Untargeted Metabolomics Reveal Parenteral Nutrition-Associated Alterations in Pediatric Patients with Short Bowel Syndrome
Abstract
1. Introduction
2. Results
2.1. Cohorts Studied
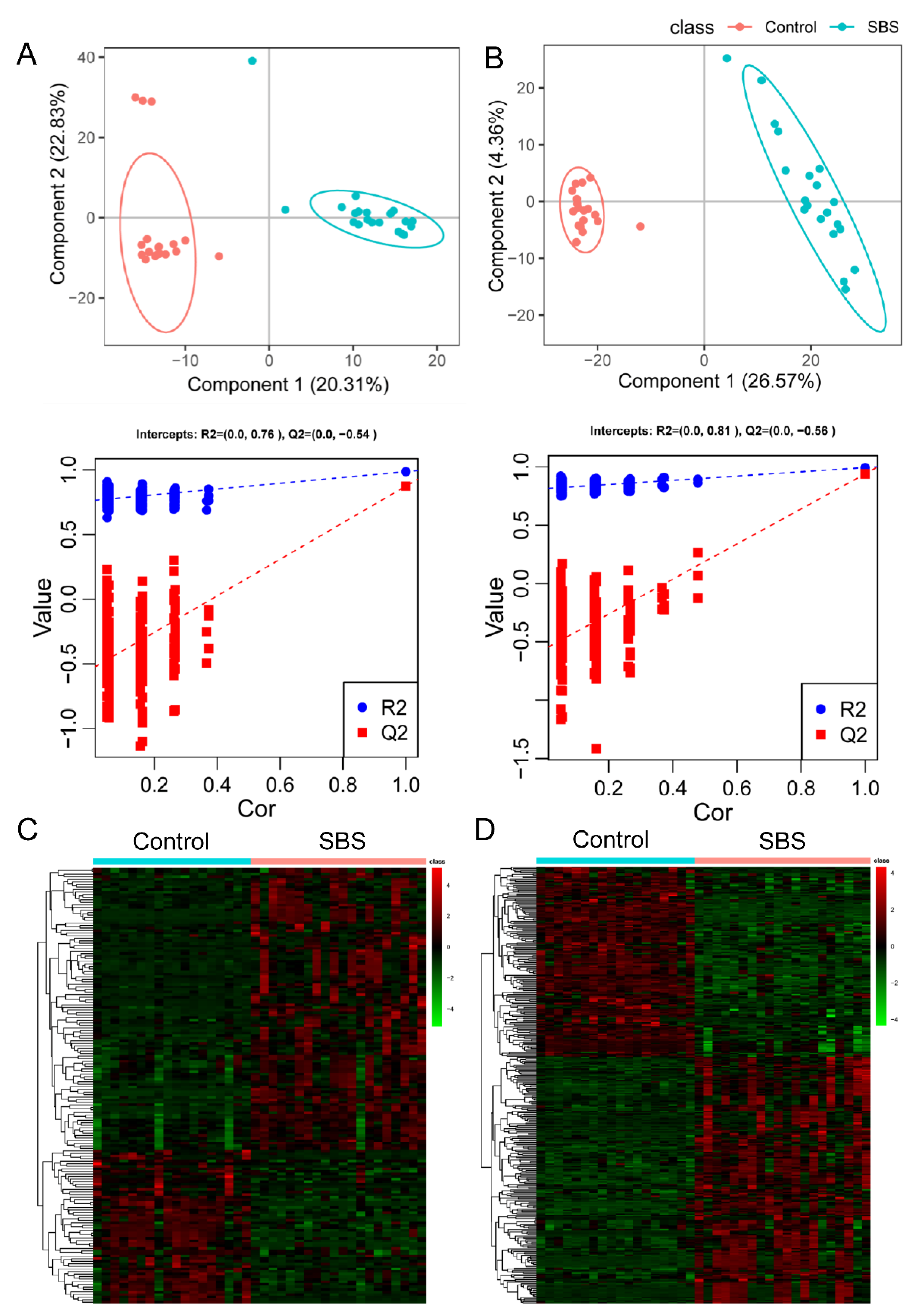
2.2. Metabolomic Profile Is Altered in Patients with SBS
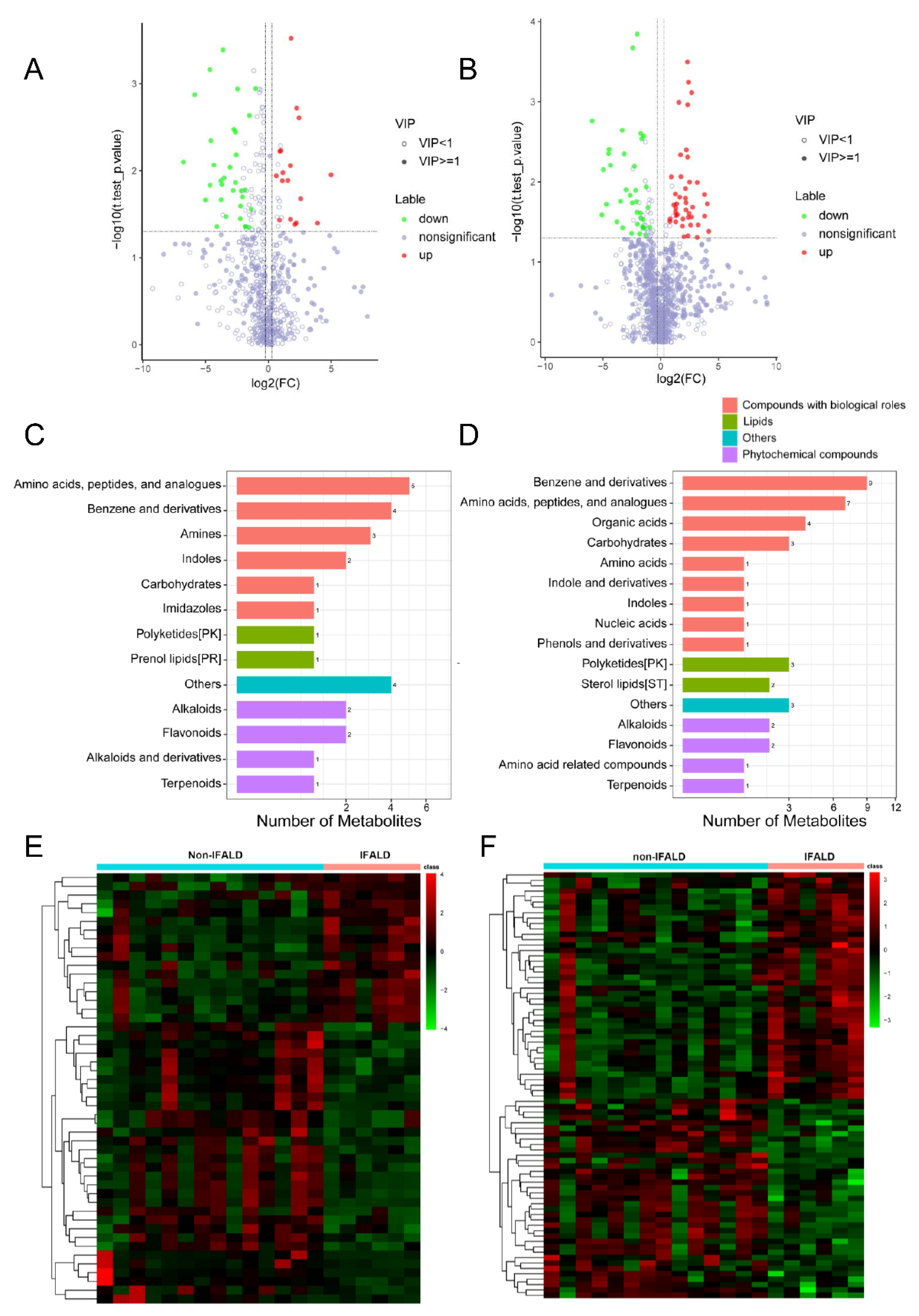
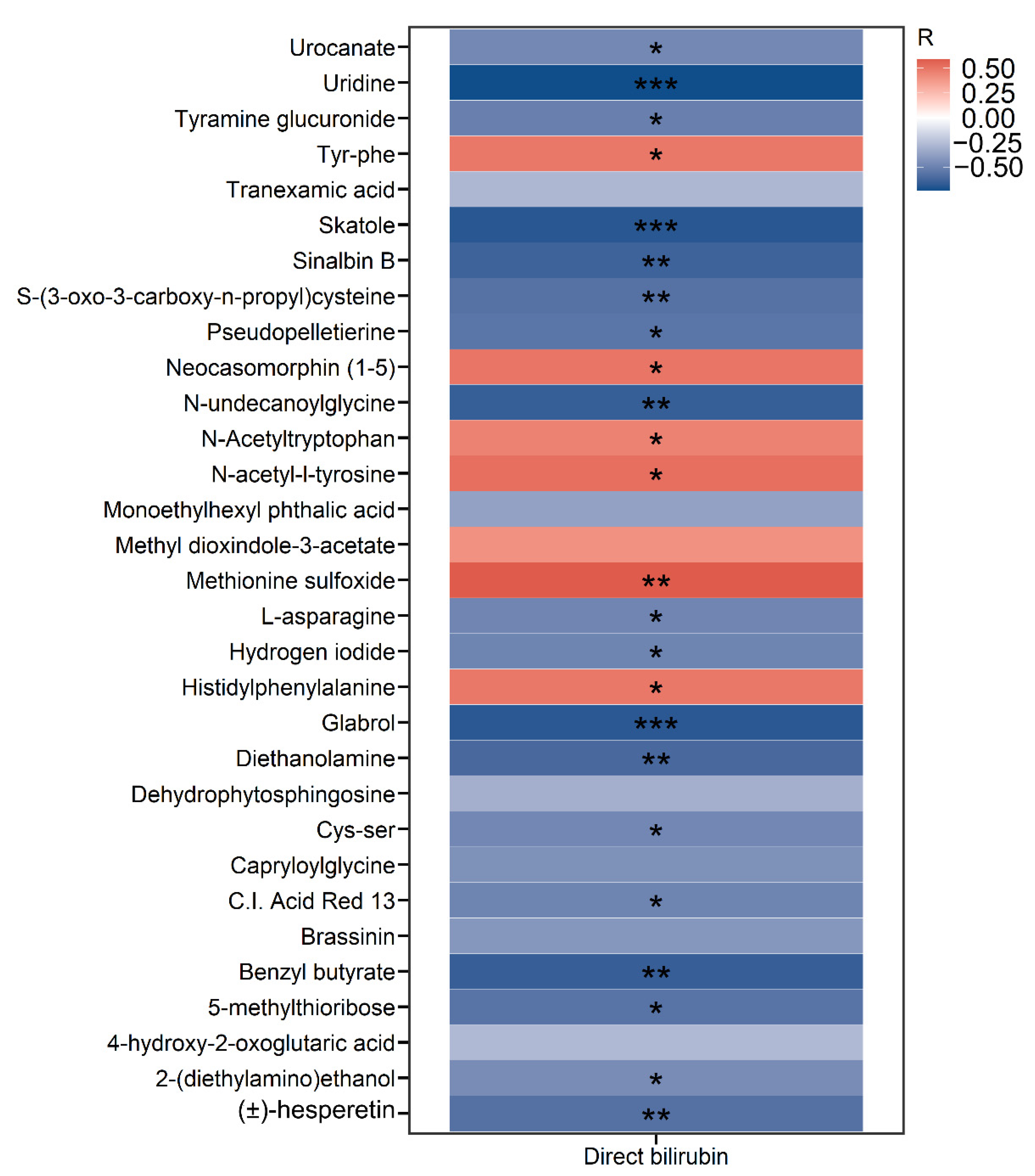
2.3. Development of IFALD in SBS Is Associated with Alterations in the Metabolomic Profiling
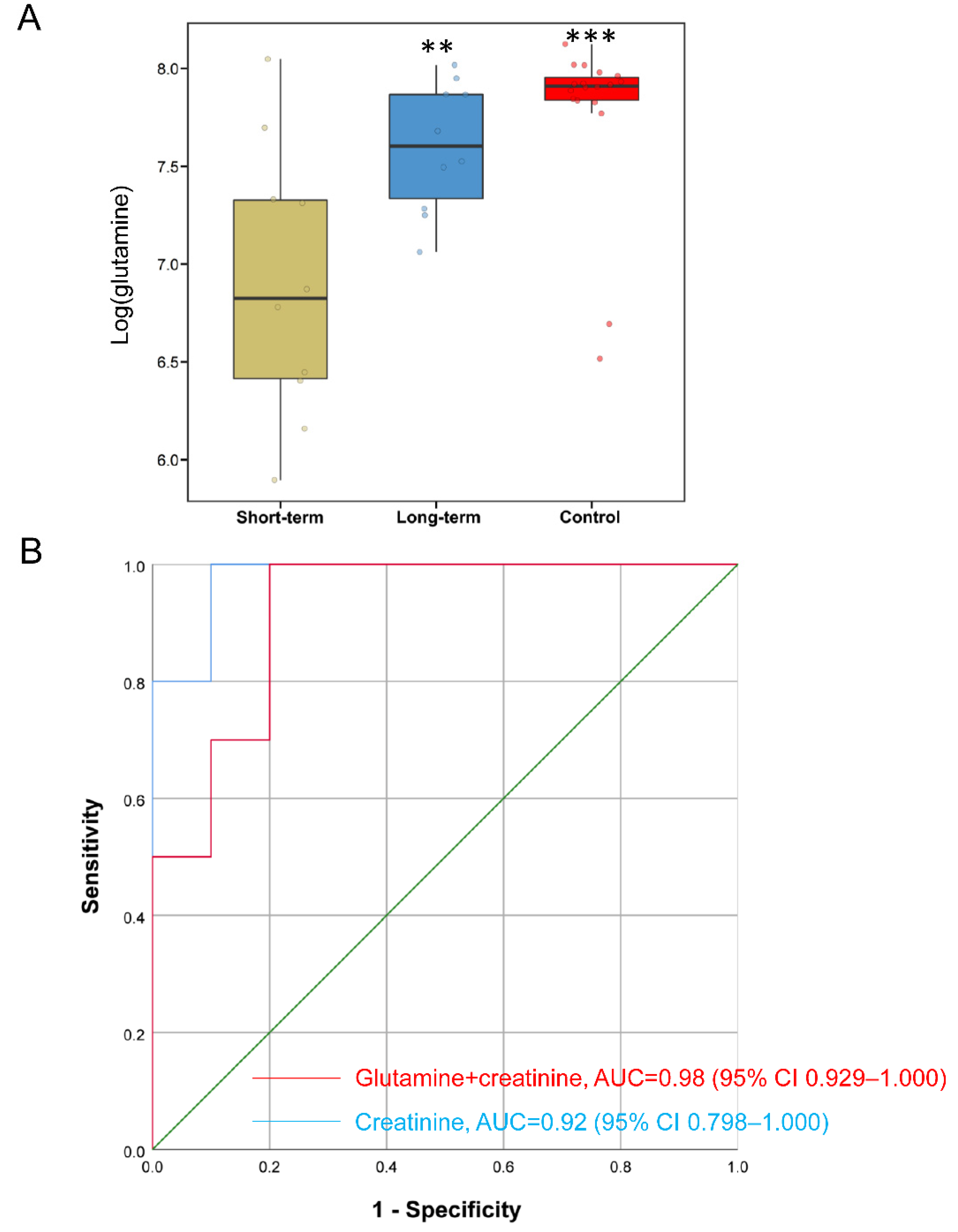
2.4. Metabolites Improve Predictive Accuracy of Long-Term PN in SBS
3. Discussion
4. Materials and Methods
4.1. Study Cohorts
4.2. Untargeted Metabolomics and Data Processing
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Khalaf, R.T.; Sokol, R.J. New Insights Into Intestinal Failure-Associated Liver Disease in Children. Hepatology 2020, 71, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Peláez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Bielawska, B.; Allard, J.P. Parenteral Nutrition and Intestinal Failure. Nutrients 2017, 9, 466. [Google Scholar] [CrossRef]
- Messing, B.; Crenn, P.; Beau, P.; Boutron-Ruault, M.C.; Rambaud, J.C.; Matuchansky, C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 1999, 117, 1043–1050. [Google Scholar] [CrossRef]
- Masoodi, M.; Gastaldelli, A.; Hyötyläinen, T.; Arretxe, E.; Alonso, C.; Gaggini, M.; Brosnan, J.; Anstee, Q.M.; Millet, O.; Ortiz, P.; et al. Metabolomics and lipidomics in NAFLD: Biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 835–856. [Google Scholar] [CrossRef]
- Kachroo, P.; Stewart, I.D.; Kelly, R.S.; Stav, M.; Mendez, K.; Dahlin, A.; Soeteman, D.I.; Chu, S.H.; Huang, M.; Cote, M.; et al. Metabolomic profiling reveals extensive adrenal suppression due to inhaled corticosteroid therapy in asthma. Nat. Med. 2022, 28, 814–822. [Google Scholar] [CrossRef]
- Moutinho, T.J.; Powers, D.A.; Hanson, G.F.; Levy, S.; Baveja, R.; Hefner, I.; Mohamed, M.; Abdelghani, A.; Baker, R.L.; Papin, J.A.; et al. Fecal sphingolipids predict parenteral nutrition-associated cholestasis in the neonatal intensive care unit. JPEN J. Parenter. Enter. Nutr. 2022. [Google Scholar] [CrossRef]
- Pereira-Fantini, P.M.; Byars, S.G.; Pitt, J.; Lapthorne, S.; Fouhy, F.; Cotter, P.D.; Bines, J.E. Unravelling the metabolic impact of SBS-associated microbial dysbiosis: Insights from the piglet short bowel syndrome model. Sci. Rep. 2017, 7, 43326. [Google Scholar] [CrossRef]
- Budinska, E.; Gojda, J.; Heczkova, M.; Bratova, M.; Dankova, H.; Wohl, P.; Bastova, H.; Lanska, V.; Kostovcik, M.; Dastych, M.; et al. Microbiome and Metabolome Profiles Associated With Different Types of Short Bowel Syndrome: Implications for Treatment. JPEN J. Parenter. Enter. Nutr. 2020, 44, 105–118. [Google Scholar] [CrossRef]
- Pichler, J.; Horn, V.; Macdonald, S.; Hill, S. Intestinal failure-associated liver disease in hospitalised children. Arch. Dis. Child. 2012, 97, 211–214. [Google Scholar] [CrossRef]
- Abi Nader, E.; Lambe, C.; Talbotec, C.; Pigneur, B.; Lacaille, F.; Garnier-Lengliné, H.; Petit, L.M.; Poisson, C.; Rocha, A.; Corriol, O.; et al. Outcome of home parenteral nutrition in 251 children over a 14-y period: Report of a single center. Am. J. Clin. Nutr. 2016, 103, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L.; Moukarzel, A.A.; Bhuta, S.; Belle, M.; Ament, M.E.; Eckhert, C.D.; Hollander, D.; Gornbein, J.; Kopple, J.D.; Vijayaroghavan, S.R. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J. Parenter. Enter. Nutr. 1995, 19, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.T.; Cao, Y.; Zhou, K.J.; Lu, L.N.; Cai, W. Altered systemic bile acid homeostasis contributes to liver disease in pediatric patients with intestinal failure. Sci. Rep. 2016, 6, 39264. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- van Goudoever, J.B.; Carnielli, V.; Darmaun, D.; Sainz de Pipaon, M. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Amino acids. Clin. Nutr. 2018, 37, 2315–2323. [Google Scholar] [CrossRef]
- Wang, B.; Wu, G.; Zhou, Z.; Dai, Z.; Sun, Y.; Ji, Y.; Li, W.; Wang, W.; Liu, C.; Han, F.; et al. Glutamine and intestinal barrier function. Amino Acids 2015, 47, 2143–2154. [Google Scholar] [CrossRef]
- Hankard, R.; Goulet, O.; Ricour, C.; Rongier, M.; Colomb, V.; Darmaun, D. Glutamine metabolism in children with short-bowel syndrome: A stable isotope study. Pediatr. Res. 1994, 36, 202–206. [Google Scholar] [CrossRef][Green Version]
- Tian, J.; Hao, L.; Chandra, P.; Jones, D.P.; Willams, I.R.; Gewirtz, A.T.; Ziegler, T.R. Dietary glutamine and oral antibiotics each improve indexes of gut barrier function in rat short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G348–G355. [Google Scholar] [CrossRef]
- Nose, K.; Yang, H.; Sun, X.; Nose, S.; Koga, H.; Feng, Y.; Miyasaka, E.; Teitelbaum, D.H. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: A potential mechanism for the preservation of epithelial barrier function. J. Interferon Cytokine Res. 2010, 30, 67–80. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gabe, S.M.; Seidner, D.L.; Lee, H.M.; Olivier, C. Citrulline correlations in short bowel syndrome-intestinal failure by patient stratification: Analysis of 24 weeks of teduglutide treatment from a randomized controlled study. Clin. Nutr. 2020, 39, 2479–2486. [Google Scholar] [CrossRef]
- Guglielmi, F.W.; Regano, N.; Mazzuoli, S.; Fregnan, S.; Leogrande, G.; Guglielmi, A.; Merli, M.; Pironi, L.; Penco, J.M.; Francavilla, A. Cholestasis induced by total parenteral nutrition. Clin. Liver Dis. 2008, 12, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Mutanen, A.; Lohi, J.; Heikkilä, P.; Jalanko, H.; Pakarinen, M.P. Loss of ileum decreases serum fibroblast growth factor 19 in relation to liver inflammation and fibrosis in pediatric onset intestinal failure. J. Hepatol. 2015, 62, 1391–1397. [Google Scholar] [CrossRef]
- Zheng, W.V.; Li, Y.; Cheng, X.; Xu, Y.; Zhou, T.; Li, D.; Xiong, Y.; Wang, S.; Chen, Z. Uridine alleviates carbon tetrachloride-induced liver fibrosis by regulating the activity of liver-related cells. J. Cell. Mol. Med. 2022, 26, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Urasaki, Y.; Pizzorno, G. Uridine prevents tamoxifen-induced liver lipid droplet accumulation. BMC Pharmacol. Toxicol. 2014, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Wesoly, R.; Weiler, U. Nutritional Influences on Skatole Formation and Skatole Metabolism in the Pig. Animals 2012, 2, 221–242. [Google Scholar] [CrossRef]
- Vhile, S.G.; Kjos, N.P.; Sørum, H.; Overland, M. Feeding Jerusalem artichoke reduced skatole level and changed intestinal microbiota in the gut of entire male pigs. Anim. Int. J. Anim. Biosci. 2012, 6, 807–814. [Google Scholar] [CrossRef]
- Deng, L.; Zhen, Q.; Gao, J.; Jin, M.; Ding, M.; Xu, B. Simultaneous determination of plasma indole and skatole in pregnant women with hepatitis B virus infection by high performance liquid chromatography. Chin. J. Chromatogr. 2017, 35, 735–740. [Google Scholar] [CrossRef]
- Fullerton, B.S.; Hong, C.R.; Jaksic, T. Long-term outcomes of pediatric intestinal failure. Semin. Pediatr. Surg. 2017, 26, 328–335. [Google Scholar] [CrossRef]
- Fallon, E.M.; Mitchell, P.D.; Nehra, D.; Potemkin, A.K.; O’Loughlin, A.A.; Gura, K.M.; Puder, M. Neonates with short bowel syndrome: An optimistic future for parenteral nutrition independence. JAMA Surg. 2014, 149, 663–670. [Google Scholar] [CrossRef]
- Peters, F.B.; Bone, J.N.; Van Oerle, R.; Albersheim, S.; Casey, L.; Piper, H. The Importance of the ileocecal valve and colon in achieving intestinal independence in infants with short bowel syndrome. J. Pediatr. Surg. 2022, 57, 117–121. [Google Scholar] [CrossRef]
- Dinesh, O.C.; Bertolo, R.F.; Brunton, J.A. Creatine supplementation to total parenteral nutrition improves creatine status and supports greater liver and kidney protein synthesis in neonatal piglets. Pediatr. Res. 2018, 83, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Y.; Lu, L.; Yan, W.; Tao, Y.; Zhou, K.; Jia, J.; Cai, W. Alterations in intestinal microbiota relate to intestinal failure-associated liver disease and central line infections. J. Pediatr. Surg. 2017, 52, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Pironi, L.; Wanten, G.; Arends, J.; Bozzetti, F.; Cuerda, C.; Joly, F.; Kelly, D.; Staun, M.; Szczepanek, K.; et al. Clinical approach to the management of Intestinal Failure Associated Liver Disease (IFALD) in adults: A position paper from the Home Artificial Nutrition and Chronic Intestinal Failure Special Interest Group of ESPEN. Clin. Nutr. 2018, 37, 1794–1797. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.J.; Revenis, M.; Kusenda, C.; Scavo, L. Parenteral nutrition-associated conjugated hyperbilirubinemia in hospitalized infants. J. Am. Diet. Assoc. 2010, 110, 1684–1695. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Chen, C.; Hou, G.; Zeng, C.; Ren, Y.; Chen, X.; Peng, C. Metabolomic profiling reveals amino acid and carnitine alterations as metabolic signatures in psoriasis. Theranostics 2021, 11, 754–767. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Núñez, E.; Steyerberg, E.W.; Núñez, J. Regression modeling strategies. Rev. Esp. De Cardiol. 2011, 64, 501–507. [Google Scholar] [CrossRef]

| Characteristics | Data Not Available | Control | SBS | p-Value |
|---|---|---|---|---|
| Total n | 18 | 20 | ||
| Demographics | ||||
| Age (months) | 48 (24.00–84.00) | 4.01 (2.42–6.93) | <0.001 | |
| Sex, male, n (%) | 11 (61.1%) | 14 (70.0%) | 0.815 | |
| Preterm birth, n (%) | 2 (11.11) | 13 (65.0) | 0.002 | |
| Antibiotics, n (%) | 0 (0.0) | 17 (85.0) | <0.001 | |
| Laboratory parameters | ||||
| RSL (cm) | 60 (47.50–70.00) | |||
| PN duration (days) | 191 (121–247) | |||
| Ileocecal valve, n (%) | 13 (65.0) | |||
| Bile acid (μmol/L) | 4.25 (2.52–5.00) | 3.85 (1.48–17.92) | 0.965 | |
| Creatinine (μmol/L) | 2 | 31.05 (22.70–37.08) | 18.60 (16.60–20.82) | <0.001 |
| ALT (U/L) | 13.25 (10.57–17.60) | 104.00 (75.12–248.00) | <0.001 | |
| AST (U/L) | 42.85 (37.95–48.37) | 143.00 (95.75–345.00) | <0.001 | |
| Sodium (mmol/L) | 2 | 138.50 (135.25–139.95) | ||
| Total bilirubin (μmol/L) | 4.65 (3.70–6.22) | 34.90 (14.70–92.55) | <0.001 | |
| Direct bilirubin (μmol/L) | 0.00 (0.00–0.00) | 0.00 (0.00–39.65) | 0.001 | |
| Albumin (g/L) | 46.85 (44.23–48.35) | 32.70 (31.15–37.23) | <0.001 | |
| GGT(U/L) | 11.00 (10.00–13.75) | 114.50 (67.25–188.75) | <0.001 | |
| White blood cell count, ×109/L | 14.45 (11.33–19.12) | |||
| Platelet counts, ×109/L | 341.00 (253.75–362.50) | |||
| Prothrombin (s) | 15.90 (14.60–16.95) | |||
| INR | 1.45 (1.33–1.54) | |||
| Name | VIP | Ratio | p-Value | Up/Down |
|---|---|---|---|---|
| Fluconazole | 2.15 | 15,234.08 | 0.004 | up |
| Cefmetazole | 1.95 | 4712.83 | 0.0011 | up |
| 1-(4-methoxyphenyl)-3-pentanyl hydrogen sulfate | 3.15 | 4278.50 | 0 | up |
| Penicilloic acid | 1.34 | 4117.74 | 0.0134 | up |
| Midazolam | 3.58 | 3119.08 | 0 | up |
| Omeprazole | 2.51 | 2981.79 | 0.0003 | up |
| Alpha-hydroxymidazolam | 3.46 | 2787.79 | 0 | up |
| Cefuroxime | 1.67 | 1597.61 | 0.0003 | up |
| 2-(4-Methyl-5-thiazolyl)ethyl butanoate | 3.48 | 1117.76 | 0 | up |
| Benzothiazole | 2.26 | 613.59 | 0 | up |
| Cystathionine | 1.46 | −1666.67 | 0.044 | down |
| Piperine | 3.20 | −555.56 | 0 | down |
| Guaiacol sulfate | 3.23 | −357.14 | 0 | down |
| Dihydronaringenin-o-sulphate | 2.36 | −312.50 | 0 | down |
| hesperetin 3′-O-sulfate | 1.92 | −312.50 | 0.0004 | down |
| Methyl indole-3-acetate | 2.75 | −270.27 | 0 | down |
| 2,4,5-Trimethoxybenzaldehyde | 1.23 | −142.86 | 0.0057 | down |
| (e)-4-methoxycinnamic acid | 1.54 | −140.85 | 0.001 | down |
| 3-(7-hydroxy-4-oxo-4h-chromen-2-yl)phenyl hydrogen sulfate | 2.25 | −129.87 | 0 | down |
| 5-sulfooxymethylfurfural | 3.15 | −123.46 | 0 | down |
| Name | VIP | Ratio | p-Value | Up/Down |
|---|---|---|---|---|
| Probucol | 2.3583 | 13.9615 | 0.0267 | up |
| 8-hydroxydemethylclomipramine | 2.2311 | 8.9335 | 0.0102 | up |
| Chlorcyclizine | 2.368 | 6.3946 | 0.0008 | up |
| Neocasomorphin (1–5) | 1.8981 | 5.6374 | 0.0101 | up |
| Bis(5-hydroxynoracronycine) | 1.6297 | 5.4574 | 0.0247 | up |
| 2-acetamido-1,5-anhydro-2-deoxy-3-o-beta-d-galactopyranosyl-d-arabino-hex-1-enitol | 2.8951 | 4.7293 | 0.0019 | up |
| Ginsenoyne B | 1.7813 | 4.6596 | 0.004 | up |
| Hydroxychloroquine | 1.5343 | 4.6471 | 0.0397 | up |
| Tropicamide | 1.7151 | 4.5564 | 0.0181 | up |
| Histidylphenylalanine | 1.6365 | 4.4079 | 0.016 | up |
| Dehydrophytosphingosine | 1.3912 | −32.3625 | 0.0216 | down |
| C.I. Acid Red 13 | 2.3328 | −31.1526 | 0.007 | down |
| Norfentanyl | 1.8038 | −25.0000 | 0.0146 | down |
| 2-ethoxy-4-(4-methyl-1,3-dioxolan-2-yl)phenol | 2.4751 | −22.0751 | 0.0039 | down |
| Benzo[a]pyrene-7,8-dihydrodiol-9,10-oxide | 1.6514 | −20.5761 | 0.0062 | down |
| (+/−)-hesperetin | 1.8447 | −17.1527 | 0.0439 | down |
| Brassinin | 1.8249 | −13.9082 | 0.0315 | down |
| Pseudopelletierine | 2.3423 | −13.7363 | 0.013 | down |
| Sinalbin B | 1.546 | −13.5318 | 0.0215 | down |
| Skatole | 1.0416 | −11.5340 | 0.012 | down |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Y.; Gao, B.; Yan, J.; Cai, W.; Jiang, L. Untargeted Metabolomics Reveal Parenteral Nutrition-Associated Alterations in Pediatric Patients with Short Bowel Syndrome. Metabolites 2022, 12, 600. https://doi.org/10.3390/metabo12070600
Wang Y, Liu Y, Gao B, Yan J, Cai W, Jiang L. Untargeted Metabolomics Reveal Parenteral Nutrition-Associated Alterations in Pediatric Patients with Short Bowel Syndrome. Metabolites. 2022; 12(7):600. https://doi.org/10.3390/metabo12070600
Chicago/Turabian StyleWang, Ying, Yang Liu, Bei Gao, Junkai Yan, Wei Cai, and Lu Jiang. 2022. "Untargeted Metabolomics Reveal Parenteral Nutrition-Associated Alterations in Pediatric Patients with Short Bowel Syndrome" Metabolites 12, no. 7: 600. https://doi.org/10.3390/metabo12070600
APA StyleWang, Y., Liu, Y., Gao, B., Yan, J., Cai, W., & Jiang, L. (2022). Untargeted Metabolomics Reveal Parenteral Nutrition-Associated Alterations in Pediatric Patients with Short Bowel Syndrome. Metabolites, 12(7), 600. https://doi.org/10.3390/metabo12070600







