Estradiol Valerate Affects Hematological and Hemorheological Parameters in Rats
Abstract
1. Introduction
2. Results
2.1. Bodyweight and General Observations
2.2. Vaginal Smears
2.3. Hormone Concentrations
2.4. Fasting Glucose and Oral Glucose Tolerance Test Results
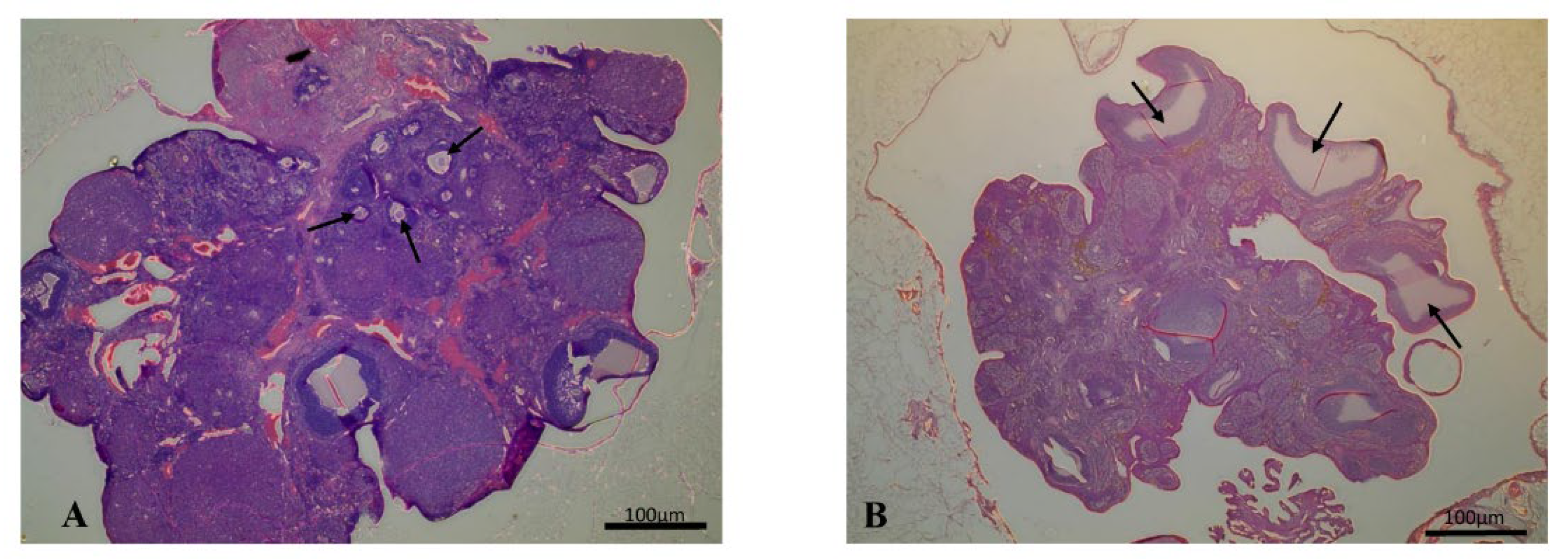
2.5. Histology
2.6. Hematological and Hemorheological Parameters
3. Discussion
4. Materials and Methods
4.1. Bodyweight Measurements and General Observations
4.2. Determining the Estrous Cycle
4.3. Blood Sampling Method
4.4. Laboratory Measurements
4.5. Histological Examination
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalczyk, K.; Franik, G.; Kowalczyk, D.; Pluta, D.; Blukacz, Ł.; Madej, P. Thyroid disorders in Polycystic Ovary Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 346–360. [Google Scholar] [PubMed]
- Mansour, A.; Hosseini, S.; Larijani, B.; Mohajeri-Tehrani, M.R. Nutrients as novel therapeutic approaches for metabolic disturbances in polycystic ovary syndrome. EXCLI J. 2016, 15, 551–564. [Google Scholar] [PubMed]
- Yang, X.; Wu, R.; Qi, D.; Fu, L.; Song, T.; Wang, Y.; Bian, Y.; Shi, Y. Profile of bile acid metabolomics in the follicular fluid of PCOS patients. Metabolites 2021, 11, 845. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc. Med. 2020, 30, 399–404. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 Consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Somogyi, V.; Peto, K.; Deak, A.; Tanczos, B.; Nemeth, N. Effects of aging and gender on micro-rheology of blood in 3 to 18 months old male and female Wistar (Crl:WI) rats. Biorheology 2018, 54, 127–140. [Google Scholar] [CrossRef]
- Mester, A.; Magyar, Z.; Molnar, A.; Somogyi, V.; Tanczos, B.; Peto, K.; Nemeth, N. Age- and gender-related hemorheological alterations in intestinal ischemia-reperfusion in the rat. J. Surg. Res. 2018, 225, 68–75. [Google Scholar] [CrossRef]
- Nemeth, N.; Kiss, F.; Magyar, Z.; Miszti-Blasius, K.; Furka, I. Following-up hemorheological consequences of gonadectomy in male and female rats. Clin. Hemorheol. Microcirc. 2012, 50, 231–243. [Google Scholar] [CrossRef]
- Akhigbe, R.E.; Azeez, M.O.; Ige, S.F.; Oyeyipo, I.P.; Ajao, F.O.; Soladoye, A.O. Hemorheological effects of long-term administration of combined oral contraceptive in rats. Int. J. Pharmacol. 2008, 4, 403–406. [Google Scholar] [CrossRef]
- Baskurt, O.K. Mechanisms of blood rheology alterations. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman., H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 170–190. [Google Scholar]
- Baskurt, O.K.; Neu, B.; Meiselman, H.J. Red Blood Cell Aggregation; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Simmonds, M.J.; Milne, N.; Ong, K.; Brotherton, E.; McNamee, A.P.; Horobin, J.; Sabapathy, S. Physical properties of blood are altered in young and lean women with polycystic ovary syndrome. PLoS ONE 2016, 11, e0167290. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.; Kiss, F.; Furka, I.; Miko, I. Gender Differences of blood rheological parameters in laboratory animals. Clin. Hemorheol. Microcirc. 2010, 45, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Paixão, L.; Ramos, R.B.; Lavarda, A.; Morsh, D.M.; Spritzer, P.M. Animal models of hyperandrogenism and ovarian morphology changes as features of polycystic ovary syndrome: A systematic review. Reprod. Biol. Endocrinol. 2017, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Jia, L.; Shen, X. Manifestation of hyperandrogenism in the continuous light exposure-induced PCOS rat model. BioMed Res. Int. 2015, 2015, 943694. [Google Scholar] [CrossRef]
- Tamura, N.; Kurabayashi, T.; Nagata, H.; Matsushita, H.; Yahata, T.; Tanaka, K. Effects of testosterone on cancellous bone, marrow adipocytes, and ovarian phenotype in a young female rat model of polycystic ovary syndrome. Fertil. Steril. 2005, 84, 1277–1284. [Google Scholar] [CrossRef]
- Wang, D.; Wang, W.; Liang, Q.; He, X.; Xia, Y.; Shen, S.; Wang, H.; Gao, Q.; Wang, Y. DHEA-induced ovarian hyperfibrosis is mediated by TGF-β signaling pathway. J. Ovarian Res. 2018, 11, 6. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, M.; Zhang, Y.; Jin, H.; Zhang, W.; Yang, J.; Yan, L.; Li, R.; Zhao, Y.; Qiao, J. High-fat diets exaggerate endocrine and metabolic phenotypes in a rat model of DHEA-induced PCOS. Reproduction 2016, 151, 431–441. [Google Scholar] [CrossRef]
- Tessaro, I.; Modina, S.C.; Franciosi, F.; Sivelli, G.; Terzaghi, L.; Lodde, V.; Luciano, A.M. Effect of oral administration of low-dose follicle stimulating hormone on hyperandrogenized mice as a model of polycystic ovary syndrome. J. Ovarian Res. 2015, 8, 64. [Google Scholar] [CrossRef]
- Sara, L.; Antal, P.; Masszi, G.; Buday, A.; Horvath, E.M.; Hamar, P.; Monos, E.; Nadasy, G.L.; Varbiro, S. Arteriolar insulin resistance in a rat model of polycystic ovary syndrome. Fertil. Steril. 2012, 97, 462–468. [Google Scholar] [CrossRef]
- Masszi, G.; Novak, A.; Tarszabo, R.; Horvath, E.M.; Buday, A.; Ruisanchez, E.; Tokes, A.-M.; Sara, L.; Benko, R.; Nadasy, G.L.; et al. Effects of vitamin D3 derivative--calcitriol on pharmacological reactivity of aortic rings in a rodent PCOS model. Pharmacol. Rep. 2013, 65, 476–483. [Google Scholar] [CrossRef]
- Dăneasă, A.; Cucolaş, C.; Lenghel, L.M.; Olteanu, D.; Orăsan, R.; Filip, G.A. Letrozole vs. estradiol valerate induced PCOS in rats: Glycemic, oxidative and inflammatory status assessment. Reproduction 2016, 151, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kafali, H.; Iriadam, M.; Ozardalı, I.; Demir, N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch. Med. Res. 2004, 35, 103–108. [Google Scholar] [CrossRef]
- Mirabolghasemi, G.; Kamyab, Z. Changes of the uterine tissue in rats with polycystic ovary syndrome induced by estradiol valerate. Int. J. Fertil. Steril. 2017, 11, 47–55. [Google Scholar]
- Osuka, S.; Nakanishi, N.; Murase, T.; Nakamura, T.; Goto, M.; Iwase, A.; Kikkawa, F. Animal models of polycystic ovary syndrome: A review of hormone-induced rodent models focused on hypothalamus-pituitary-ovary axis and neuropeptides. Reprod. Med. Biol. 2019, 18, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Vervita, V.; Saltamavros, A.D.; Adonakis, G.; Tsapanos, V.; Decavalas, G.; Georgopoulos, N.A. Obesity and insulin resistance increase plasma viscosity in young women with polycystic ovary syndrome. Gynecol. Endocrinol. 2009, 25, 640–646. [Google Scholar] [CrossRef]
- Haydardedeoglu, F.; Haydardedeoglu, B.; Kozanoglu, I.; Tutuncu, N.; Anaforoglu, I.; Ertorer, M. High plasma viscosity may be a predictor of cardiovascular risk in women with polycystic ovary syndrome. IVF Lite 2014, 1, 100. [Google Scholar] [CrossRef]
- Laganà, A.S.; Rossetti, P.; Buscema, M.; La Vignera, S.; Condorelli, R.S.; Gullo, G.; Granese, R.; Triolo, O. Metabolism and Ovarian Function in PCOS Women: A Therapeutic Approach with Inositols. Int. J. Endocrinol. 2016, 2016, 6306410. [Google Scholar] [CrossRef]
- Paul, C.; Laganà, A.S.; Maniglio, P.; Triolo, P.; Brady, D.M. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: State-of-the-art and future perspectives. Gynecol. Endocrinol. 2016, 32, 431–438. [Google Scholar] [CrossRef]
- Noroozzadeh, M.; Behboudi-Gandevani, S.; Zadeh-Vakili, A.; Ramezani Tehrani, F. Hormone-induced rat model of polycystic ovary syndrome: A systematic review. Life Sci. 2017, 191, 259–272. [Google Scholar] [CrossRef]
- Shi, D.; Vine, D.F. Animal models of polycystic ovary syndrome: A focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil. Steril. 2012, 98, 185–193.e2. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, S.; Bardei, L.K.; Hojati, V.; Ghorbani, A.; Nabiuni, M. Anti-inflammatory effects of curcumin on insulin resistance index, levels of interleukin-6, C-reactive protein, and liver histology in polycystic ovary syndrome-induced rats. Cell J. 2017, 19, 425–433. [Google Scholar]
- Komal, F.; Khan, M.K.; Imran, M.; Ahmad, M.H.; Anwar, H.; Ashfaq, U.A.; Ahmad, N.; Masroor, A.; Ahmad, R.S.; Nadeem, M.; et al. Impact of different omega-3 fatty acid sources on lipid, hormonal, blood glucose, weight gain and histopathological damages profile in PCOS rat model. J. Transl. Med. 2020, 18, 349. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal cytology of the laboratory rat and mouse: Review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef]
- Lara, H.E.; Ferruz, J.L.; Luza, S.; Bustamante, D.A.; Borges, Y.; Ojeda, S.R. Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology 1993, 133, 2690–2695. [Google Scholar] [CrossRef]
- van Zutphen, L.F.M.; Baumans, V.; Beynen, A.C. Principles of Laboratory Animal Science: A Contribution to the Humane Use and Care of Animals and to the Quality of Experimental Results; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 2001. [Google Scholar]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Anzalone, C.R.; Hong, L.-S.; Lu, J.K.H.; LaPolt, P.S. Influences of age and ovarian follicular reserve on estrous cycle patterns, ovulation, and hormone secretion in the Long-Evans rat. Biol. Reprod. 2001, 64, 1056–1062. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Ploj, K.; Larsson, B.-M.; Holmäng, A. Rats with steroid-induced polycystic ovaries develop hypertension and increased sympathetic nervous system activity. Reprod. Biol. Endocrinol. 2005, 3, 44. [Google Scholar] [CrossRef]
- Lara, H.E.; Dissen, G.A.; Leyton, V.; Paredes, A.; Fuenzalida, H.; Fiedler, J.L.; Ojeda, S.R. An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology 2000, 141, 1059–1072. [Google Scholar] [CrossRef]
- Brun, J.F.; Varlet-Marie, E.; Myzia, J.; Raynaud de Mauverger, E.; Pretorius, E. Metabolic influences modulating erythrocyte deformability and eryptosis. Metabolites 2021, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Bogar, L.; Juricskay, I.; Kesmarky, G.; Feher, G.; Kenyeres, P.; Toth, K. Gender differences in hemorheological parameters of coronary artery disease patients. Clin. Hemorheol. Microcirc. 2006, 35, 99–103. [Google Scholar] [PubMed]
- Kameneva, M.V.; Watach, M.J.; Borovetz, H.S. Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin. Hemorheol. Microcirc. 1999, 21, 357–363. [Google Scholar] [PubMed]
- Guillet, R.; Driss, F.; Perrotin, P.; Pautou, C.; Nalpas, B.; Boynard, M. Gender, menstrual cycle, oral contraceptives and red blood cell deformability in healthy adult subjects. Clin. Hemorheol. Microcirc. 1998, 19, 83–88. [Google Scholar] [PubMed]
- Pehlivanoglu, B.; Dikmenoglu, N.; Balkanci, D.Z. Effect of stress on erythrocyte deformability, influence of gender and menstrual cycle. Clin. Hemorheol. Microcirc. 2007, 37, 301–308. [Google Scholar] [PubMed]
- Brun, J. Hormones, metabolism and body composition as major determinants of blood rheology: Potential pathophysiological meaning. Clin. Hemorheol. Microcirc. 2002, 26, 63–79. [Google Scholar]
- Valensi, P.; Gaudey, F.; Parries, J.; Attali, J.R. Glucagon and noradrenaline reduce erythrocyte deformability. Metabolism 1992, 42, 1169–1172. [Google Scholar] [CrossRef]
- Grady, D.; Wenger, N.K.; Herrington, D.; Khan, S.; Furberg, C.; Hunninghake, D.; Vittinghoff, E.; Hulley, S. Postmenopausal hormone therapy increases risk for venous thromboembolic disease. The heart and estrogen/progestin replacement study. Ann. Intern. Med. 2000, 132, 689–696. [Google Scholar] [CrossRef]
- Lewis, D.A.; Bracamonte, M.P.; Rud, K.S.; Miller, V.M. Selected contribution: Effects of sex and ovariectomy on responses to platelets in porcine femoral veins. J. Appl. Physiol. 2001, 91, 2823–2830. [Google Scholar] [CrossRef][Green Version]
- Spengler, M.I.; Goni, G.M.; Mengarelli, G.; Bravo Luna, M.; Bocanera, R.; Tozzini, R.; Rasia, M.L. Effect of hormone replacement therapy upon haemorheological variables. Clin. Hemorheol. Microcirc. 2003, 28, 13–19. [Google Scholar]
- Rajan, R.K.; M, S.S.; Balaji, B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm. Biol. 2017, 55, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Jing, T.; Meng, Q.; Liu, C.; Hu, S.; Ma, Y.; Liu, Y.; Lu, J.; Cheng, Y.; Wang, D.; et al. Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-Dawley Rats. BioMed Res. Int. 2014, 2014, 160980. [Google Scholar] [CrossRef] [PubMed]
- Hardeman, M.; Goedhart, P.; Shin, S. Methods in hemorheology. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 242–266. [Google Scholar]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Parameterization of red blood cell elongation index--shear stress curves obtained by ektacytometry. Scand. J. Clin. Lab. Investig. 2009, 69, 777–788. [Google Scholar] [CrossRef] [PubMed]

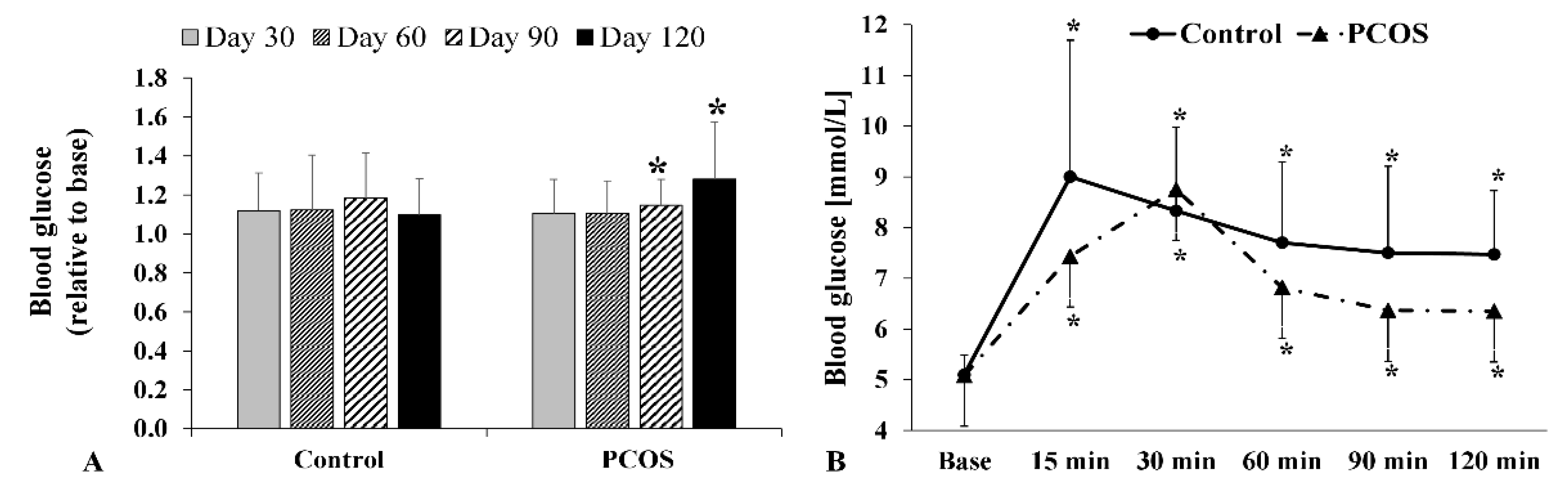

| Phase | Base | Day 30 | Day 60 | Day 90 | Day 120 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | PCOS | Control | PCOS | Control | PCOS | Control | PCOS | Control | PCOS | |
| Pro-estrous | - | 33.3% | 22.22% | 33.33% | 44.44% | 55.55% | 62.5% | 77.78% | 12.5% | 77.78% |
| Estrous | 66.6% | 33.3% | 55.56% | - | 22.22% | 11.11% | 25% | - | 62.5% | - |
| Met-estrous | 33.3% | - | 22.22% | 44.44% | 11.11% | 33.33% | 12.5% | 22.22% | 25% | 22.22% |
| Di-estrous | - | 33.3% | - | 22.22% | 22.22% | - | - | - | - | - |
| Hormone | Group | Day 60 | Day 120 |
|---|---|---|---|
| LH | Control | 0.996 ± 0.021 | 0.998 ± 0.029 |
| PCOS | 1.00 ± 0.012 | 1.008 ± 0.022 + | |
| FSH | Control | 0.986 ± 0.039 | 0.988 ± 0.056 |
| PCOS | 0.985 ± 0.037 | 1.018 ± 0.062 | |
| Testosterone | Control | 1.376 ± 0.292 | 1.533 ± 0.677 |
| PCOS | 1.206 ± 1.032 | 1.472 ± 1.000 |
| Structure or Variable | Control | PCOS |
|---|---|---|
| Follicle | 6.71 ± 6.01 | 1.5 ± 1.0 |
| Corpus Luteum | 2.83 ± 2.13 | 1.63 ± 0.9 |
| Cyst | 0 | 5 ± 4.42 * |
| Cyst diameter (μm) | - | 690 ± 92.14 * |
| Variable | Group | Base | Day 30 | Day 60 | Day 90 | Day 120 |
|---|---|---|---|---|---|---|
| WBC [109/L] | Control | 4.37 ± 0.83 | 5.27 ± 0.87 | 5.11 ± 1.61 | 5.41 ± 1.73 | 4.61 ± 1.1 |
| PCOS | 5.25 ± 1.05 # | 5.1.4 | 4.8 ± 1.48 | 3.97 ± 0.97 *,# | 3.96 ± 1.3 * | |
| RBC [1012/L] | Control | 7.12 ± 0.71 | 6.86 ± 0.42 | 7.38 ± 0.49 | 7.42 ± 0.38 * | 7.48 ± 0.32 * |
| PCOS | 7.33 ± 0.34 | 6.68 ± 0.3 * | 6.41 ± 0.55 *,# | 6.8 ± 0.39 *,# | 6.85 ± 0.54 *,# | |
| Hct [%] | Control | 41.56 ± 4.2 | 40.24 ± 2.6 | 43.01 ± 2.48 | 43.11 ± 1.96 | 44 ± 2 * |
| PCOS | 42.89 ± 1.82 | 38.37 ± 1.74 *,# | 37.34 ± 3.89 *,# | 40.12 ± 2.17 *,# | 40.17 ± 2.56 *,# | |
| Hbg [g/dL] | Control | 13.8 ± 1.4 | 13.37 ± 0.94 | 14.34 ± 0.86 | 14.29 ± 0.68 * | 14.56 ± 0.73 * |
| PCOS | 14 ± 0.58 | 12.68 ± 0.61 # | 12.39 ± 1.31 # | 13.46 ± 0.81 *,# | 13.47 ± 0.83 *,# | |
| MCV [fL] | Control | 58.3 ± 0.74 | 58.6 ± 1.05 | 58.33 ± 1.22 | 58.14 ± 1.12 | 58.85 ± 1.3 |
| PCOS | 58.5 ± 1.17 | 57.43 ± 1.7 *,# | 58.1 ± 2.00 | 58.94 ± 0.91 # | 58.75 ± 1.71 | |
| MCH [pg] | Control | 19.4 ± 0.52 | 19.5 ± 0.61 | 19.45 ± 0.65 | 19.28 ± 0.56 | 19.48 ± 0.57 |
| PCOS | 19.11 ± 0.45 | 18.93 ± 0.69 # | 19.27 ± 0.63 | 19.8 ± 0.67 *,# | 19.71 ± 0.66 * | |
| MCHC [g/L] | Control | 33.2 ± 0.82 | 33.2 ± 0.68 | 33.34 ± 0.75 | 33.14 ± 0.44 | 33.09 ± 0.39 |
| PCOS | 32.66 ± 0.41 # | 32.97 ± 0.48 | 33.18 ± 0.44 * | 33.54 ± 0.96 * | 33.56 ± 0.68 * | |
| Plt [109/L] | Control | 608.6 ± 120.7 | 597.33 ± 213.45 | 659 ± 118.9 | 559.9 ± 195.3 | 600.6 ± 185.4 |
| PCOS | 534.29 ± 87.69 # | 665.2 ± 204.4 * | 595.94 ± 136.97 | 578.67 ± 145.7 | 645.53 ± 92.57 * |
| Variable | Group | Base | Day 30 | Day 60 | Day 90 | Day 120 |
|---|---|---|---|---|---|---|
| EI at 3 Pa | Control | 0.38 ± 0.01 | 0.38 ± 0.02 | 0.4 ± 0.01 * | 0.38 ± 0.01 | 0.39 ± 0.01 * |
| PCOS | 0.38 ± 0.02 | 0.38 ± 0.01 | 0.39 ± 0.01 * | 0.39 ± 0.01 *,# | 0.39 ± 0.01 | |
| EImax | Control | 0.55 ± 0.02 | 0.56 ± 0.02 | 0.57 ± 0.02 * | 0.56 ± 0.03 | 0.57 ± 0.03 * |
| PCOS | 0.54 ± 0.02 | 0.57 ± 0.02 * | 0.57 ± 0.01 * | 0.56 ± 0.03 * | 0.56 ± 0.24 * | |
| SS1/2 [Pa] | Control | 1.78 ± 0.22 | 1.79 ± 0.3 | 1.73 ± 0.22 | 1.73 ± 0.19 | 1.14 ± 0.33 * |
| PCOS | 1.62 ± 0.2 # | 1.75 ± 0.2 * | 1.78 ± 0.18 * | 1.16 ± 0.34 *,# | 1.24 ± 0.24 * | |
| EImax/SS1/2 [Pa−1] | Control | 0.31 ± 0.04 | 0.33 ± 0.08 | 0.33 ± 0.05 | 0.32 ± 0.03 | 0.49 ± 0.11 * |
| PCOS | 0.34 ± 0.04 | 0.33 ± 0.04 | 0.33 ± 0.04 | 0.47 ± 0.1 *,# | 0.46 ± 0.09 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barath, B.; Varga, A.; Matrai, A.A.; Deak-Pocsai, K.; Nemeth, N.; Deak, A. Estradiol Valerate Affects Hematological and Hemorheological Parameters in Rats. Metabolites 2022, 12, 602. https://doi.org/10.3390/metabo12070602
Barath B, Varga A, Matrai AA, Deak-Pocsai K, Nemeth N, Deak A. Estradiol Valerate Affects Hematological and Hemorheological Parameters in Rats. Metabolites. 2022; 12(7):602. https://doi.org/10.3390/metabo12070602
Chicago/Turabian StyleBarath, Barbara, Adam Varga, Adam Attila Matrai, Krisztina Deak-Pocsai, Norbert Nemeth, and Adam Deak. 2022. "Estradiol Valerate Affects Hematological and Hemorheological Parameters in Rats" Metabolites 12, no. 7: 602. https://doi.org/10.3390/metabo12070602
APA StyleBarath, B., Varga, A., Matrai, A. A., Deak-Pocsai, K., Nemeth, N., & Deak, A. (2022). Estradiol Valerate Affects Hematological and Hemorheological Parameters in Rats. Metabolites, 12(7), 602. https://doi.org/10.3390/metabo12070602







