PPARβ/δ Augments IL-1β-Induced COX-2 Expression and PGE2 Biosynthesis in Human Mesangial Cells via the Activation of SIRT1
Abstract
1. Introduction
2. Results
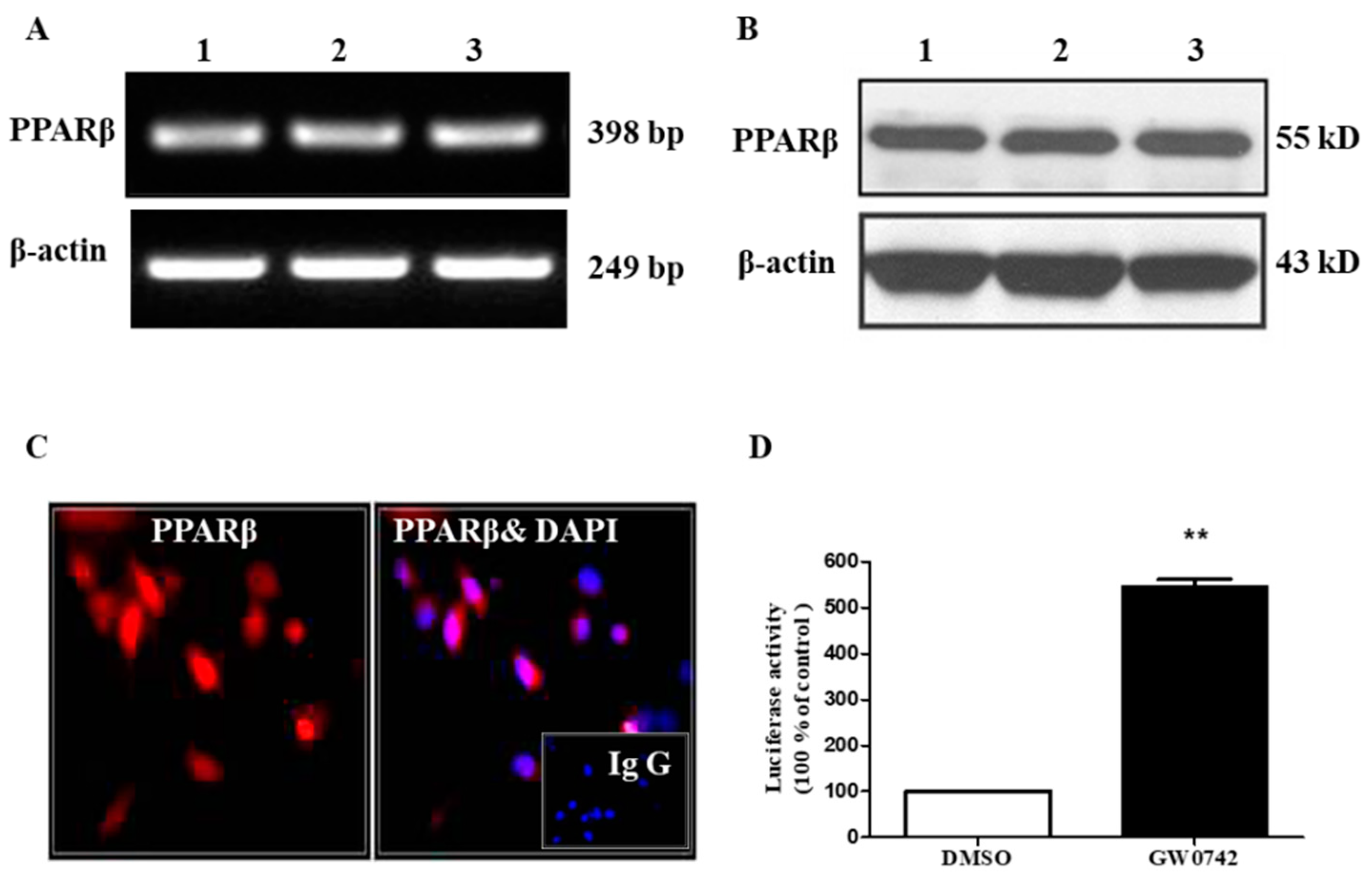
2.1. PPARβ/δ Is Functionally Expressed in hMCs
2.2. PPARβ/δ Increases COX-2 Expression and PGE2 Production in hMCs
2.3. IL-1β Induces PPARβ/δ Expression in hMCs
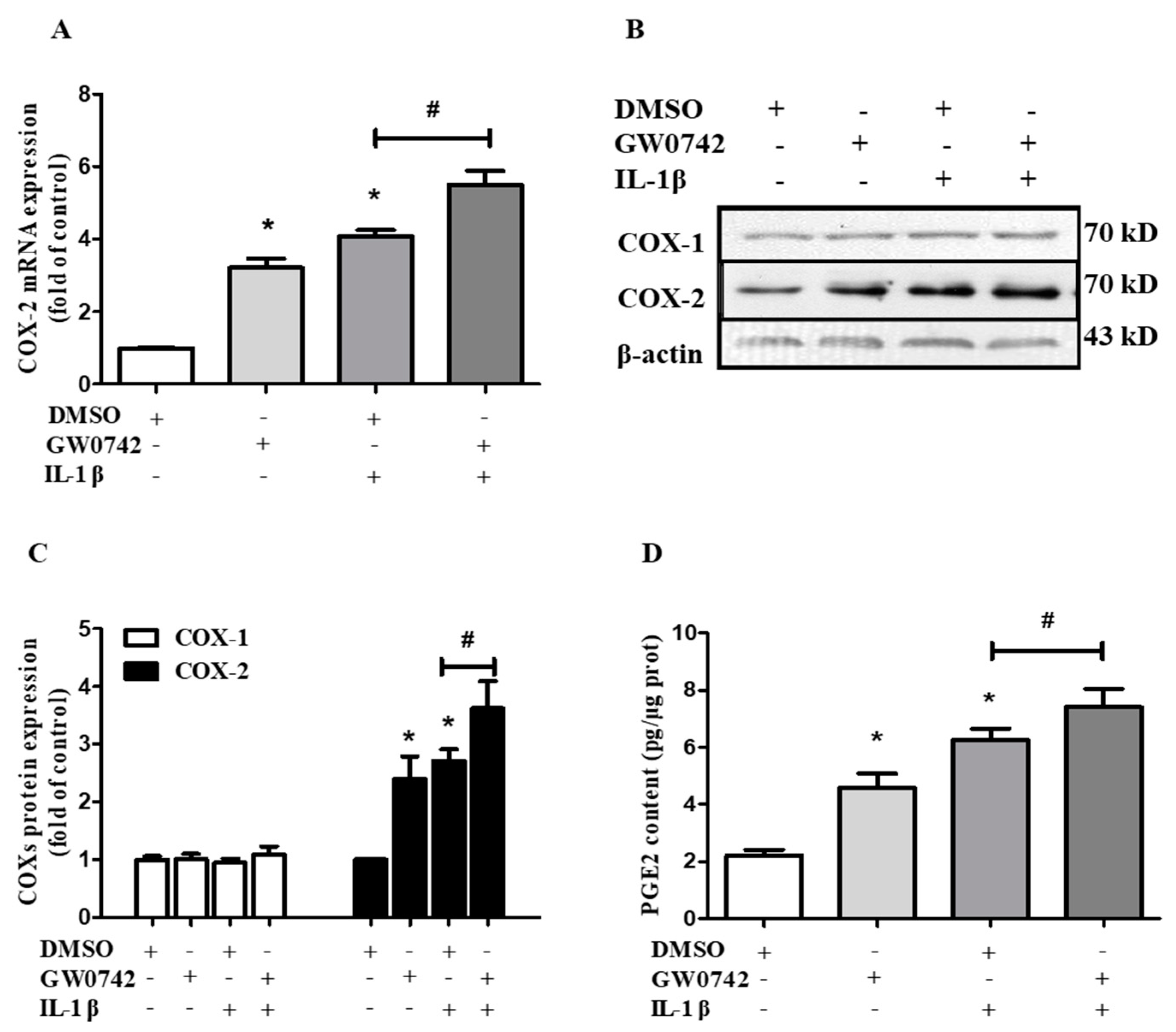
2.4. PPARβ/δ Activation Augments IL-1β-Induced COX-2 Expression and PGE2 Production in hMCs
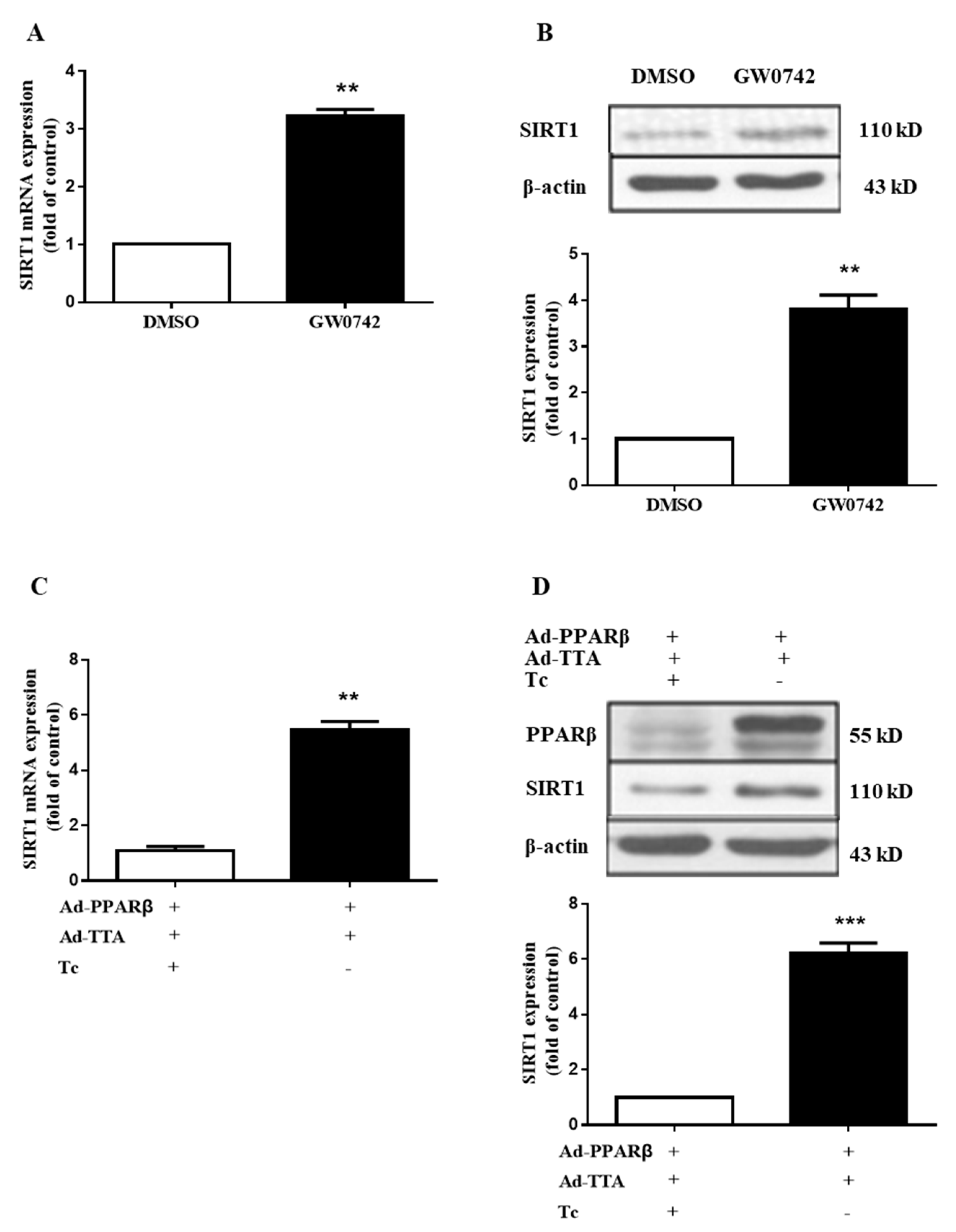
2.5. PPARβ/δ Increases the Expression of SIRT1 in hMCs
2.6. SIRT1 Inhibition Blocks the Stimulatory Effect of PPARβ/δ on IL-1β-Induced COX-2 Expression and PGE2 Production in hMCs
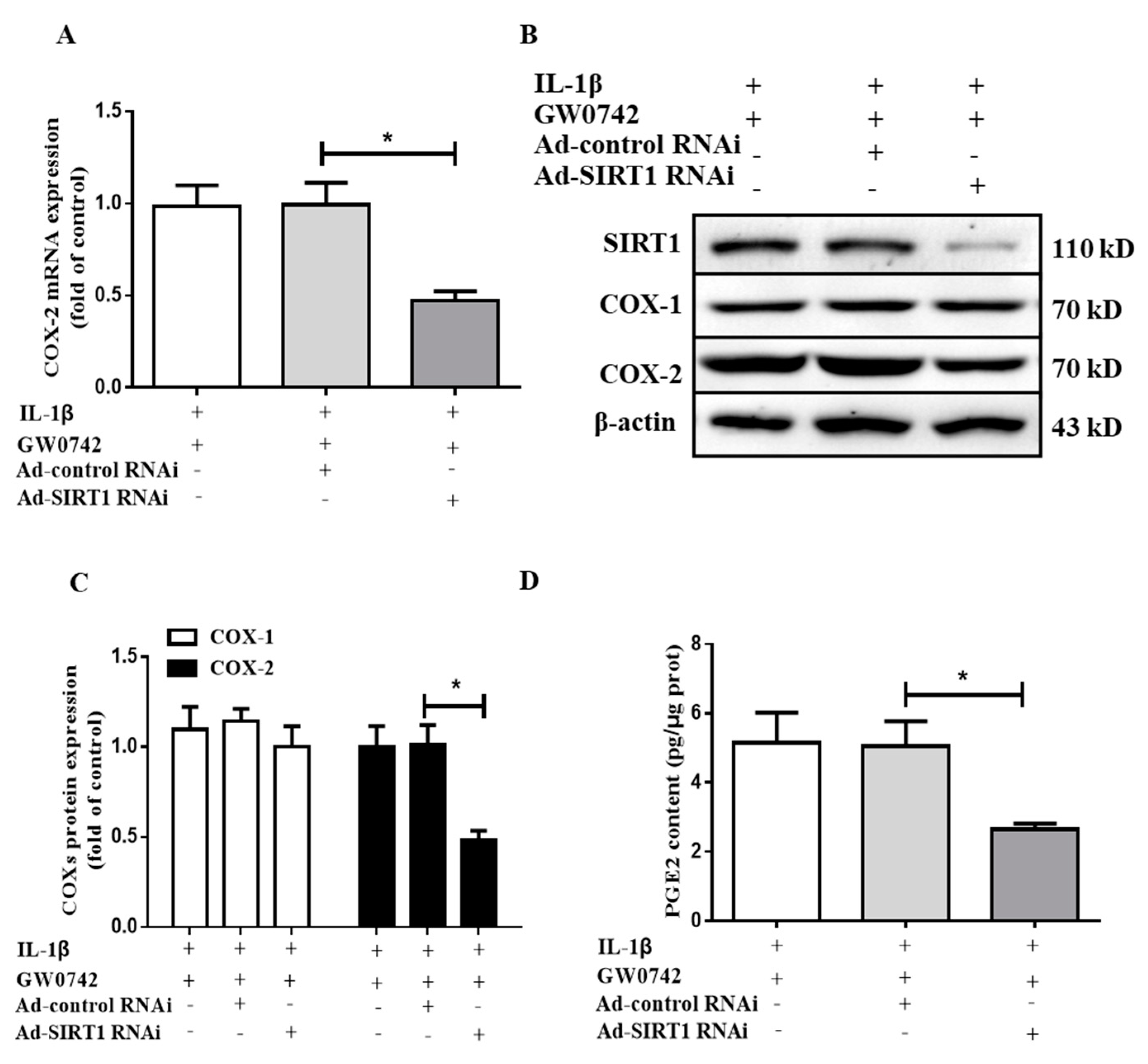
2.7. SIRT1 Knockdown Blocks the Effect of PPARβ/δ on IL-1β-Induced COX2 Expression and PGE2 Production in hMCs
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Human Mesangial Cell Culture
4.3. Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.4. Western Blot Analysis
4.5. Transient Transfection and Luciferase Assay
4.6. Adenovirus Infection of hMCS
4.7. Measurement of Prostaglandin E2 Levels
4.8. Immunofluorescence Staining
4.9. Statistical Analysis
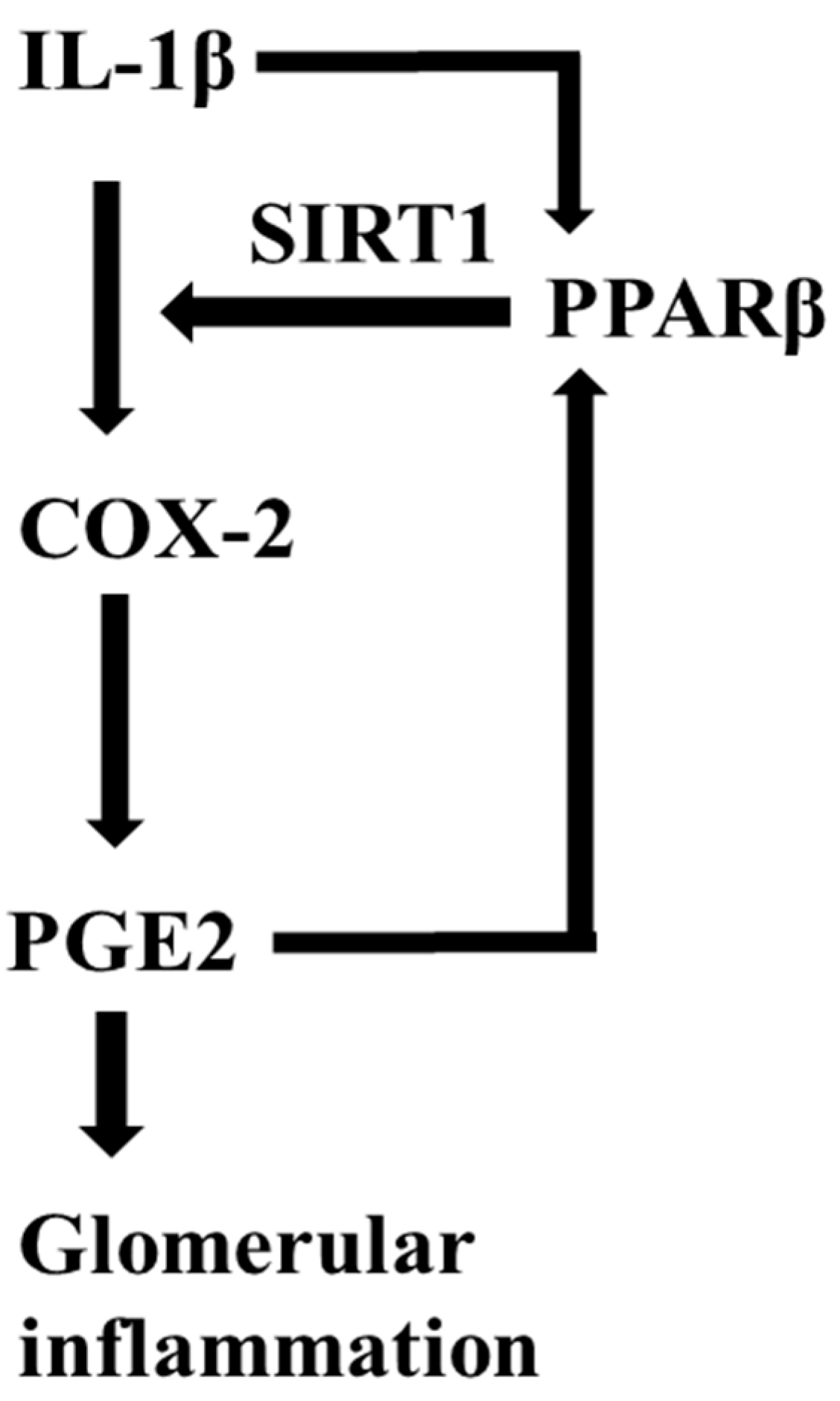
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luan, Z.L.; Zhang, C.; Ming, W.H.; Huang, Y.Z.; Guan, Y.F.; Zhang, X.Y. Nuclear receptors in renal health and disease. EBioMedicine 2022, 76, 103855. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, Z. The Role of Peroxisome Proliferator-Activated Receptors in Kidney Diseases. Front. Pharmacol. 2022, 13, 832732. [Google Scholar] [CrossRef] [PubMed]
- Kadayat, T.M.; Shrestha, A.; Jeon, Y.H.; An, H.; Kim, J.; Cho, S.J.; Chin, J. Targeting Peroxisome Proliferator-Activated Receptor Delta (PPARdelta): A Medicinal Chemistry Perspective. J. Med. Chem. 2020, 63, 10109–10134. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.L.; Nerurkar, S.S.; Bao, W.; Jucker, B.M.; Sarov-Blat, L.; Steplewski, K.; Willette, R.N. In vivo activation of peroxisome proliferator-activated receptor-delta protects the heart from ischemia/reperfusion injury in Zucker fatty rats. J. Pharmacol. Exp. Ther. 2008, 325, 466–474. [Google Scholar] [CrossRef]
- Nahle, Z.; Hsieh, M.; Pietka, T.; Coburn, C.T.; Grimaldi, P.A.; Zhang, M.Q.; Abumrad, N.A. CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta-mediated adaptation to metabolic stress. J. Biol. Chem. 2008, 283, 14317–14326. [Google Scholar] [CrossRef]
- Kim, D.J.; Bility, M.T.; Billin, A.N.; Willson, T.M.; Gonzalez, F.J.; Peters, J.M. PPARbeta/delta selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006, 13, 53–60. [Google Scholar] [CrossRef]
- Nadra, K.; Anghel, S.I.; Joye, E.; Tan, N.S.; Basu-Modak, S.; Trono, D.; Desvergne, B. Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor beta/delta. Mol. Cell Biol. 2006, 26, 3266–3281. [Google Scholar] [CrossRef]
- Planavila, A.; Rodriguez-Calvo, R.; Jove, M.; Michalik, L.; Wahli, W.; Laguna, J.C.; Vazquez-Carrera, M. Peroxisome proliferator-activated receptor beta/delta activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc. Res. 2005, 65, 832–841. [Google Scholar] [CrossRef]
- Gupta, R.A.; Tan, J.; Krause, W.F.; Geraci, M.W.; Willson, T.M.; Dey, S.K.; DuBois, R.N. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc. Natl. Acad. Sci. USA 2000, 97, 13275–13280. [Google Scholar] [CrossRef]
- Barish, G.D.; Atkins, A.R.; Downes, M.; Olson, P.; Chong, L.W.; Nelson, M.; Lee, C.H. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc. Natl. Acad. Sci. USA 2008, 105, 4271–4276. [Google Scholar] [CrossRef]
- Rodriguez-Calvo, R.; Serrano, L.; Coll, T.; Moullan, N.; Sanchez, R.M.; Merlos, M.; Vazquez-Carrera, M. Activation of peroxisome proliferator-activated receptor beta/delta inhibits lipopolysaccharide-induced cytokine production in adipocytes by lowering nuclear factor-kappaB activity via extracellular signal-related kinase 1/2. Diabetes 2008, 57, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Hollingshead, H.E.; Morimura, K.; Adachi, M.; Kennett, M.J.; Billin, A.N.; Willson, T.M.; Peters, J.M. PPARbeta/delta protects against experimental colitis through a ligand-independent mechanism. Dig. Dis. Sci. 2007, 52, 2912–2919. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Bajwa, P.J.; Carson, M.J.; Jeske, D.R.; Cong, Y.; Elson, C.O.; Straus, D.S. Fenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient mice. Gastroenterology 2007, 133, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Salzman, N.H.; Porter, E.; Nuding, S.; Weichenthal, M.; Petras, R.E.; Bevins, C.L. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 18129–18134. [Google Scholar] [CrossRef]
- Man, M.Q.; Barish, G.D.; Schmuth, M.; Crumrine, D.; Barak, Y.; Chang, S.; Feingold, K.R. Deficiency of PPARbeta/delta in the epidermis results in defective cutaneous permeability barrier homeostasis and increased inflammation. J. Investig. Dermatol. 2008, 128, 370–377. [Google Scholar] [CrossRef]
- Schmuth, M.; Haqq, C.M.; Cairns, W.J.; Holder, J.C.; Dorsam, S.; Chang, S.; Feingold, K.R. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J. Investig. Dermatol. 2004, 122, 971–983. [Google Scholar] [CrossRef]
- Kharroubi, I.; Lee, C.H.; Hekerman, P.; Darville, M.I.; Evans, R.M.; Eizirik, D.L.; Cnop, M. BCL-6: A possible missing link for anti-inflammatory PPAR-delta signalling in pancreatic beta cells. Diabetologia 2006, 49, 2350–2358. [Google Scholar] [CrossRef]
- Berry, E.B.; Keelan, J.A.; Helliwell, R.J.; Gilmour, R.S.; Mitchell, M.D. Nanomolar and micromolar effects of 15-deoxy-delta 12,14-prostaglandin J2 on amnion-derived WISH epithelial cells: Differential roles of peroxisome proliferator-activated receptors gamma and delta and nuclear factor kappa B. Mol. Pharmacol. 2005, 68, 169–178. [Google Scholar] [CrossRef]
- Ding, G.; Cheng, L.; Qin, Q.; Frontin, S.; Yang, Q. PPARdelta modulates lipopolysaccharide-induced TNFalpha inflammation signaling in cultured cardiomyocytes. J. Mol. Cell Cardiol. 2006, 40, 821–828. [Google Scholar] [CrossRef]
- Haskova, Z.; Hoang, B.; Luo, G.; Morgan, L.A.; Billin, A.N.; Barone, F.C.; Kilgore, K.S. Modulation of LPS-induced pulmonary neutrophil infiltration and cytokine production by the selective PPARbeta/delta ligand GW0742. Inflamm. Res. 2008, 57, 314–321. [Google Scholar] [CrossRef]
- Neels, J.G.; Grimaldi, P.A. Physiological functions of peroxisome proliferator-activated receptor beta. Physiol. Rev. 2014, 94, 795–858. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Ogawa, D.; Wada, J.; Yamamoto, N.; Shikata, K.; Sato, C.; Makino, H. Activation of peroxisome proliferator-activated receptor delta inhibits streptozotocin-induced diabetic nephropathy through anti-inflammatory mechanisms in mice. Diabetes 2011, 60, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Collino, M.; Benetti, E.; Miglio, G.; Castiglia, S.; Rosa, A.C.; Aragno, M.; Fantozzi, R. Peroxisome proliferator-activated receptor beta/delta agonism protects the kidney against ischemia/reperfusion injury in diabetic rats. Free Radic. Biol. Med. 2011, 50, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Floege, J.; Bienert, A. Glomerulonephritis. Dtsch. Med. Wochenschr. 2013, 138, 1515–1528. [Google Scholar] [CrossRef]
- Kiryluk, K.; Novak, J. The genetics and immunobiology of IgA nephropathy. J. Clin. Investig. 2014, 124, 2325–2332. [Google Scholar] [CrossRef]
- Schlondorff, D. The glomerular mesangial cell: An expanding role for a specialized pericyte. FASEB J. 1987, 1, 272–281. [Google Scholar] [CrossRef]
- Migliorini, A.; Ebid, R.; Scherbaum, C.R.; Anders, H.J. The danger control concept in kidney disease: Mesangial cells. J. Nephrol. 2013, 26, 437–449. [Google Scholar] [CrossRef]
- Ghilardi, J.R.; Svensson, C.I.; Rogers, S.D.; Yaksh, T.L.; Mantyh, P.W. Constitutive Spinal Cyclooxygenase-2 Participates in the Initiation of Tissue Injury-Induced Hyperalgesia. J. Neurosci. 2004, 24, 2727–2732. [Google Scholar] [CrossRef]
- Paul, A.G.; Chandran, B.; Sharma-Walia, N. Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: A key player in Kaposi’s sarcoma-associated herpes virus associated malignancies. Transl. Res. 2013, 162, 77–92. [Google Scholar] [CrossRef]
- Kitahara, M.; Eitner, F.; Ostendorf, T.; Kunter, U.; Janssen, U.; Westenfeld, R.; Floege, J. Selective Cyclooxygenase-2 Inhibition Impairs Glomerular Capillary Healing in Experimental Glomerulonephritis. J. Am. Soc. Nephrol. 2002, 13, 1261–1270. [Google Scholar] [CrossRef]
- Radeke, H.H.; Meier, B.; Topley, N.; Floge, J.; Habermehl, G.G.; Resch, K. Interleukin 1-α and tumor necrosis factor-α induce oxygen radical production in mesangial cells. Kidney Int. 1990, 37, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.F. Secretory products of macrophages. J. Clin. Investig. 1987, 79, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Atkins, R.C. Interleukin-1 in crescentic glomerulonephritis. Kidney Int. 1995, 48, 576–586. [Google Scholar] [CrossRef]
- Sedor, J.R.; Nakazato, Y.; Konieczkowski, M. Interleukin-1 and the mesangial cell. Kidney Int. 1992, 41, 595–599. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, L.; Han, C.; Wu, T. A novel positive feedback loop between peroxisome proliferator-activated receptor-delta and prostaglandin E2 signaling pathways for human cholangiocarcinoma cell growth. J. Biol. Chem. 2006, 281, 33982–33996. [Google Scholar] [CrossRef]
- Xu, L.; Han, C.; Lim, K.; Wu, T. Cross-talk between peroxisome proliferator-activated receptor delta and cytosolic phospholipase A(2)alpha/cyclooxygenase-2/prostaglandin E(2) signaling pathways in human hepatocellular carcinoma cells. Cancer Res. 2006, 66, 11859–11868. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Zhang, M.Z.; You, L.; Davis, L.S.; Fan, H.; Hao, C.M. Sirt1 activation protects the mouse renal medulla from oxidative injury. J. Clin. Investig. 2010, 120, 1056–1068. [Google Scholar] [CrossRef]
- Phua, W.; Tan, W.R.; Yip, Y.S.; Hew, I.D.; Wee, J.; Cheng, H.S.; Tan, N.S. PPARbeta/delta Agonism Upregulates Forkhead Box A2 to Reduce Inflammation in C2C12 Myoblasts and in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 1747. [Google Scholar] [CrossRef]
- Sawano, H.; Haneda, M.; Sugimoto, T.; Inoki, K.; Koya, D.; Kikkawa, R. 15-Deoxy-Delta12,14-prostaglandin J2 inhibits IL-1beta-induced cyclooxygenase-2 expression in mesangial cells. Kidney Int. 2002, 61, 1957–1967. [Google Scholar] [CrossRef][Green Version]
- Xu, J.; Zhang, Y.; Xiao, Y.; Ma, S.; Liu, Q.; Dang, S.; Zhang, Y. Inhibition of 12/15-lipoxygenase by baicalein induces microglia PPARbeta/delta: A potential therapeutic role for CNS autoimmune disease. Cell Death Dis. 2013, 4, e569. [Google Scholar] [CrossRef]
- Smith, W.L.; Dewitt, D.L. ; Dewitt, D.L. Prostaglandin endoperoxide H synthases-1 and -2. Adv. Immunol. 1996, 62, 167–215. [Google Scholar] [PubMed]
- Neeb, L.; Hellen, P.; Boehnke, C.; Hoffmann, J.; Schuh-Hofer, S.; Dirnagl, U.; Reuter, U. IL-1beta stimulates COX-2 dependent PGE(2) synthesis and CGRP release in rat trigeminal ganglia cells. PLoS ONE 2011, 6, e17360. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kanamaru, Y.; Watanabe, T.; Tada, N.; Horikoshi, S.; Suzuki, Y.; Tomino, Y. Targeted IgA Fc receptor I (FcalphaRI) therapy in the early intervention and treatment of pristane-induced lupus nephritis in mice. Clin. Exp. Immunol. 2015, 181, 407–416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Werber, H.I.; Emancipator, S.N.; Tykocinski, M.L.; Sedor, J.R. The interleukin 1 gene is expressed by rat glomerular mesangial cells and is augmented in immune complex glomerulonephritis. J. Immunol. 1987, 138, 3207–3212. [Google Scholar] [PubMed]
- Matsumoto, K.; Hatano, M. Production of interleukin 1 in glomerular cell cultures from rats with nephrotoxic serum nephritis. Clin. Exp. Immunol. 1989, 75, 123–128. [Google Scholar]
- Yoshioka, K.; Takemura, T.; Murakami, K.; Okada, M.; Yagi, K.; Miyazato, H.; Maki, S. In situ expression of cytokines in IgA nephritis. Kidney Int. 1993, 44, 825–833. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, K.; Dowling, J.; Atkins, R.C. Production of Interleukin 1 in Glomerular Cell Cultures from Patients with Rapidly Progressive Crescentic Glomerulonephritis. Am. J. Nephrol. 1988, 8, 463–470. [Google Scholar] [CrossRef]
- Takemura, T.; Yoshioka, K.; Murakami, K.; Akano, N.; Okada, M.; Aya, N.; Maki, S. Cellular localization of inflammatory cytokines in human glomerulonephritis. Virchows Arch. 1994, 424, 459–464. [Google Scholar] [CrossRef]
- Cernuda-Morollon, E.; Rodriguez-Pascual, F.; Klatt, P.; Lamas, S.; Perez-Sala, D. PPAR agonists amplify iNOS expression while inhibiting NF-kappaB: Implications for mesangial cell activation by cytokines. J. Am. Soc. Nephrol. 2002, 13, 2223–2231. [Google Scholar] [CrossRef]
- Zhou, D.; Jin, J.; Liu, Q.; Shi, J.; Hou, Y. PPARdelta agonist enhances colitis-associated colorectal cancer. Eur. J. Pharmacol. 2019, 842, 248–254. [Google Scholar] [CrossRef]
- Kim, M.S.; Sweeney, T.R.; Shigenaga, J.K.; Chui, L.G.; Moser, A.; Grunfeld, C.; Feingold, K.R. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism 2007, 56, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Perico, L.; Benigni, A. Sirtuins in Renal Health and Disease. J. Am. Soc. Nephrol. 2018, 29, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, T.; Milne, J.C.; Imamura, T.; Schenk, S.; Sonoda, N.; Babendure, J.L.; Olefsky, J.M. SIRT1 Exerts Anti-Inflammatory Effects and Improves Insulin Sensitivity in Adipocytes. Mol. Cell. Biol. 2009, 29, 1363–1374. [Google Scholar] [CrossRef]
- Okazaki, M.; Iwasaki, Y.; Nishiyama, M.; Taguchi, T.; Tsugita, M.; Nakayama, S.; Terada, Y. PPARbeta/delta regulates the human SIRT1 gene transcription via Sp1. Endocr. J. 2010, 57, 403–413. [Google Scholar] [CrossRef]
- Regnault, T.R.; Zhao, L.; Chiu, J.S.; Gottheil, S.K.; Foran, A.; Yee, S.P. Peroxisome Proliferator-Activated Receptor-beta/delta, -gamma Agonists and Resveratrol Modulate Hypoxia Induced Changes in Nuclear Receptor Activators of Muscle Oxidative Metabolism. PPAR Res. 2010, 2010, 129173. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Shi, Q.; Katkuri, S.; Walhi, W.; Desvergne, B.; DuBois, R.N. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell 2004, 6, 285–295. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cao, R.; Gu, T.; Cao, C.; Chen, T.; Guan, Y.; Zhang, X. PPARβ/δ Augments IL-1β-Induced COX-2 Expression and PGE2 Biosynthesis in Human Mesangial Cells via the Activation of SIRT1. Metabolites 2022, 12, 595. https://doi.org/10.3390/metabo12070595
Li Y, Cao R, Gu T, Cao C, Chen T, Guan Y, Zhang X. PPARβ/δ Augments IL-1β-Induced COX-2 Expression and PGE2 Biosynthesis in Human Mesangial Cells via the Activation of SIRT1. Metabolites. 2022; 12(7):595. https://doi.org/10.3390/metabo12070595
Chicago/Turabian StyleLi, Yaqing, Rong Cao, Tingting Gu, Cong Cao, Tingyue Chen, Youfei Guan, and Xiaoyan Zhang. 2022. "PPARβ/δ Augments IL-1β-Induced COX-2 Expression and PGE2 Biosynthesis in Human Mesangial Cells via the Activation of SIRT1" Metabolites 12, no. 7: 595. https://doi.org/10.3390/metabo12070595
APA StyleLi, Y., Cao, R., Gu, T., Cao, C., Chen, T., Guan, Y., & Zhang, X. (2022). PPARβ/δ Augments IL-1β-Induced COX-2 Expression and PGE2 Biosynthesis in Human Mesangial Cells via the Activation of SIRT1. Metabolites, 12(7), 595. https://doi.org/10.3390/metabo12070595






