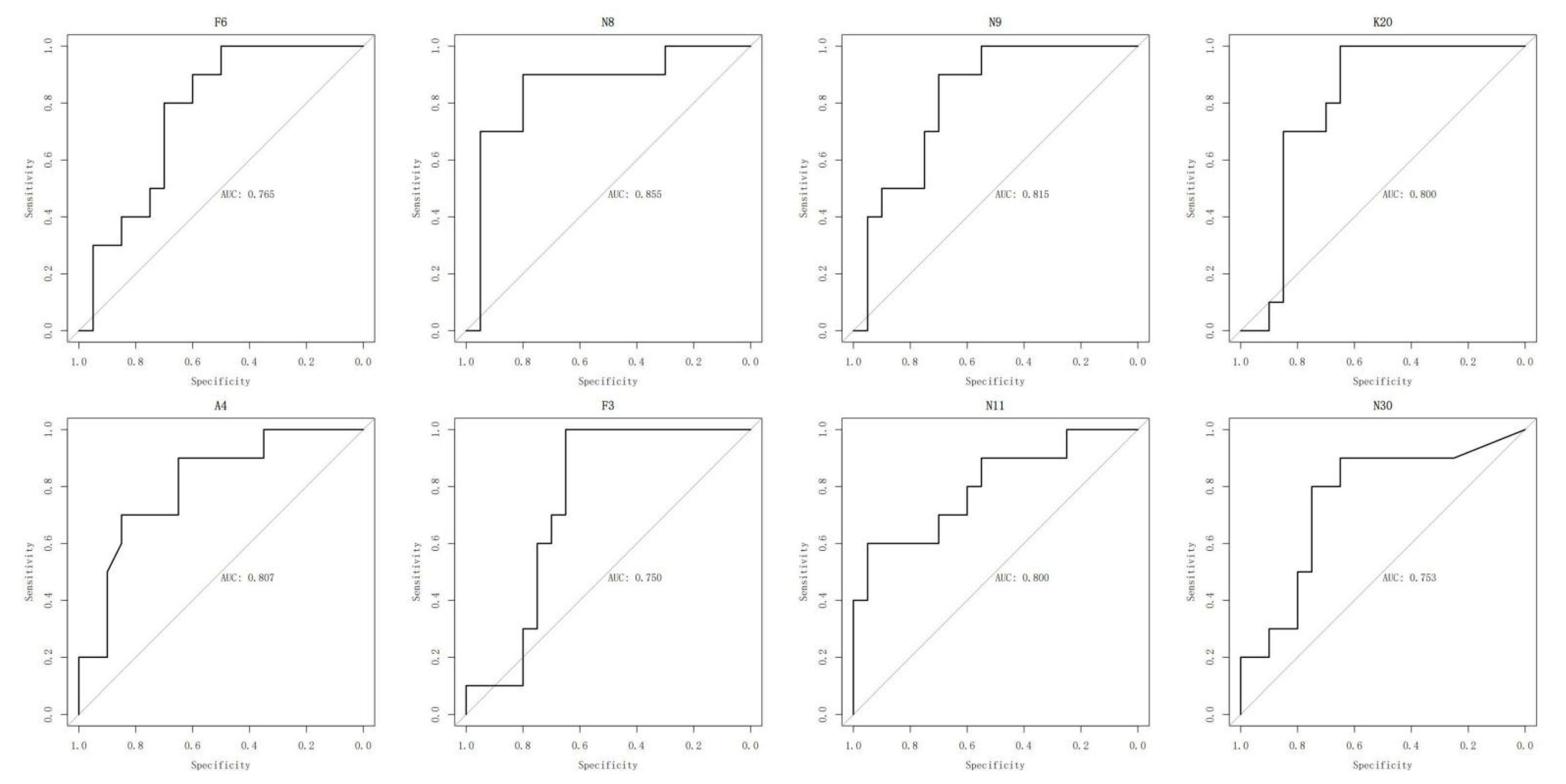
2.5. ROC Analysis of Oxylipins
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Sample Collection and Preparation for Oxylipin Analysis
4.3. Detection of Oxylipins
4.4. Data Acquisition and Processing
4.5. Statistical Analysis
4.6. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Abdominal Aortic Aneurysm; |
| ACS | Acute Coronary Syndromes; |
| AD | Aortic Dissection; |
| ALA | α-Linolenic Acid; |
| ANOVA | Analysis of Variance; |
| ARA | Arachidonic Acid; |
| ATGL | Adipose Triglyceride Lipase; |
| AUC | Area under the ROC Curve; |
| BKCa | Large conductance Ca2+—activated K+ channels; |
| BMI | Body Mass Index; |
| CHD | Coronary Heart Disease; |
| COX | Cyclooxygenase; |
| CT | Computed Tomography; |
| CTA | Computed Tomography Angiography; |
| CYP450 | Cytochrome P450; |
| DGLA | Dihomo-γ-Linolenic Acid; |
| DHA | Docosahexaenoic Acid; |
| EPA | Eicosapentaenoic Acid; |
| GLA | γ-Linolenic Acid; |
| HCA | Hierarchical Cluster Analysis; |
| IKCa | Intermediate conductance Ca2+—activated K+ channels; |
| IL-6 | Interleukin-6; |
| KEGG | Kyoto Encyclopedia of Genes and Genomes; |
| LA | Linolenic Acid; |
| LC-MS/MS | Liquid Chromatography Linked to Tandem Mass Spectrometry; |
| LOX | Lipoxygenase; |
| OPLS-DA | Orthogonal Partial Least Squares-Discriminant Analysis; |
| PCA | Principal Component Analysis; |
| PPARs | Peroxisome Proliferators-Activated Receptors; |
| PUFA | Polyunsaturated Fatty Acid; |
| ROC | Receiver Operating Characteristic Curve; |
| TAAD | Type A Aortic Dissection; |
| TBAD | Type B Aortic Dissection; |
| TIC | Total Ion Chromatogram; |
| VIP | Variable Importance in Projection; |
| XIC | Extracted Ion Chromatogram. |
References
- Mussa, F.F.; Horton, J.D.; Moridzadeh, R.; Nicholson, J.; Trimarchi, S.; Eagle, K.A. Acute aortic dissection and intramural hematoma: A systematic review. JAMA 2016, 316, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, S.A.; Russell, L. Epidemiology of thoracic aortic dissection. Nat. Rev. Cardiol. 2011, 8, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Karmy-Jones, R.; Aldea, G.; Boyle, E.J. The continuing evolution in the management of thoracic aortic dissection. Chest 2000, 117, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, C.A.; Clough, R.E. Management of acute aortic dissection. Lancet 2015, 385, 800–811. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Clough, R.E.; Sakalihasan, N.; Suzuki, T.; Gibbs, R.; Mussa, F.; Jenkins, M.P.; Thompson, M.M.; Evangelista, A.; Yeh, J.S.; et al. Aortic dissection. Nat. Rev. Dis. Primers 2016, 2, 16053. [Google Scholar] [CrossRef]
- Cifani, N.; Proietta, M.; Tritapepe, L.; Di Gioia, C.; Ferri, L.; Taurino, M.; Del, P.F. Stanford-A acute aortic dissection, inflammation, and metalloproteinases: A review. Ann. Med. 2015, 47, 441–446. [Google Scholar] [CrossRef]
- Suzuki, T.; Eagle, K.A. Biomarker-Assisted diagnosis of acute aortic dissection. Circulation 2018, 137, 270–272. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, X.; Gao, H.; Yuan, H.; Hu, R.; Jia, L.; Zhu, J.; Sun, L.; Zhang, H.; Huang, L.; et al. Magnitude of soluble ST2 as a novel biomarker for acute aortic dissection. Circulation 2018, 137, 259–269. [Google Scholar] [CrossRef]
- Suzuki, T.; Distante, A.; Zizza, A.; Trimarchi, S.; Villani, M.; Salerno, U.J.; de Luca, T.S.L.; Renzulli, A.; Sabino, F.; Nowak, R.; et al. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur. Heart J. 2008, 29, 1439–1445. [Google Scholar] [CrossRef]
- Wen, D.; Du, X.; Dong, J.Z.; Zhou, X.L.; Ma, C.S. Value of D-dimer and C reactive protein in predicting inhospital death in acute aortic dissection. Heart 2013, 99, 1192–1197. [Google Scholar] [CrossRef]
- Del, P.F.; Proietta, M.; Tritapepe, L.; Miraldi, F.; Koverech, A.; Cardelli, P.; Tabacco, F.; de Santis, V.; Vecchione, A.; Mitterhofer, A.P.; et al. Inflammation and immune response in acute aortic dissection. Ann. Med. 2010, 42, 622–629. [Google Scholar]
- Luo, F.; Zhou, X.L.; Li, J.J.; Hui, R.T. Inflammatory response is associated with aortic dissection. Ageing Res. Rev. 2009, 8, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C.M.; Pitt, A.R. Oxidative lipidomics coming of age: Advances in analysis of oxidized phospholipids in physiology and pathology. Antioxid. Redox Signal. 2015, 22, 1646–1666. [Google Scholar] [CrossRef] [PubMed]
- Pauls, S.D.; Du, Y.; Clair, L.; Winter, T.; Aukema, H.M.; Taylor, C.G.; Zahradka, P. Impact of age, menopause, and obesity on oxylipins linked to vascular health. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 883–897. [Google Scholar] [CrossRef]
- Pauls, S.D.; Rodway, L.R.; Sidhu, K.K.; Winter, T.; Sidhu, N.; Aukema, H.M.; Zahradka, P.; Taylor, C.G. Oils Rich in alpha-Linolenic Acid or Docosahexaenoic Acid Have Distinct Effects on Plasma Oxylipin and Adiponectin Concentrations and on Monocyte Bioenergetics in Women with Obesity. J. Nutr. 2021, 151, 3053–3066. [Google Scholar] [CrossRef]
- Larsson, N.; Lundstrom, S.L.; Pinto, R.; Rankin, G.; Karimpour, M.; Blomberg, A.; Sandstrom, T.; Pourazar, J.; Trygg, J.; Behndig, A.F.; et al. Lipid mediator profiles differ between lung compartments in asthmatic and healthy humans. Eur. Respir. J. 2014, 43, 453–463. [Google Scholar] [CrossRef]
- Lundstrom, S.L.; Yang, J.; Brannan, J.D.; Haeggstrom, J.Z.; Hammock, B.D.; Nair, P.; O’Byrne, P.; Dahlen, S.E.; Wheelock, C.E. Lipid mediator serum profiles in asthmatics significantly shift following dietary supplementation with omega-3 fatty acids. Mol. Nutr. Food Res. 2013, 57, 1378–1389. [Google Scholar] [CrossRef]
- Augimeri, G.; Plastina, P.; Gionfriddo, G.; Rovito, D.; Giordano, C.; Fazio, A.; Barone, I.; Catalano, S.; Ando, S.; Bonofiglio, D.; et al. N-Eicosapentaenoyl dopamine, a conjugate of dopamine and eicosapentaenoic acid (EPA), exerts anti-inflammatory properties in mouse and human macrophages. Nutrients 2019, 11, 2247. [Google Scholar] [CrossRef]
- Wolfer, A.M.; Scott, A.J.; Rueb, C.; Gaudin, M.; Darzi, A.; Nicholson, J.K.; Holmes, E.; Kinross, J.M. Longitudinal analysis of serum oxylipin profile as a novel descriptor of the inflammatory response to surgery. J. Transl. Med. 2017, 15, 83. [Google Scholar] [CrossRef][Green Version]
- Chistyakov, D.V.; Gavrish, G.E.; Goriainov, S.V.; Chistyakov, V.V.; Astakhova, A.A.; Azbukina, N.V.; Sergeeva, M.G. Oxylipin profiles as functional characteristics of acute inflammatory responses in astrocytes Pre-Treated with IL-4, IL-10, or LPS. Int. J. Mol. Sci. 2020, 21, 1780. [Google Scholar] [CrossRef]
- Schunck, W.H.; Konkel, A.; Fischer, R.; Weylandt, K.H. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018, 183, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Maly, M.; Hajsl, M.; Bechynska, K.; Kucerka, O.; Sramek, M.; Suttnar, J.; Hlavackova, A.; Hajslova, J.; Kosek, V. Lipidomic analysis to assess oxidative stress in acute coronary syndrome and acute stroke patients. Metabolites 2021, 11, 412. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, S.; Aukema, H.M.; Ravandi, A.; Lavallee, R.; Guzman, R.; Pierce, G.N. Specific plasma oxylipins increase the odds of cardiovascular and cerebrovascular events in patients with peripheral artery disease. Can. J. Physiol. Pharmacol. 2017, 95, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, S.; Parikh, M.; Stamenkovic, A.; Pierce, G.N.; Aukema, H.M. Dietary modulation of oxylipins in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H903–H918. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, M.A. Role of oxylipins in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1142–1154. [Google Scholar] [CrossRef]
- Vilacosta, I.; San, R.J.; di Bartolomeo, R.; Eagle, K.; Estrera, A.L.; Ferrera, C.; Kaji, S.; Nienaber, C.A.; Riambau, V.; Schafers, H.J.; et al. Acute aortic syndrome revisited: JACC State-of-the-Art review. J. Am. Coll. Cardiol. 2021, 78, 2106–2125. [Google Scholar] [CrossRef]
- Bossone, E.; Eagle, K.A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 2021, 18, 331–348. [Google Scholar] [CrossRef]
- Zhu, Y.; Lingala, B.; Baiocchi, M.; Tao, J.J.; Toro, A.V.; Khoo, J.W.; Williams, K.M.; Traboulsi, A.A.; Hammond, H.C.; Lee, A.M.; et al. Type a aortic Dissection-Experience over 5 decades: JACC historical breakthroughs in perspective. J. Am. Coll. Cardiol. 2020, 76, 1703–1713. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Trybus, K.M.; Guo, D.C.; Sweeney, H.L.; Regalado, E.; Kamm, K.; Stull, J.T. Altered smooth muscle cell force generation as a driver of thoracic aortic aneurysms and dissections. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 26–34. [Google Scholar] [CrossRef]
- Vrsalovic, M. Prognostic effect of cardiac troponin elevation in acute aortic dissection: A meta-analysis. Int. J. Cardiol. 2016, 214, 277–278. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Fu, W.; Guo, D.; Jiang, J.; Wang, Y. Association of smooth muscle cell phenotypes with extracellular matrix disorders in thoracic aortic dissection. J. Vasc. Surg. 2012, 56, 1698–1709, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Fu, W.; Wang, C.; Guo, D.; Jiang, J.; Wang, Y. Smooth muscle cell phenotypic diversity between dissected and unaffected thoracic aortic media. J. Cardiovasc. Surg. 2013, 54, 511–521. [Google Scholar]
- Li, G.; Wu, X.W.; Lu, W.H.; Cheng, J.; Wu, X.Y.; Ai, R.; Zhou, Z.H.; Tang, Z.Z.; Liao, Y.H. High-sensitivity cardiac troponin T: A biomarker for the early risk stratification of type-A acute aortic dissection? Arch. Cardiovasc. Dis. 2016, 109, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lu, L.; Qiu, Z.; Huang, Q.; Chen, Y.; Chen, L. Mechanical stretch aggravates aortic dissection by regulating MAPK pathway and the expression of MMP-9 and inflammation factors. Biomed. Pharmacother. 2018, 108, 1294–1302. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.; Zhang, T. The analysis of the levels of plasma inflammation-related cytokines and endotoxins in patients with acute aortic dissection. Clin. Hemorheol. Microcirc. 2020, 76, 1–7. [Google Scholar] [CrossRef]
- Wang, X.L.; Liu, O.; Qin, Y.W.; Zhang, H.J.; Lv, Y. Association of the polymorphisms of MMP-9 and TIMP-3 genes with thoracic aortic dissection in Chinese Han population. Acta Pharmacol. Sin. 2014, 35, 351–355. [Google Scholar] [CrossRef]
- Erdolu, B.; As, A.K. C-Reactive protein and neutrophil to lymphocyte ratio values in predicting inhospital death in patients with stanford type a acute aortic dissection. Heart Surg. Forum 2020, 23, E488–E492. [Google Scholar] [CrossRef]
- Yuan, S.M. Profiles and predictive values of interleukin-6 in aortic dissection: A review. Braz. J. Cardiovasc. Surg. 2019, 34, 596–604. [Google Scholar] [CrossRef]
- Liu, H.; Luo, Z.; Liu, L.; Yang, X.; Zhuang, Y.; Tu, G.; Ma, G.; Zhang, Y.; Zheng, J.; Zhu, D.; et al. Inflammatory biomarkers to predict adverse outcomes in postoperative patients with acute type a aortic dissection. Scand. Cardiovasc. J. 2020, 54, 37–46. [Google Scholar] [CrossRef]
- Mori, K.; Tamune, H.; Tanaka, H.; Nakamura, M. Admission values of d-dimer and c-reactive protein (CRP) predict the long-term outcomes in acute aortic dissection. Intern. Med. 2016, 55, 1837–1843. [Google Scholar] [CrossRef]
- Surendran, A.; Zhang, H.; Winter, T.; Edel, A.; Aukema, H.; Ravandi, A. Oxylipin profile of human low-density lipoprotein is dependent on its extent of oxidation. Atherosclerosis 2019, 288, 101–111. [Google Scholar] [CrossRef]
- Coras, R.; Kavanaugh, A.; Kluzniak, A.; Holt, D.; Weilgosz, A.; Aaron, A.; Quehenberger, O.; Ritchlin, C.; Guma, M. Differences in oxylipin profile in psoriasis versus psoriatic arthritis. Arthritis Res. Ther. 2021, 23, 200. [Google Scholar] [CrossRef]
- Tans, R.; Bande, R.; van Rooij, A.; Molloy, B.J.; Stienstra, R.; Tack, C.J.; Wevers, R.A.; Wessels, H.; Gloerich, J.; van Gool, A.J. Evaluation of cyclooxygenase oxylipins as potential biomarker for obesity-associated adipose tissue inflammation and type 2 diabetes using targeted multiple reaction monitoring mass spectrometry. Prostaglandins Leukot. Essent. Fat. Acids 2020, 160, 102157. [Google Scholar] [CrossRef]
- Huang, H.; Ye, G.; Lai, S.Q.; Zou, H.X.; Yuan, B.; Wu, Q.C.; Wan, L.; Wang, Q.; Zhou, X.L.; Wang, W.J.; et al. Plasma lipidomics identifies unique lipid signatures and potential biomarkers for patients with aortic dissection. Front. Cardiovasc. Med. 2021, 8, 757022. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Zhang, T.; Liu, F.; Zhang, W.; Wang, G.; Gu, G.; Han, Q.; Xu, D.; Yao, C.; et al. Identification of lysophosphatidylcholines and sphingolipids as potential biomarkers for acute aortic dissection via serum metabolomics. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 434–441. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Yang, W.; Yu, H.; Zhang, L.; Ma, P.; Wu, P.; Li, X.; Cho, K.; Xue, S.; et al. Plasma amino acid profile in patients with aortic dissection. Sci. Rep. 2017, 7, 40146. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, X.; Tan, X.R.; Chen, Y.Q. Admission LDL-C and long-term mortality in patients with acute aortic dissection: A survival analysis in China. Ann. Transl. Med. 2021, 9, 1345. [Google Scholar] [CrossRef]
- Liu, X.T.; He, X.W.; Tan, R.; Liu, W.J.; Wang, B.; Liu, Y.J.; Wang, T.; Liu, C.W.; Su, X.; Zeng, H.S. High-density lipoprotein cholesterol and in-hospital mortality in patients with acute aortic dissection. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 364–367. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, G.; He, H.; Pan, X.; Peng, W.; Chai, X. Triglyceride/High-Density lipoprotein cholesterol ratio is associated with In-Hospital mortality in acute type b aortic dissection. BioMed Res. Int. 2020, 2020, 5419846. [Google Scholar] [CrossRef]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef]
- Weinberg, R.L.; Brook, R.D.; Rubenfire, M.; Eagle, K.A. Cardiovascular impact of nutritional supplementation with omega-3 fatty acids: JACC focus seminar. J. Am. Coll. Cardiol. 2021, 77, 593–608. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 polyunsaturated fatty acid (Fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the american heart association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Kamata, R.; Bumdelger, B.; Kokubo, H.; Fujii, M.; Yoshimura, K.; Ishida, T.; Ishida, M.; Yoshizumi, M. EPA prevents the development of abdominal aortic aneurysms through Gpr-120/Ffar-4. PLoS ONE 2016, 11, e165132. [Google Scholar] [CrossRef]
- Abe, S.; Sugimura, H.; Watanabe, S.; Murakami, Y.; Ebisawa, K.; Ioka, T.; Takahashi, T.; Ando, T.; Kono, K.; Inoue, T. Eicosapantaenoic acid treatment based on the EPA/AA ratio in patients with coronary artery disease: Follow-up data from the Tochigi Ryomo EPA/AA Trial in Coronary Artery Disease (TREAT-CAD) study. Hypertens. Res. 2018, 41, 939–946. [Google Scholar] [CrossRef]
- Aikawa, T.; Miyazaki, T.; Shimada, K.; Sugita, Y.; Shimizu, M.; Ouchi, S.; Kadoguchi, T.; Yokoyama, Y.; Shiozawa, T.; Hiki, M.; et al. Low serum levels of EPA are associated with the size and growth rate of abdominal aortic aneurysm. J. Atheroscler. Thromb. 2017, 24, 912–920. [Google Scholar] [CrossRef]
- Takaki, A.; Umemoto, S.; Ono, K.; Seki, K.; Ryoke, T.; Fujii, A.; Itagaki, T.; Harada, M.; Tanaka, M.; Yonezawa, T.; et al. Add-on therapy of EPA reduces oxidative stress and inhibits the progression of aortic stiffness in patients with coronary artery disease and statin therapy: A randomized controlled study. J. Atheroscler. Thromb. 2011, 18, 857–866. [Google Scholar] [CrossRef]
- Watanabe, K.; Taketomi, Y.; Miki, Y.; Kugiyama, K.; Murakami, M. Group V secreted phospholipase A2 plays a protective role against aortic dissection. J. Biol. Chem. 2020, 295, 10092–10111. [Google Scholar] [CrossRef]
- Fan, F.; Roman, R.J. Effect of cytochrome p450 metabolites of arachidonic acid in nephrology. J. Am. Soc. Nephrol. 2017, 28, 2845–2855. [Google Scholar] [CrossRef]
- Cerk, I.K.; Wechselberger, L.; Oberer, M. Adipose triglyceride lipase regulation: An overview. Curr. Protein Pept. Sci. 2018, 19, 221–233. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Kristensen, K.L.; Burillo, E.; Martinez-Lopez, D.; Calvo, C.; Ros, E.; Martin-Ventura, J.L.; Sala-Vila, A. Arachidonic acid, but not omega-3 index, relates to the prevalence and progression of abdominal aortic aneurysm in a Population-Based study of danish men. J. Am. Heart Assoc. 2018, 7, e007790. [Google Scholar] [CrossRef]
- Morita, I.; Saito, Y.; Chang, W.C.; Murota, S. Effects of purified eicosapentaenoic acid on arachidonic acid metabolism in cultured murine aortic smooth muscle cells, vessel walls and platelets. Lipids 1983, 18, 42–49. [Google Scholar] [CrossRef]
- Riederer, M.; Lechleitner, M.; Kofeler, H.; Frank, S. Reduced expression of adipose triglyceride lipase decreases arachidonic acid release and prostacyclin secretion in human aortic endothelial cells. Arch Physiol. Biochem. 2017, 123, 249–253. [Google Scholar] [CrossRef]
- Saravanan, P.; Davidson, N.C.; Schmidt, E.B.; Calder, P.C. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010, 376, 540–550. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef]
- Limbu, R.; Cottrell, G.S.; Mcneish, A.J. Characterisation of the vasodilation effects of DHA and EPA, n-3 PUFAs (fish oils), in rat aorta and mesenteric resistance arteries. PLoS ONE 2018, 13, e192484. [Google Scholar] [CrossRef]
- Boivin, A.; Burban, M.; Clere-Jehl, R.; Le Borgne, P.; Merdji, H.; Auger, C.; Schini-Kerth, V.; Meziani, F.; Helms, J. Docosahexaenoic acid, but not eicosapentaenoic acid, improves septic shock-induced arterial dysfunction in rats. PLoS ONE 2017, 12, e189658. [Google Scholar] [CrossRef]
- Yoshihara, T.; Shimada, K.; Fukao, K.; Sai, E.; Sato-Okabayashi, Y.; Matsumori, R.; Shiozawa, T.; Alshahi, H.; Miyazaki, T.; Tada, N.; et al. Omega 3 polyunsaturated fatty acids suppress the development of aortic aneurysms through the inhibition of Macrophage-Mediated inflammation. Circ. J. 2015, 79, 1470–1478. [Google Scholar] [CrossRef]
- Pisaniello, A.D.; Psaltis, P.J.; King, P.M.; Liu, G.; Gibson, R.A.; Tan, J.T.; Duong, M.; Nguyen, T.; Bursill, C.A.; Worthley, M.I.; et al. Omega-3 fatty acids ameliorate vascular inflammation: A rationale for their atheroprotective effects. Atherosclerosis 2021, 324, 27–37. [Google Scholar] [CrossRef]
- Meital, L.T.; Windsor, M.T.; Perissiou, M.; Schulze, K.; Magee, R.; Kuballa, A.; Golledge, J.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci. Rep. 2019, 9, 12978. [Google Scholar] [CrossRef]



| TAAD (n = 10) | TBAD (n = 10) | CONTROL (n = 10) | ||
|---|---|---|---|---|
| Group A | Group B | Group C | p | |
| Age (years) | 47.40 ± 12.77 | 52.20 ± 13.05 | 49.10 ± 4.91 | 0.790 |
| Gender (%) | ||||
| Male (n) | 10 (100.0) | 7 (70.0) | 7 (70.0) | 0.153 |
| Female (n) | 0 (0.0) | 3 (30.0) | 3 (30.0) | |
| BMI (kg/m2) | 28.09 ± 6.26 | 27.78 ± 9.04 | 24.81 ± 3.73 | 0.491 |
| Hypertension (%) | 6 (60.0) | 7 (70.0) | 9 (90.0) | 0.303 |
| Diabetes (%) | 0 (0.0) | 2 (20.0) | 1 (10.0) | 0.329 |
| WBC (109/L) | 9.90 [7.50, 15.68] | 11.85 [7.82, 13.38] | 6.05 [5.45, 6.47] | 0.003 |
| NEU percentage (%) | 86.65 [80.00, 88.35] | 85.40 [81.55, 91.10] | 59.50 [56.60, 64.20] | <0.001 |
| RBC (1012/L) | 4.31 [3.47, 4.68] | 4.78 [3.97, 5.05] | 4.90 [4.65, 5.12] | 0.046 |
| Hemoglobin (g/L) | 139.50 [109.25, 144.00] | 146.00 [117.75, 147.00] | 156.50 [149.25, 163.25] | 0.010 |
| Platelet (109/L) | 153.00 [111.50, 184.00] | 155.50 [120.00, 235.25] | 237.50 [198.25, 266.75] | 0.010 |
| GPT (U/L) | 24.80 [21.00, 28.00] | 15.25 [14.03, 19.75] | 26.30 [18.38, 32.27] | 0.159 |
| GOT (U/L) | 28.00 [21.00, 41.40] | 21.15 [17.80, 31.42] | 22.95 [19.73, 24.80] | 0.342 |
| ALP (U/L) | 62.60 [55.48, 67.03] | 76.10 [65.10, 88.80] | 62.40 [54.15, 74.53] | 0.173 |
| LDH (U/L) | 262.00 [194.00, 366.00] | 251.00 [187.00, 270.25] | 179.00 [175.25, 186.00] | 0.025 |
| Total bilirubin (μmol/L) | 17.15 [9.27, 25.25] | 14.50 [10.50, 16.65] | 12.75 [10.15, 16.52] | 0.753 |
| Direct bilirubin (μmol/L) | 3.05 [2.72, 4.47] | 3.70 [3.10, 4.20] | 2.10 [1.75, 2.80] | 0.009 |
| Cholinesterase (KU/L) | 7.20 [6.07, 8.27] | 7.40 [6.10, 8.90] | 9.85 [9.50, 10.83] | 0.004 |
| Total protein (g/L) | 65.90 [47.40, 67.50] | 70.55 [65.80, 72.95] | 75.70 [73.95, 77.85] | <0.001 |
| Albumin (g/L) | 38.60 [31.70, 41.10] | 42.35 [41.85, 43.38] | 45.80 [45.12, 46.65] | <0.001 |
| Globulin (g/L) | 24.80 [19.30, 27.80] | 27.75 [24.70, 31.63] | 29.80 [28.33, 32.73] | 0.012 |
| A/G Rate | 1.39 [1.36, 1.73] | 1.50 [1.32, 1.72] | 1.52 [1.39, 1.62] | 0.993 |
| Urea (mmol/L) | 5.81 [5.15, 6.40] | 6.40 [5.00, 7.83] | 4.90 [4.12, 5.47] | 0.166 |
| Creatinine (μmol/L) | 77.95 [63.25, 90.17] | 71.50 [59.40, 85.00] | 65.50 [60.00, 74.50] | 0.253 |
| Uric acid (μmol/L) | 396.00 [319.00, 445.25] | 322.50 [265.25, 466.50] | 389.50 [318.50, 443.50] | 0.830 |
| Triglycerides (mmol/L) | 1.07 [0.81, 1.65] | 0.56 [0.44, 1.14] | 2.08 [1.52, 2.89] | 0.007 |
| cholesterol (mmol/L) | 3.81 [3.38, 4.02] | 4.48 [3.94, 4.89] | 5.79 [5.41, 6.69] | 0.001 |
| H-cholesterol (mmol/L) | 1.00 [0.95, 1.04] | 1.14 [0.95, 1.56] | 1.06 [1.02, 1.55] | 0.612 |
| L-cholesterol (mmol/L) | 2.18 [1.89, 2.30] | 2.74 [2.51, 2.94] | 3.49 [3.42, 4.31] | 0.006 |
| Apo AI (g/L) | 0.98 [0.86, 1.04] | 1.03 [0.93, 1.15] | 1.08 [1.07, 1.09] | 0.372 |
| Apo B (g/L) | 0.70 [0.61, 0.72] | 0.81 [0.78, 0.92] | 0.76 [0.58, 0.93] | 0.335 |
| eGFR | 77.00 [51.80, 102.20] | 106.60 [75.00, 119.30] | 108.35 [99.12, 125.17] | 0.369 |
| CRP (mg/L) | 7.50 [2.47, 31.00] | 6.70 [3.90, 17.40] | 1.55 [1.52, 1.58] | 0.302 |
| CK (U/L) | 116.00 [82.00, 267.00] | 76.00 [43.00, 111.00] | — | 0.093 |
| CKMB (U/L) | 8.00 [3.00, 17.00] | 6.00 [4.00, 12.00] | — | 0.789 |
| cTNT (ug/L) | 0.03 [0.01, 0.04] | 0.01 [0.01, 0.02] | — | 0.289 |
| D-Dimer (μg/mL) | 4.14 [3.62, 8.04] | 4.22 [1.67, 8.08] | — | 0.929 |
| BNP (pg/mL) | 35.90 [23.60, 56.70] | 65.10 [27.77, 115.70] | — | 0.431 |
| PCT (ng/mL) | 0.70 [0.37, 22.24] | 0.04 [0.04, 0.05] | — | 0.248 |
| Variable Name | OR | 2.5% CI | 97.5% CI | B | Wald | p Value |
|---|---|---|---|---|---|---|
| N9 | 2.467 | 1.256 | 7.245 | 0.903 | 4.454 | 0.035 |
| A4 | 0.872 | 0.734 | 0.974 | −0.137 | 4.001 | 0.045 |
| F3 | 1.000 | 1.000 | 1.000 | 0.000 | 4.764 | 0.029 |
| N11 | 0.015 | 0.000 | 0.324 | −4.231 | 5.761 | 0.016 |
| N30 | 0.003 | 0.000 | 0.613 | −5.986 | 3.943 | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Tang, X.; Wang, Y.; Chen, W.; Xue, Y.; Cao, H.; Zhang, B.; Pan, J.; Zhou, Q.; Wang, D.; et al. Serum Oxylipin Profiles Identify Potential Biomarkers in Patients with Acute Aortic Dissection. Metabolites 2022, 12, 587. https://doi.org/10.3390/metabo12070587
Jiang Y, Tang X, Wang Y, Chen W, Xue Y, Cao H, Zhang B, Pan J, Zhou Q, Wang D, et al. Serum Oxylipin Profiles Identify Potential Biomarkers in Patients with Acute Aortic Dissection. Metabolites. 2022; 12(7):587. https://doi.org/10.3390/metabo12070587
Chicago/Turabian StyleJiang, Yi, Xinlong Tang, Yali Wang, Wei Chen, Yunxing Xue, Hailong Cao, Bomin Zhang, Jun Pan, Qing Zhou, Dongjin Wang, and et al. 2022. "Serum Oxylipin Profiles Identify Potential Biomarkers in Patients with Acute Aortic Dissection" Metabolites 12, no. 7: 587. https://doi.org/10.3390/metabo12070587
APA StyleJiang, Y., Tang, X., Wang, Y., Chen, W., Xue, Y., Cao, H., Zhang, B., Pan, J., Zhou, Q., Wang, D., & Fan, F. (2022). Serum Oxylipin Profiles Identify Potential Biomarkers in Patients with Acute Aortic Dissection. Metabolites, 12(7), 587. https://doi.org/10.3390/metabo12070587