Differential Metabolic Responses of Lettuce Grown in Soil, Substrate and Hydroponic Cultivation Systems under NH4+/NO3− Application
Abstract
1. Introduction
2. Results
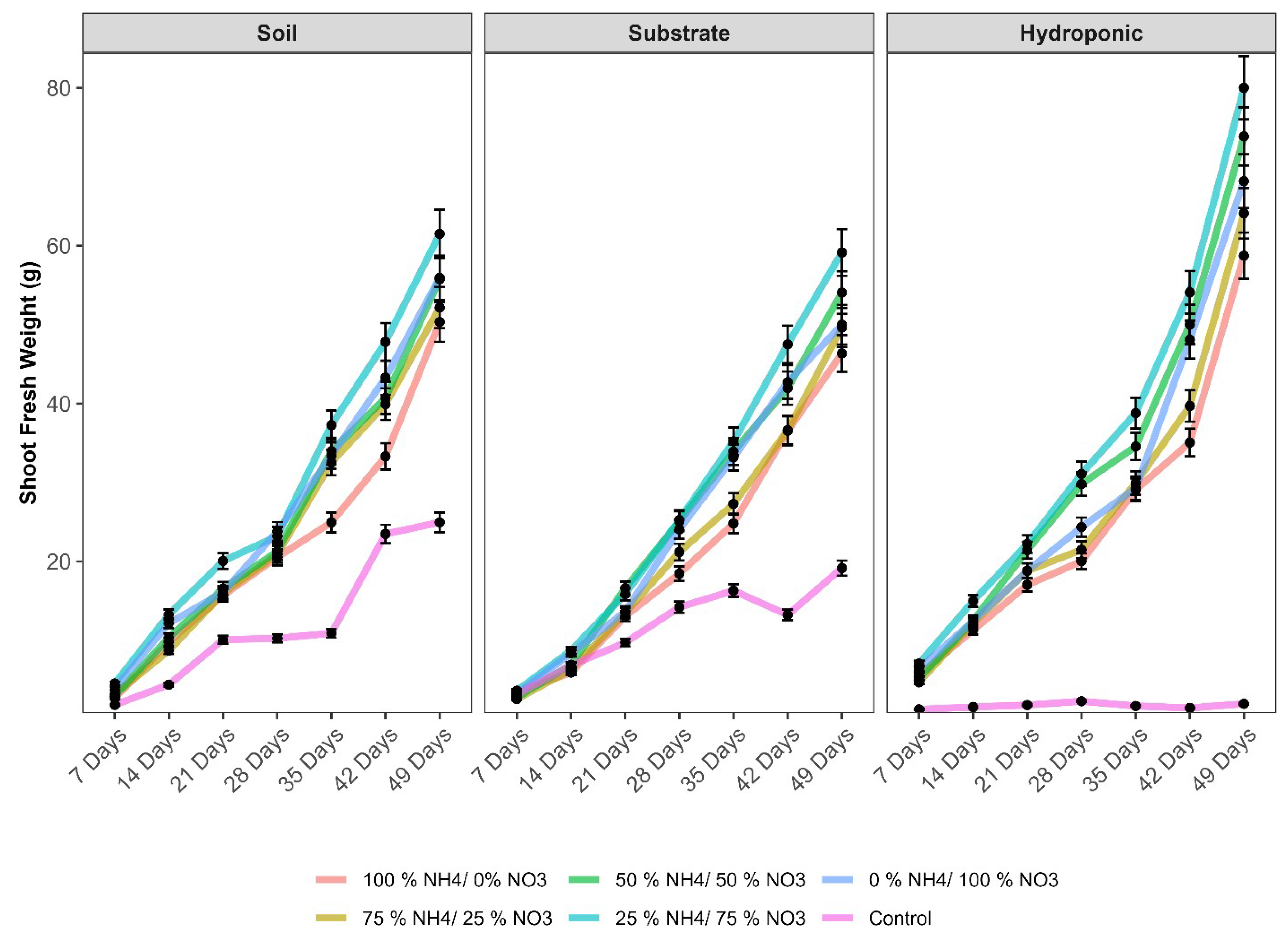
2.1. Physiological and Growth Parameters of Lettuce
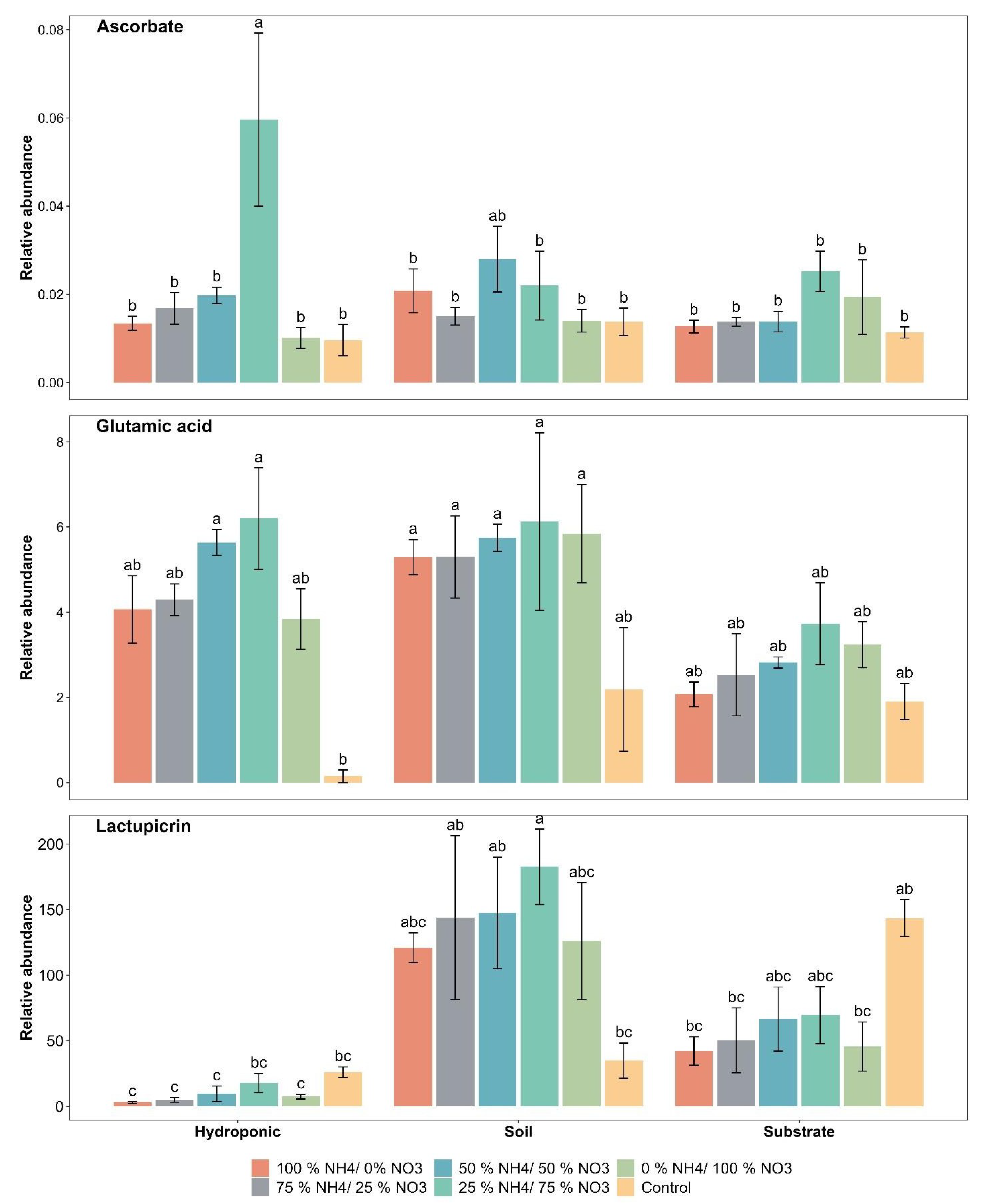
2.2. Taste and Quality-Related Compounds
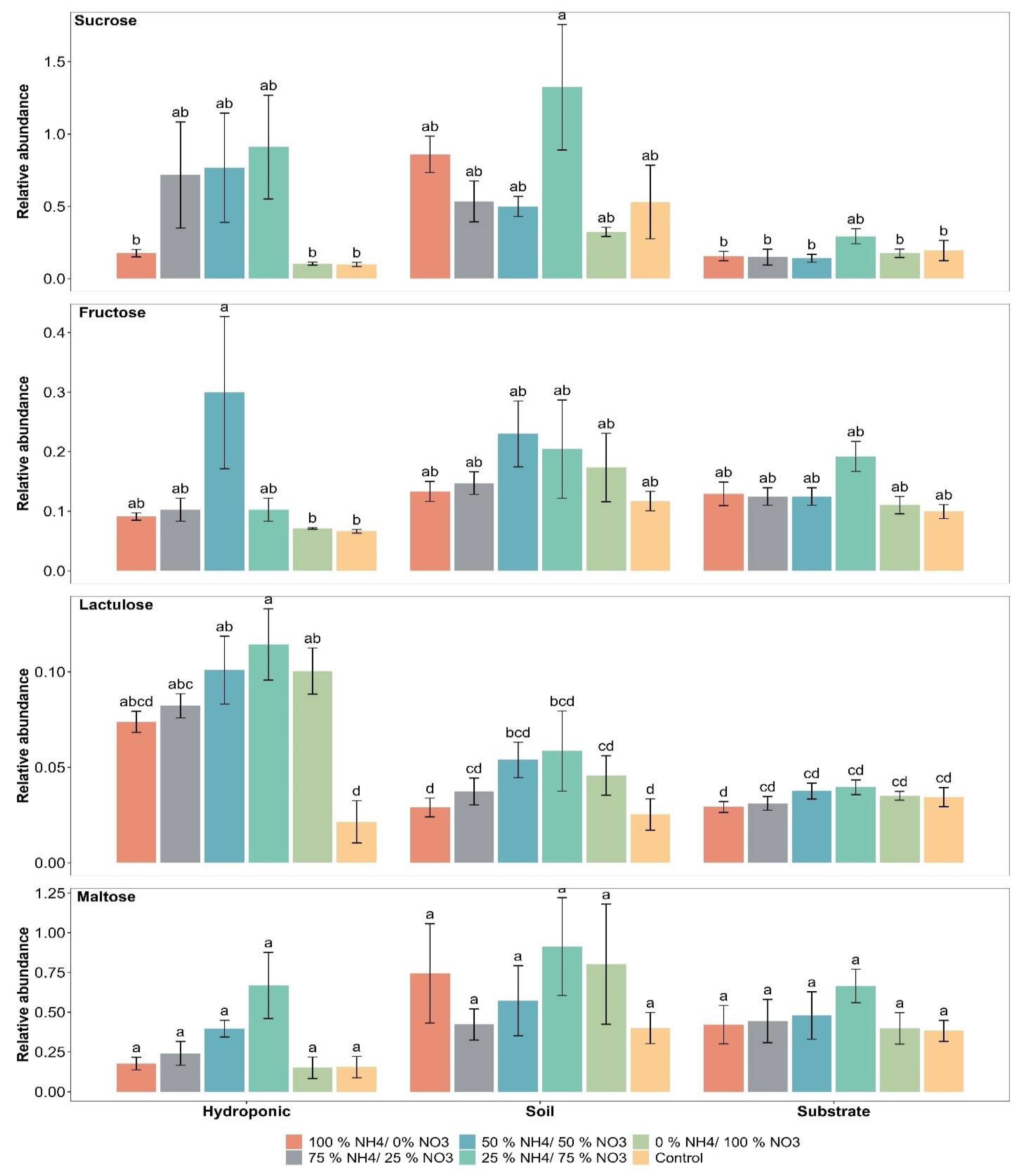
2.3. Sugar Compounds
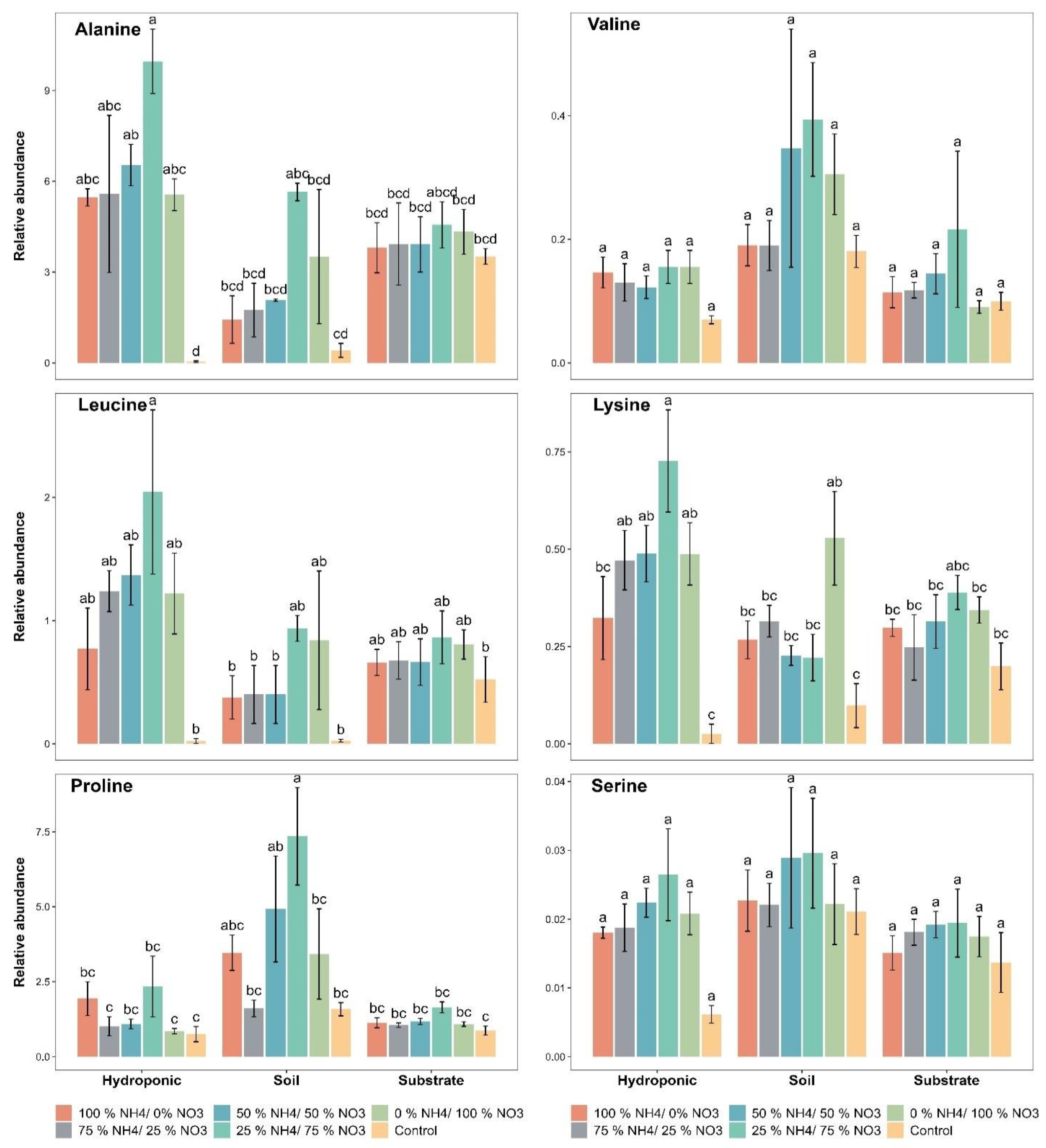
2.4. Amino Acid Contents
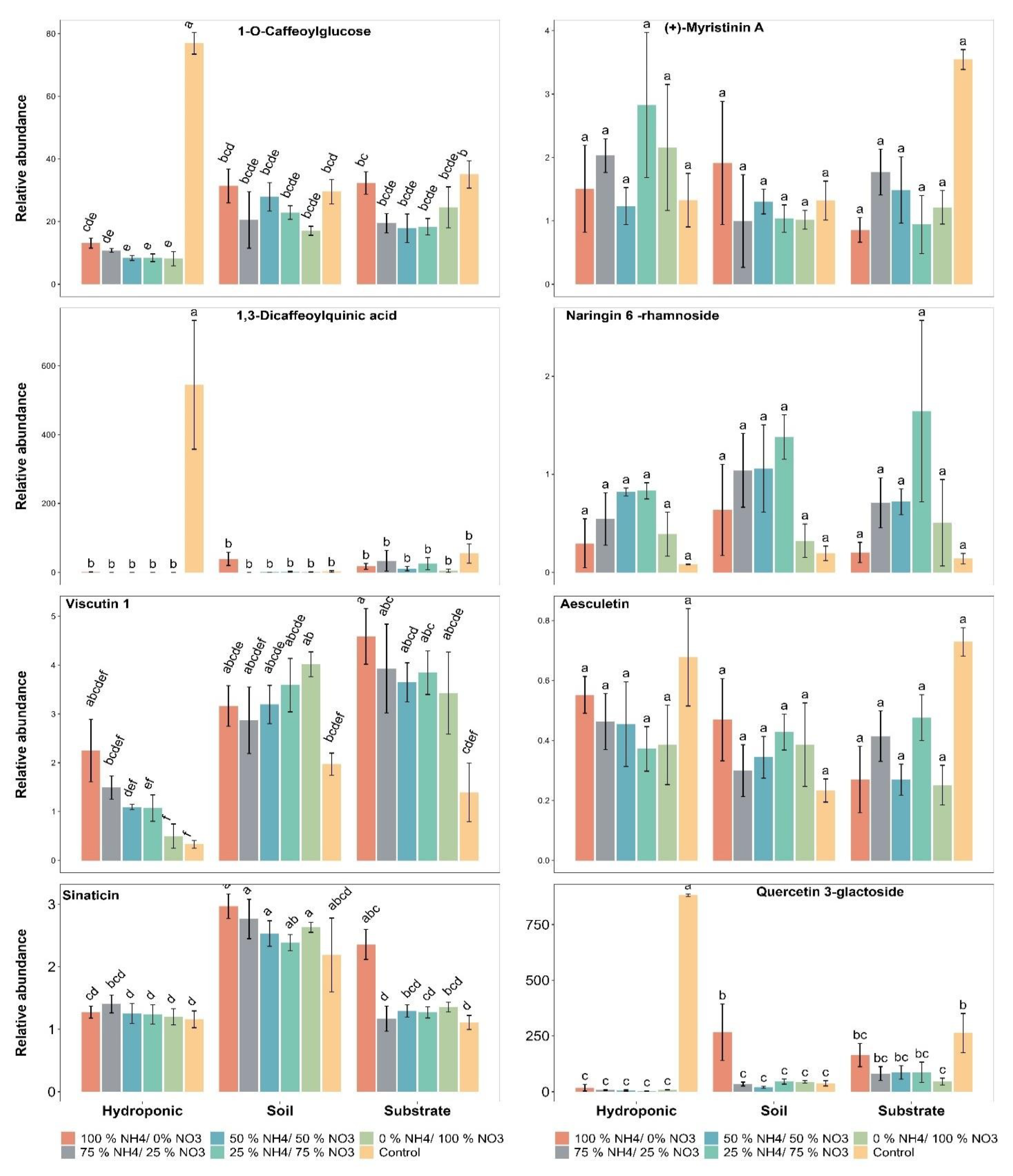
2.5. Polyphenolic Compounds
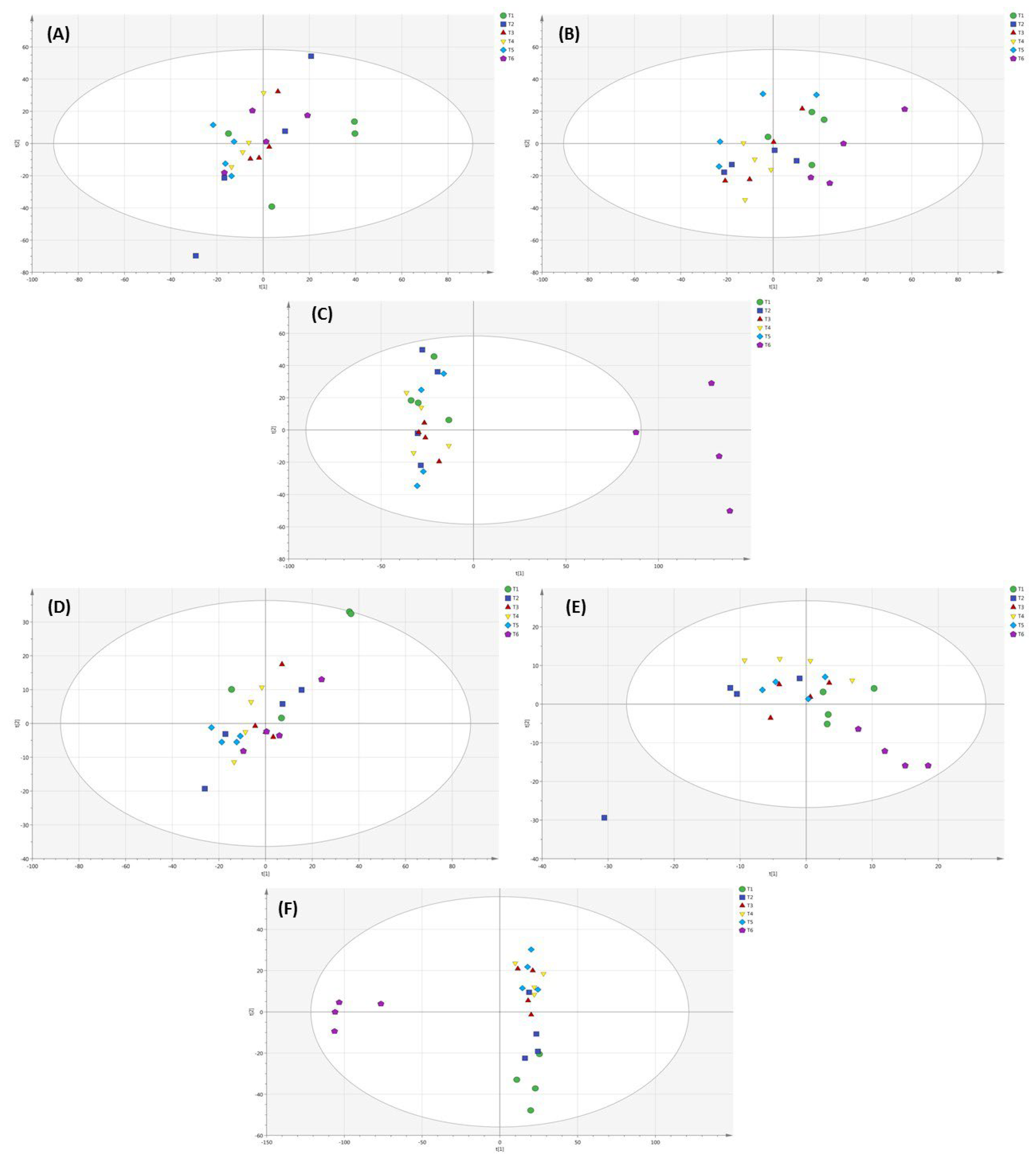
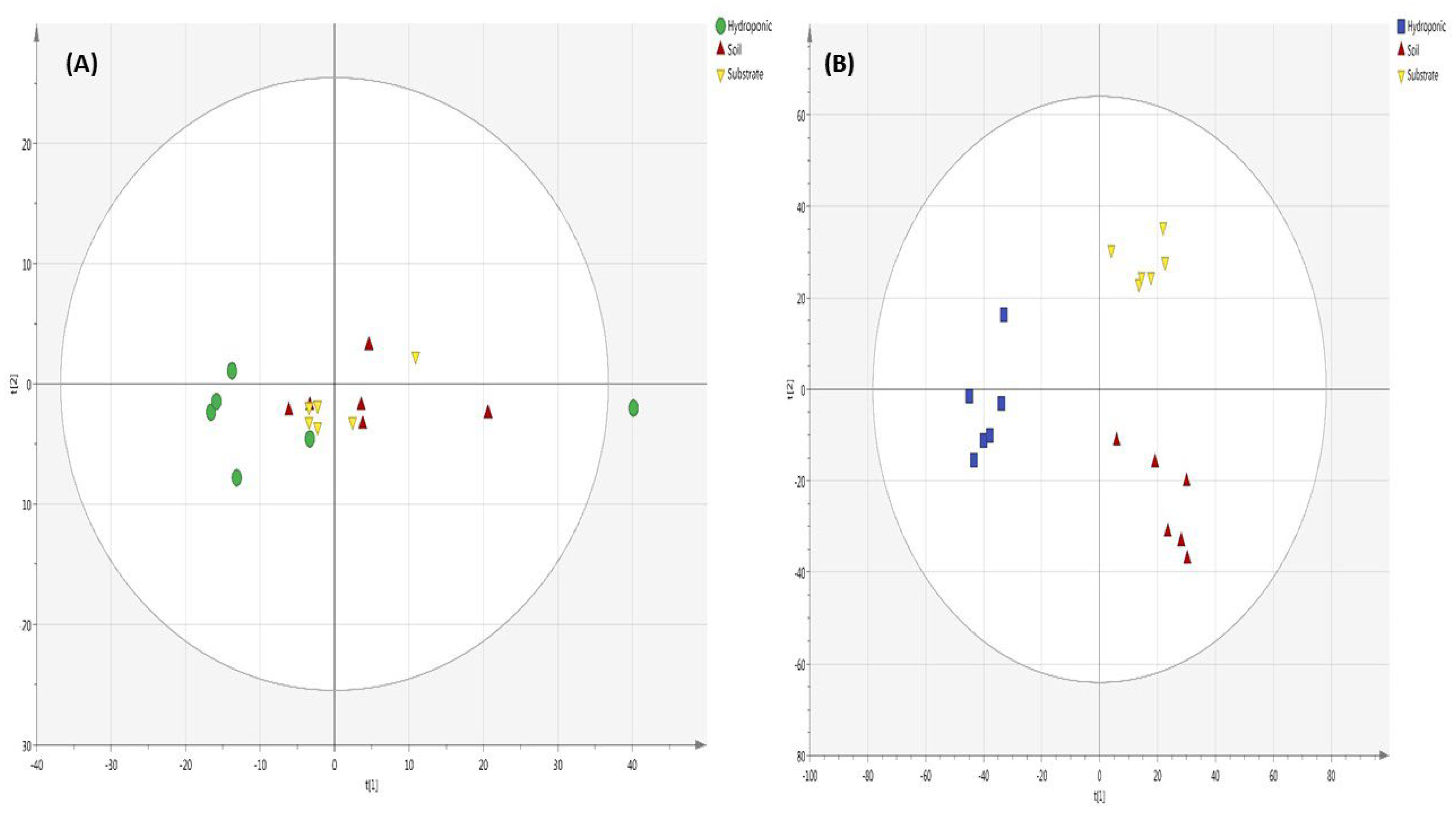
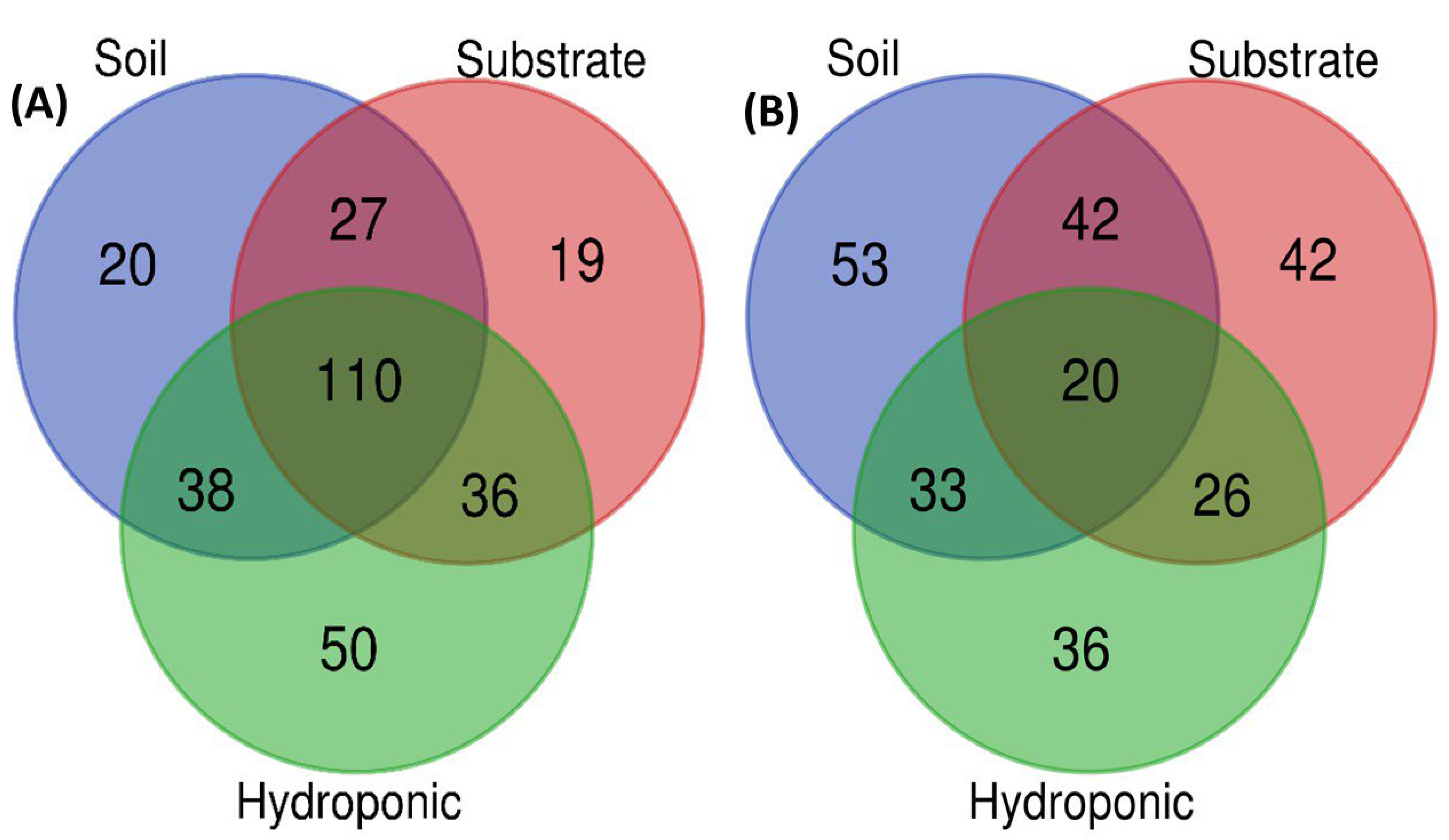
2.6. Metabolomic Profiling of Lettuce Treated with Different NH4+/NO3− Ratios in Soil, Substrate and Hydroponic Cultivation System
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Untargeted Metabolomic Analysis through GC-MS and UPLC VION IMS QTOF-MS/MS
4.2.1. Sample Preparation for GC-MS and UPLC VION IMS QTOF-MS/MS
4.2.2. Analysis Using GC-MS and UPLC VION IMS QTOF-MS/MS
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.-L.; Tsay, Y.-F. Influence of differing nitrate and nitrogen availability on flowering control in Arabidopsis. J. Exp. Bot. 2017, 68, 2603–2609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, T.; Lassaletta, L.; Mueller, N.D.; Tubiello, F.N.; Lisk, M.D.; Lu, C.; Conant, R.T.; Dorich, C.D.; Gerber, J.; et al. Quantification of global and national nitrogen budgets for crop production. Nat. Food 2021, 2, 529–540. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Zong, J.; Guo, H.; Li, J.; Wang, J.; Wang, H.; Li, L.; Chen, J. Integration of the metabolome and transcriptome reveals the mechanism of resistance to low nitrogen supply in wild bermudagrass (Cynodon dactylon (L.) Pers.) roots. BMC Plant Biol. 2021, 21, 480. [Google Scholar] [CrossRef]
- Zhou, T.; Hua, Y.; Yue, C.; Huang, J.; Zhang, Z. Physiologic, metabolomic, and genomic investigations reveal distinct glutamine and mannose metabolism responses to ammonium toxicity in allotetraploid rapeseed genotypes. Plant Sci. 2021, 310, 110963. [Google Scholar] [CrossRef]
- Chowdhury, N.B.; Schroeder, W.L.; Sarkar, D.; Amiour, N.; Quilleré, I.; Hirel, B.; Maranas, C.D.; Saha, R. Dissecting the metabolic reprogramming of maize root under nitrogen-deficient stress conditions. J. Exp. Bot. 2021, 73, 275–291. [Google Scholar] [CrossRef]
- Sun, C.; Hao, L.; Wang, D.; Li, C.; Zhang, C.; Chen, X.; Fu, J.; Zhang, Y. Nitrogen utilisation and metabolism in maize ( Zea mays L.) plants under different rates of biochar addition and nitrogen input conditions. Plant Biol. 2019, 21, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Guo, S.W.; Shinmachi, F.; Sunairi, M.; Noguchi, A.; Hasegawa, I.; Shen, R.F. Aluminium tolerance in rice is antagonistic with nitrate preference and synergistic with ammonium preference. Ann. Bot. 2012, 111, 69–77. [Google Scholar] [CrossRef]
- Qin, J.; Yue, X.; Fang, S.; Qian, M.; Zhou, S.; Shang, X.; Yang, W. Responses of nitrogen metabolism, photosynthetic parameter and growth to nitrogen fertilization inCyclocarya paliurus. For. Ecol. Manag. 2021, 502, 119715. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, B.; Hao, Y.; Liu, H.; Sun, G.; Chen, R.; Song, S. Appropriate NH4+/NO3− Ratio Triggers Plant Growth and Nutrient Uptake of Flowering Chinese Cabbage by Optimizing the pH Value of Nutrient Solution. Front. Plant Sci. 2021, 12, 656144. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional quality and health benefits of microgreens, a crop of modern agriculture. J. Futur. Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Hachiya, T.; Inaba, J.; Wakazaki, M.; Sato, M.; Toyooka, K.; Miyagi, A.; Kawai-Yamada, M.; Sugiura, D.; Nakagawa, T.; Kiba, T.; et al. Excessive ammonium assimilation by plastidic glutamine synthetase causes ammonium toxicity in Arabidopsis thaliana. Nat. Commun. 2021, 12, 4944. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Su, W.; Hao, Y.; Liu, H.; Sun, G.; Chen, R.; Song, S. Effect of Nitrate Concentration on the Growth, Bolting and Related Gene Expression in Flowering Chinese Cabbage. Agronomy 2021, 11, 936. [Google Scholar] [CrossRef]
- De Laporte, A.; Banger, K.; Weersink, A.; Wagner-Riddle, C.; Grant, B.; Smith, W. Economic and environmental consequences of nitrogen application rates, timing and methods on corn in Ontario. Agric. Syst. 2021, 188, 103018. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Răchișan, A.L.; Negrean, V.; Perné, M.-G.; Donca, V.I.; Alexescu, T.-G.; Para, I.; et al. The effects of favonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Khan, H.; Belwal, T.; Efferth, T.; Farooqi, A.A.; Sanches-Silva, A.; Vacca, R.A.; Nabavi, S.F.; Khan, F.; Devkota, H.P.; Barreca, D.; et al. Targeting epigenetics in cancer: Therapeutic potential of flavonoids. Crit. Rev. Food Sci. Nutr. 2020, 61, 1616–1639. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Stefanelli, D.; Goodwin, I.; Jones, R. Minimal nitrogen and water use in horticulture: Effects on quality and content of selected nutrients. Food Res. Int. 2010, 43, 1833–1843. [Google Scholar] [CrossRef]
- Becker, C.; Klaering, H.-P.; Kroh, L.W.; Krumbein, A. Cool-cultivated red leaf lettuce accumulates cyanidin-3-O-(6”-O-malonyl)-glucoside and caffeoylmalic acid. Food Chem. 2014, 146, 404–411. [Google Scholar] [CrossRef]
- Tavarini, S.; Sgherri, C.; Ranieri, A.M.; Angelini, L.G. Effect of Nitrogen Fertilization and Harvest Time on Steviol Glycosides, Flavonoid Composition, and Antioxidant Properties in Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 7041–7050. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Tamura, Y.; Mori, T.; Nakabayashi, R.; Kobayashi, M.; Saito, K.; Okazaki, S.; Wang, N.; Kusano, M. Metabolomic Evaluation of the Quality of Leaf Lettuce Grown in Practical Plant Factory to Capture Metabolite Signature. Front. Plant Sci. 2018, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Uraisami, K.; Kai, K.; Hayashi, E. Productivity: Definition and application. In Plant Factory Basics, Applications and Advances; Academic Press: Cambridge, MA, USA, 2021; pp. 197–216. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, W.; Yu, H.; Zheng, G. Development of eco-organic type soilless culture in Mainland China. Acta Hortic. 2004, 295–300. [Google Scholar] [CrossRef]
- Majid, M.; Khan, J.N.; Shah, Q.M.A.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of hydroponic systems for the cultivation of Lettuce (Lactuca sativa L., var. Longifolia) and comparison with protected soil-based cultivation. Agric. Water Manag. 2021, 245, 106572. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.; Gaspari, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Nutrient Supplementation Configures the Bioactive Profile and Production Characteristics of three Brassica Microgreens Species Grown in Peat-Based Media. Agronomy 2021, 11, 346. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Battistelli, A.; Moscatello, S.; Proietti, S.; Rea, E. Yield, water requirement, nutrient uptake and fruit quality of zucchini squash grown in soil and closed soilless culture. J. Hortic. Sci. Biotechnol. 2004, 79, 423–430. [Google Scholar] [CrossRef]
- Mouroutoglou, C.; Kotsiras, A.; Ntatsi, G.; Savvas, D. Impact of the Hydroponic Cropping System on Growth, Yield, and Nutrition of a Greek Sweet Onion (Allium cepa L.) Landrace. Horticulturae 2021, 7, 432. [Google Scholar] [CrossRef]
- Sardare, M.D.; Admane, S.V. A review on plant without soil-hydroponic. Int. J. Res. Eng. Tech. 2013, 2, 299–304. [Google Scholar]
- Alawiye, T.T.; Babalola, O.O. Metabolomics: Current application and prospects in crop production. Biologia 2021, 76, 227–239. [Google Scholar] [CrossRef]
- Valdés, A.; Álvarez-Rivera, G.; Socas-Rodríguez, B.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Foodomics: Analytical Opportunities and Challenges. Anal. Chem. 2021, 94, 366–381. [Google Scholar] [CrossRef]
- Razzaq, A.; Sadia, B.; Raza, A.; Hameed, M.K.; Saleem, F. Metabolomics: A Way Forward for Crop Improvement. Metabolites 2019, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gaquerel, E. Next-Generation Mass Spectrometry Metabolomics Revives the Functional Analysis of Plant Metabolic Diversity. Annu. Rev. Plant Biol. 2021, 72, 867–891. [Google Scholar] [CrossRef] [PubMed]
- Maguire, W.J.; Call, C.W.; Cerbu, C.; Jambor, K.L.; Benavides-Montes, V.E. Comprehensive Determination of Unregulated Pesticide Residues in Oregon Cannabis Flower by Liquid Chromatography Paired with Triple Quadrupole Mass Spectrometry and Gas Chromatography Paired with Triple Quadrupole Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 12670–12674. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- de Souza, L.P.; Alseekh, S.; Scossa, F.; Fernie, A.R. Ultra-high-performance liquid chromatography high-resolution mass spectrometry variants for metabolomics research. Nat. Methods 2021, 18, 733–746. [Google Scholar] [CrossRef]
- Letertre, M.P.M.; Dervilly, G.; Giraudeau, P. Combined Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry Approaches for Metabolomics. Anal. Chem. 2020, 93, 500–518. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Huang, K.; Liu, Y.; Liu, M.; Wang, J. Effect of LED Spectrum on the Quality and Nitrogen Metabolism of Lettuce Under Recycled Hydroponics. Front. Plant Sci. 2021, 12, 1159. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, X.; Li, K.; Dai, P.; Li, J.; Liang, B.; Sun, C.; Lin, X. Metabolomics analysis reveals potential mechanisms of phenolic accumulation in lettuce (Lactuca sativa L.) induced by low nitrogen supply. Plant Physiol. Biochem. 2021, 158, 446–453. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, R.; Han, Y.; Hao, J.; Liu, C.; Fan, S. Effects of different NO3−:NH4+ ratios on the photosynthesis and ultrastructure of lettuce seedlings. Hortic. Environ. Biotechnol. 2020, 61, 459–472. [Google Scholar] [CrossRef]
- Wang, N.; Fu, F.; Wang, H.; Wang, P.; He, S.; Shao, H.; Ni, Z.; Zhang, X. Effects of irrigation and nitrogen on chlorophyll content, dry matter and nitrogen accumulation in sugar beet (Beta vulgaris L.). Sci. Rep. 2021, 11, 16651. [Google Scholar] [CrossRef]
- Song, S.; Yi, L.; Zhu, Y.; Liu, H.; Sun, G.; Chen, R. Effects of ammonium and nitrate ratios on plant growth, nitrate concentration and nutrient uptake in flowering Chinese cabbage. Bangladesh J. Bot. 2017, 46, 1259–1267. [Google Scholar]
- Xing, S.; Wang, J.; Zhou, Y.; Bloszies, S.A.; Tu, C.; Hu, S. Effects of NH4+–N/NO3−–N ratios on photosynthetic characteristics, dry matter yield and nitrate concentration of spinach. Exp. Agric. 2015, 51, 151–160. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Dawuda, M.M.; Xie, J.; Yu, J.; Li, J.; Zhang, X.; Tang, C.; Wang, C.; Gan, Y. Appropriate Ammonium-Nitrate Ratio Improves Nutrient Accumulation and Fruit Quality in Pepper (Capsicum annuum L.). Agronomy 2019, 9, 683. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Gonnella, M.; Serio, F.; Santamaria, P. Effects of ammonium and nitrate nutrition on yield and quality in endive. J. Hortic. Sci. Biotechnol. 2008, 83, 64–70. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D. Nitrogen form and ratio impact Swiss chard (Beta vulgaris subsp. cicla) shoot tissue carotenoid and chlorophyll concentrations. Sci. Hortic. 2016, 204, 99–105. [Google Scholar] [CrossRef]
- Bybordi, A. Effects of Salinity and N on the Growth, Photosynthesis and N Status of Canola (Brassica napus L.). Not. Sci. Biol. 2010, 2, 92–97. [Google Scholar] [CrossRef][Green Version]
- Cao, Y.-W.; Qu, R.-J.; Miao, Y.-J.; Tang, X.-Q.; Zhou, Y.; Wang, L.; Geng, L. Untargeted liquid chromatography coupled with mass spectrometry reveals metabolic changes in nitrogen-deficient Isatis indigotica Fortune. Phytochemistry 2019, 166, 112058. [Google Scholar] [CrossRef]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato ( Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef]
- Harborne, J.; Williams, C. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Hu, L.; Li, Y.; Wu, Y.; Lv, J.; Dawuda, M.M.; Tang, Z.; Liao, W.; Calderón-Urrea, A.; Xie, J.; Yu, J. Nitric Oxide Is Involved in the Regulation of the Ascorbate–Glutathione Cycle Induced by the Appropriate Ammonium: Nitrate to Mitigate Low Light Stress in Brassica pekinensis. Plants 2019, 8, 489. [Google Scholar] [CrossRef]
- Page, M.; Sultana, N.; Paszkiewicz, K.; Florance, H.; Smirnoff, N. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: Further evidence for redox control of anthocyanin synthesis. Plant Cell Environ. 2011, 35, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Longo, L.; Vasapollo, G. Extraction and identification of anthocyanins from Smilax aspera L. berries. Food Chem. 2006, 94, 226–231. [Google Scholar] [CrossRef]
- Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. JU Chem. Technol. Metal. 2005, 40, 255–260. [Google Scholar]
- Wong, C.-C.; Li, H.-B.; Cheng, K.-W.; Chen, F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006, 97, 705–711. [Google Scholar] [CrossRef]
- Kopsell, D.E.; Kopsell, D.A.; Randle, W.M.; Coolong, T.W.; Sams, C.E.; Curran-Celentano, J. Kale Carotenoids Remain Stable while Flavor Compounds Respond to Changes in Sulfur Fertility. J. Agric. Food Chem. 2003, 51, 5319–5325. [Google Scholar] [CrossRef]
- Stewart, A.J.; Chapman, W.; Jenkins, G.I.; Graham, I.; Martin, T.; Crozier, A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell Environ. 2001, 24, 1189–1197. [Google Scholar] [CrossRef]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC Plant Biol. 2015, 15, 96. [Google Scholar] [CrossRef]
- Assefa, A.D.; Choi, S.; Lee, J.-E.; Sung, J.-S.; Hur, O.-S.; Ro, N.-Y.; Lee, H.-S.; Jang, S.-W.; Rhee, J.-H. Identification and quantification of selected metabolites in differently pigmented leaves of lettuce (Lactuca sativa L.) cultivars harvested at mature and bolting stages. BMC Chem. 2019, 13, 56. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, D.W.; Liu, D.H.; Geng, M.J.; Zhou, W.B.; Mi, W.J.; Yang, T.W.; Hamilton, D. Influence of nitrogen on the primary and secondary metabolism and synthesis of flavonoids in chrysanthemum morifolium ramat. J. Plant Nutr. 2010, 33, 240–254. [Google Scholar] [CrossRef]
- Radušienė, J.; Marksa, M.; Ivanauskas, L.; Jakštas, V.; Çalişkan, Ö.; Kurt, D.; Odabaş, M.S.; Çirak, C. Effect of nitrogen on herb production, secondary metabolites and antioxidant activities of Hypericum pruinatum under nitrogen application. Ind. Crop. Prod. 2019, 139, 111519. [Google Scholar] [CrossRef]
- Tilsner, J.; Kassner, N.; Struck, C.; Lohaus, G. Amino acid contents and transport in oilseed rape (Brassica napus L.) under different nitrogen conditions. Planta 2005, 221, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk-Wochniak, E.; Fernie, A.R. Metabolic profiling reveals altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. J. Exp. Bot. 2004, 56, 309–321. [Google Scholar] [CrossRef] [PubMed]
- McAllister, C.H.; Beatty, P.; Good, A.G. Engineering nitrogen use efficient crop plants: The current status. Plant Biotechnol. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef]
- Zhen, S.; Zhou, J.; Deng, X.; Zhu, G.; Cao, H.; Wang, Z.; Yan, Y. Metabolite profiling of the response to high-nitrogen fertilizer during grain development of bread wheat (Triticum aestivum L.). J. Cereal Sci. 2016, 69, 85–94. [Google Scholar] [CrossRef]
- Fetike, J.; Fernie, A.R. Intracellular and cell-to-apoplast compartmentation of carbohydrate metabolism. Trends Plant Sci. 2015, 20, 490–497. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, S.; Liang, Y.; Li, B.; Ma, S.; Wang, Z.; Ma, B.; Li, M. Nitrogen Levels Regulate Sugar Metabolism and Transport in the Shoot Tips of Crabapple Plants. Front. Plant Sci. 2021, 12, 626149. [Google Scholar] [CrossRef]
- De-Ávila-Silva, L.; Condori-Apfata, J.A.; Marcelino, M.M.; Tavares, A.C.A.; Raimundi, S.C.J.; Martino, P.B.; Araújo, W.L.; Zsögön, A.; Sulpice, R.; Nunes-Nesi, A. Nitrogen differentially modulates photosynthesis, carbon allocation and yield related traits in two contrasting Capsicum chinense cultivars. Plant Sci. 2019, 283, 224–237. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.C.; Gai, J.Y.; Zhu, Y.L.; Yang, L.F.; Wei, G.P. Effects of nitrogen forms on the growth, ascorbate-glutathione cycle and lipid peroxidation in developing seeds of vegetable soybean. Afr. J. Agric. Res. 2009, 4, 1178–1188. [Google Scholar]
- Caifeng, L.; Fengming, M.; Yue, Z.H.A.O.; Wenhua, L. Effects of nitrogen forms on key en-zyme activities and related products in sugar and nitrogen metabolism of sugar beet (Beta vulgaris L.). Zuo Wu Xue Bao 2003, 29, 128–132. [Google Scholar]
- Awad, M.A.; De-Jager, A. Relationships between fruit nutrients and concentrations of flavo-noids and chlorogenic acid in ‘Elstar’apple skin. Sci. Hortic. 2002, 92, 265–276. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. Integrative physiological, transcriptome, and metabolome analysis reveals the effects of nitrogen sufficiency and deficiency conditions in apple leaves and roots. Environ. Exp. Bot. 2021, 192, 104633. [Google Scholar] [CrossRef]
- Zhang, L.; Garneau, M.G.; Majumdar, R.; Grant, J.; Tegeder, M. Improvement of pea biomass and seed productivity by simultaneous increase of phloem and embryo loading with amino acids. Plant J. 2015, 81, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.P.; Tegeder, M. Connecting Source with Sink: The Role of Arabidopsis AAP8 in Phloem Loading of Amino Acids. Plant Physiol. 2016, 171, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Barneix, A.J.; Causin, H. The central role of amino acids on nitrogen utilization and plant growth. J. Plant Physiol. 1996, 149, 358–362. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Weigelt, K.; Küster, H.; Radchuk, R.; Müller, M.; Weichert, H.; Fait, A.; Fernie, A.R.; Saalbach, I.; Weber, H. Increasing amino acid supply in pea embryos reveals specific interactions of N and C metabolism, and highlights the importance of mitochondrial metabolism. Plant J. 2008, 55, 909–926. [Google Scholar] [CrossRef]
- Yang, L.; Cao, W.; Thorup-Kristensen, K.; Bai, J.; Gao, S.; Chang, D. Effect of Orychophrag-mus violaceus incorporation on nitrogen uptake in succeeding maize. Plant Soil Environ. 2015, 61, 260–265. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Improving Plant Nitrogen Use Efficiency through Alteration of Amino Acid Transport Processes. Plant Physiol. 2017, 175, 235–247. [Google Scholar] [CrossRef]
- Grant, J.E.; Ninan, A.; Cripps-Guazzone, N.; Shaw, M.; Song, J.; Petřík, I.; Novák, O.; Tegeder, M.; Jameson, P.E. Concurrent overexpression of amino acid permease AAP1(3a) and SUT1 sucrose transporter in pea resulted in increased seed number and changed cytokinin and protein levels. Funct. Plant Biol. 2021, 48, 889. [Google Scholar] [CrossRef]
- Bongue-Bartelsman, M.; Phillips, D.A. Nitrogen stress regulates gene expression of enzymes in the flavonoid biosynthetic pathway of tomato. Plant Physiol. Biochem. 1995, 33, 539–546. [Google Scholar]
- Aires, A.; Rosa, E.; Carvalho, R. Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts (Brassica oleracea var.italica). J. Sci. Food Agric. 2006, 86, 1512–1516. [Google Scholar] [CrossRef]
- Arnold, A.; Sajitz-Hermstein, M.; Nikoloski, Z. Effects of Varying Nitrogen Sources on Amino Acid Synthesis Costs in Arabidopsis thaliana under Different Light and Carbon-Source Conditions. PLoS ONE 2015, 10, e0116536. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; An, S.H.; Hwang, B.K. Pepper asparagine synthetase 1 (CaAS1) is required for plant nitrogen assimilation and defense responses to microbial pathogens. Plant J. 2011, 67, 749–762. [Google Scholar] [CrossRef]
- Oaks, A. A Re-Evaluation of Nitrogen Assimilation in Roots. BioScience 1992, 42, 103–111. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Beard, K.F.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Liu, M.; Liu, Y.; Strappe, P.; Sun, H.; Zhou, Z. Comparative non-targeted metabolomic analysis reveals insights into the mechanism of rice yellowing. Food Chem. 2020, 308, 125621. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; He, S.; Zhu, D.; Huang, Y.; Zhou, Q. Widely targeted metabolomics analysis reveals new biomarkers and mechanistic insights on chestnut (Castanea mollissima Bl.) calcification process. Food Res. Int. 2021, 141, 110128. [Google Scholar] [CrossRef]
- He, L.; Teng, L.; Tang, X.; Long, W.; Wang, Z.; Wu, Y.; Liao, L. Agro-morphological and metabolomics analysis of low nitrogen stress response in Axonopus compressus. AoB Plants 2021, 13, plab022. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Qin, Y.; Yan, H.; Jiang, X.; Qin, Y. Nitrogen deficiency maintains the yield and improves the antioxidant activity of Coreopsis tinctoria Nutt. Biosci. Biotechnol. Biochem. 2021, 85, 1492–1505. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. Involvement of Nitrogen on Flavonoids, Glutathione, Anthocyanin, Ascorbic Acid and Antioxidant Activities of Malaysian Medicinal Plant Labisia pumila Blume (Kacip Fatimah). Int. J. Mol. Sci. 2011, 13, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, B.; Ni, X.; He, Y. Ammonium/nitrate ratio affects the growth and glucosinolates content of pakchoi. Hortic. Bras. 2020, 38, 246–253. [Google Scholar] [CrossRef]
- Lin, Z.H.; Chen, C.S.; Zhong, Q.S.; Ruan, Q.C.; Chen, Z.H.; You, X.M.; Shan, R.Y.; Li, X.L. The GC-TOF/MS-based Metabolomic analysis reveals altered metabolic profiles in nitro-gen-deficient leaves and roots of tea plants (Camellia sinensis). BMC Plant Biol. 2021, 21, 506. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, C.; Van Elderen, C.W. Chemical analysis of peaty growing media by means of water extraction. Commun. Soil Sci. Plant Anal. 1994, 25, 3199–3208. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region: Third Edition; International Center for Agriculture Research in the Dry Area (ICARDA): Beirut, Lebanon, 2013; p. 244. [Google Scholar]
- Cerovic, Z.G.; Masdoumier, G.; Ben Ghozlen, N.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Du, H.; Wang, Z.; Yu, W.; Liu, Y.; Huang, B. Differential metabolic responses of perennial grass Cynodon transvaalensis × Cynodon dactylon (C4) and Poa Pratensis (C3) to heat stress. Physiol. Plant. 2010, 141, 251–264. [Google Scholar] [CrossRef]
- Abu-Reidah, I.; Contreras, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Reversed-phase ultra-high-performance liquid chromatography coupled to electrospray ionization-quadrupole-time-of-flight mass spectrometry as a powerful tool for metabolic profiling of vegetables: Lactuca sativa as an example of its application. J. Chromatogr. A 2013, 1313, 212–227. [Google Scholar] [CrossRef]
- Bijlsma, L.; Bade, R.; Celma, A.; Mullin, L.; Cleland, G.; Stead, S.; Hernandez, F.; Sancho, J.V. Prediction of Collision Cross-Section Values for Small Molecules: Application to Pesticide Residue Analysis. Anal. Chem. 2017, 89, 6583–6589. [Google Scholar] [CrossRef]



| Parameters (Units) | Soil Values | Substrate Values |
|---|---|---|
| pH | 6.5 ± 0.02 | 5.7 ± 0.04 |
| ECe (dS m−1) | 1.54 ± 0.23 | 0.83 ± 0.19 |
| Organic matter content (g kg−1) | 33.5 ± 0.43 | 488.3 ± 1.14 |
| Total nitrogen (g 100g−1) | 0.19 ± 0.11 | 0.33 ± 0.05 |
| Ammonium (mg kg−1) | 42.8 ± 0.29 | 38 ± 0.12 |
| Nitrate (mg kg−1) | 225.9 ± 1.43 | 379.5 ± 1.03 |
| Potassium (mg kg−1) | 22530 ± 20.67 | 3512 ± 5.82 |
| Phosphorus (mg kg−1) | 1297 ± 15.76 | 260.2 ± 1.35 |
| Calcium (mg kg−1) | 9332 ± 35.05 | 6528 ± 9.41 |
| Magnesium (mg kg−1) | 8411 ± 12.24 | 6274 ± 15.65 |
| Textural class | Silt Loam | |
| Sand (%) | 11.91 ± 0.90 | |
| Clay (%) | 12.67 ± 0.55 | |
| Silt (%) | 75.42 ± 0.81 |
| NH4+/NO3− | 100/0% | 75/25% | 50/50% | 25/75% | 0/100% | Control |
|---|---|---|---|---|---|---|
| Soil (mg Kg−1) | 306.6/0 | 229.95/35.32 | 153.3/70.65 | 76.65/105.97 | 0/141.3 | 0 |
| Hydroponic (mmol L−1) | 5/0 | 3.75/1.25 | 2.5/2.5 | 1.25/3.75 | 5 | 0 |
| Substrate (mmol L−1) | 5/0 | 3.75/1.25 | 2.5/2.5 | 1.25/3.75 | 5 | 0 |
| Other nutrients applied in hydroponic system (mmol L−1) | ||||||
| K | 1 | |||||
| P | 0.25 | |||||
| Mg | 0.36 | |||||
| Ca | 0.15 | |||||
| Cu | 0.016 | |||||
| Zn | 0.015 | |||||
| Mn | 0.01 | |||||
| Fe | 0.008 | |||||
| B | 0.002 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, M.K.; Umar, W.; Razzaq, A.; Aziz, T.; Maqsood, M.A.; Wei, S.; Niu, Q.; Huang, D.; Chang, L. Differential Metabolic Responses of Lettuce Grown in Soil, Substrate and Hydroponic Cultivation Systems under NH4+/NO3− Application. Metabolites 2022, 12, 444. https://doi.org/10.3390/metabo12050444
Hameed MK, Umar W, Razzaq A, Aziz T, Maqsood MA, Wei S, Niu Q, Huang D, Chang L. Differential Metabolic Responses of Lettuce Grown in Soil, Substrate and Hydroponic Cultivation Systems under NH4+/NO3− Application. Metabolites. 2022; 12(5):444. https://doi.org/10.3390/metabo12050444
Chicago/Turabian StyleHameed, Muhammad Khalid, Wajid Umar, Ali Razzaq, Tariq Aziz, Muhammad Aamer Maqsood, Shiwei Wei, Qingliang Niu, Danfeng Huang, and Liying Chang. 2022. "Differential Metabolic Responses of Lettuce Grown in Soil, Substrate and Hydroponic Cultivation Systems under NH4+/NO3− Application" Metabolites 12, no. 5: 444. https://doi.org/10.3390/metabo12050444
APA StyleHameed, M. K., Umar, W., Razzaq, A., Aziz, T., Maqsood, M. A., Wei, S., Niu, Q., Huang, D., & Chang, L. (2022). Differential Metabolic Responses of Lettuce Grown in Soil, Substrate and Hydroponic Cultivation Systems under NH4+/NO3− Application. Metabolites, 12(5), 444. https://doi.org/10.3390/metabo12050444







