Untargeted Metabolomics Identifies a Novel Panel of Markers for Autologous Blood Transfusion
Abstract
1. Introduction
2. Results
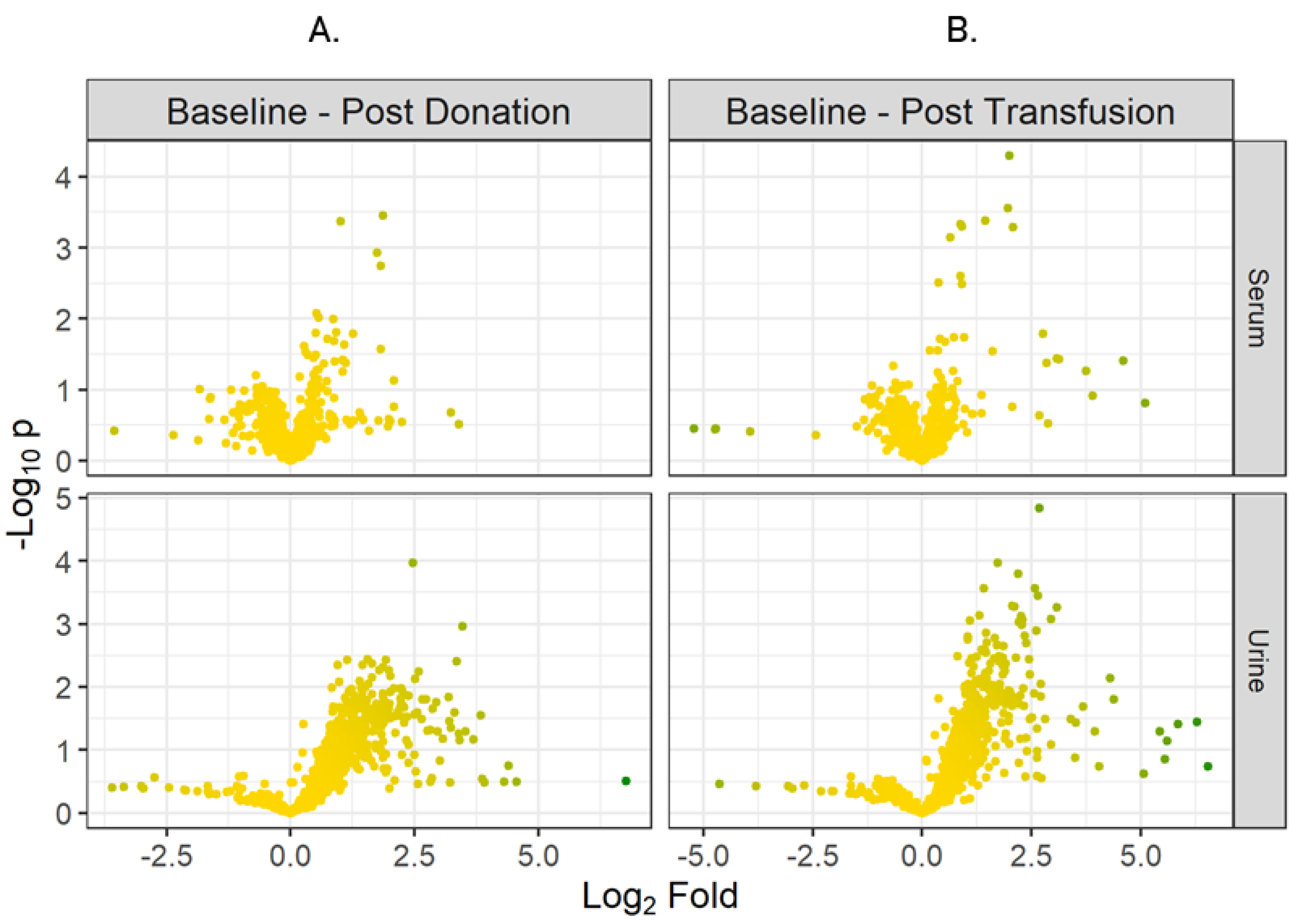
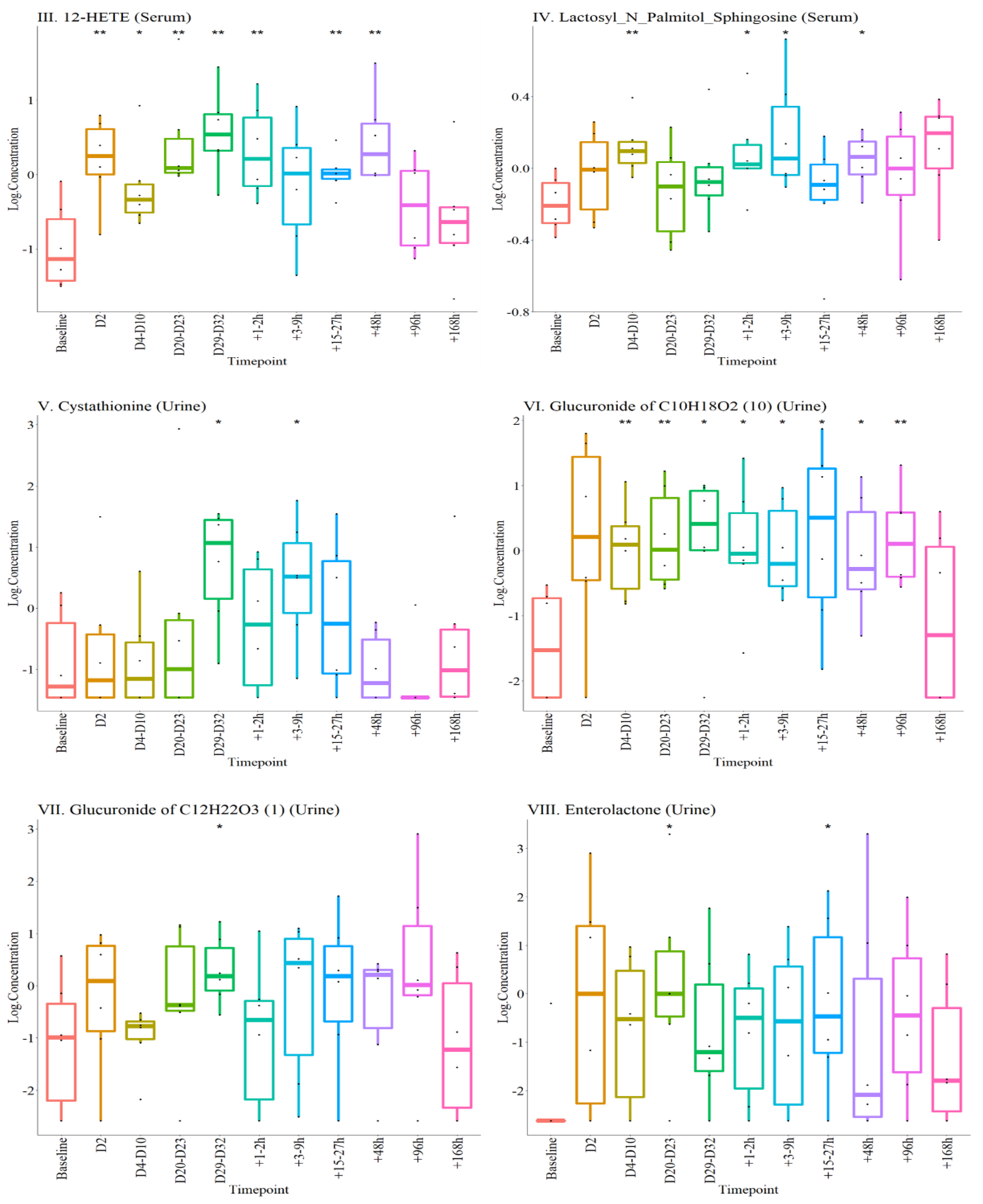
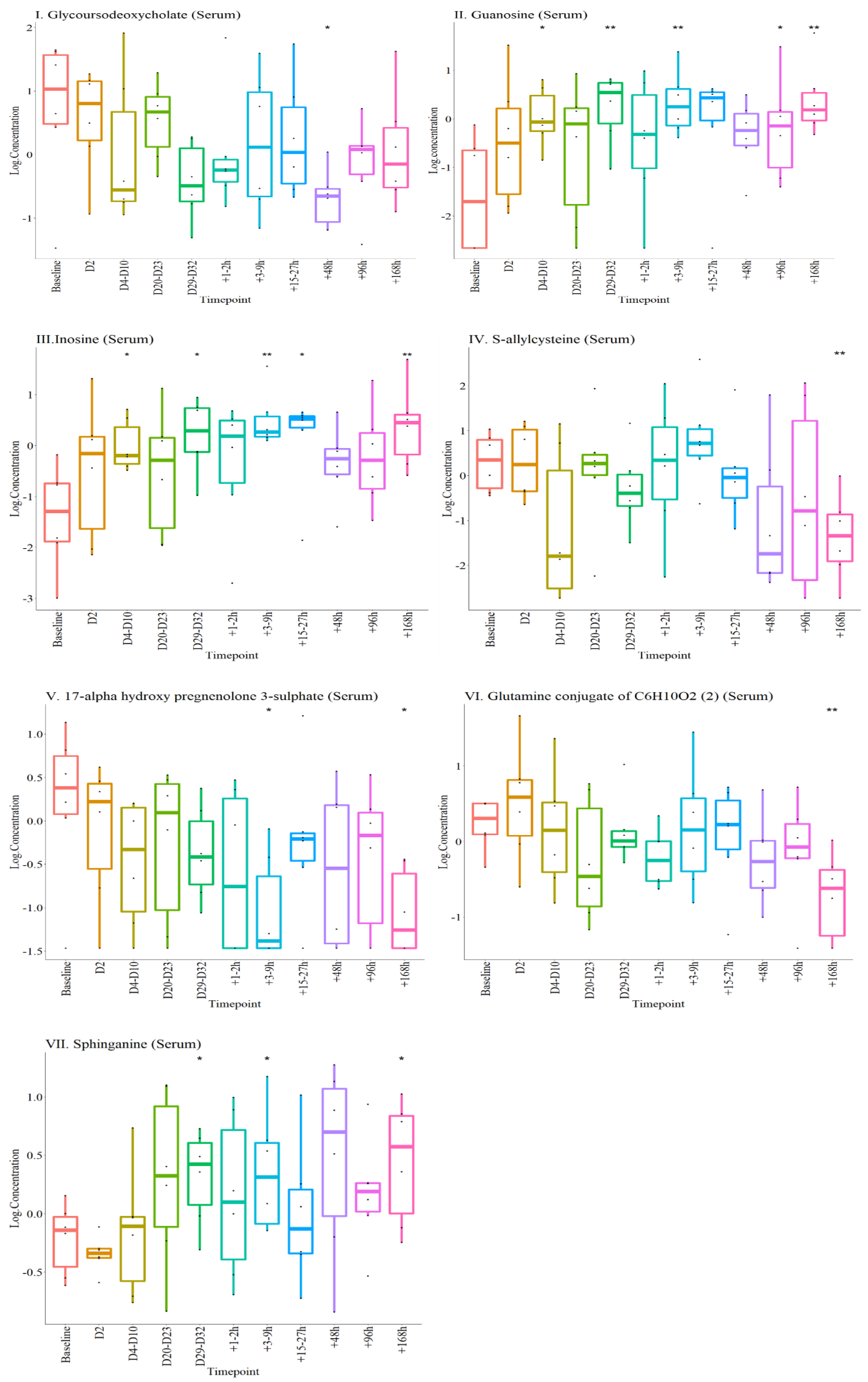
2.1. Biomarker Discovery
2.2. Plasticizers
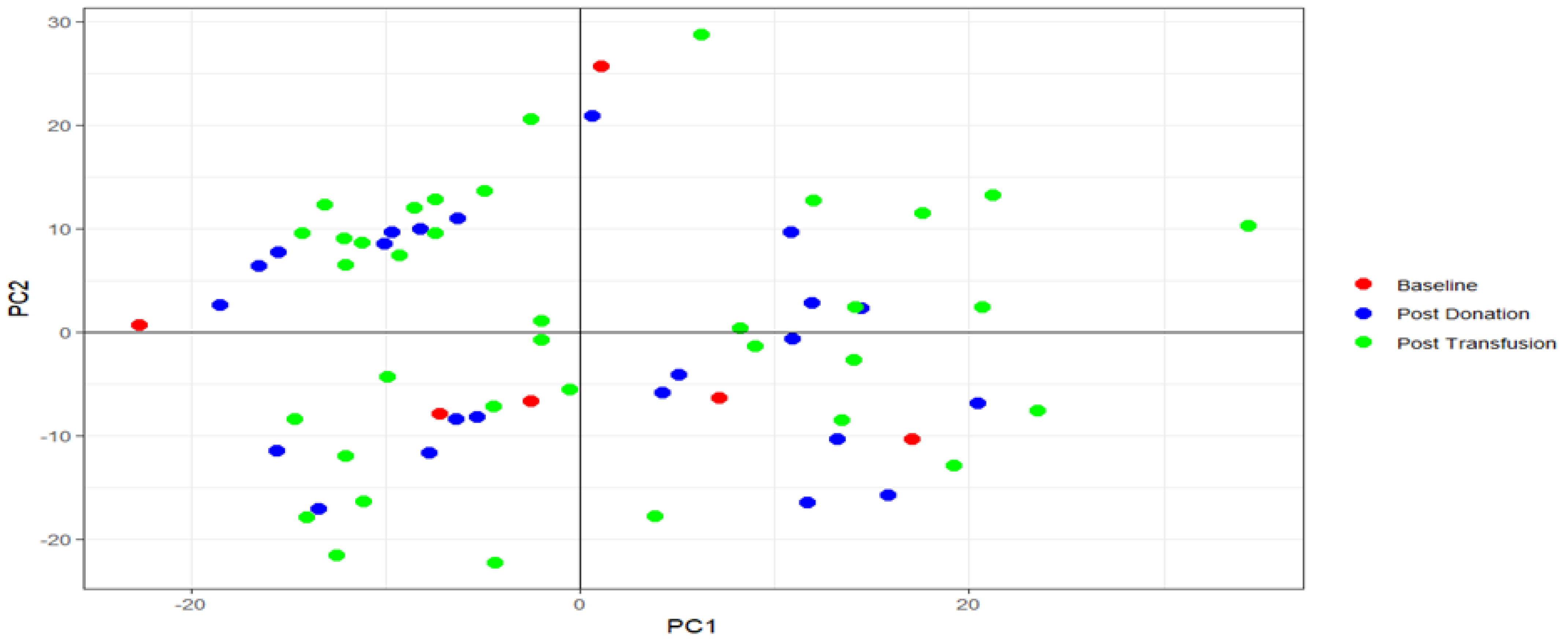
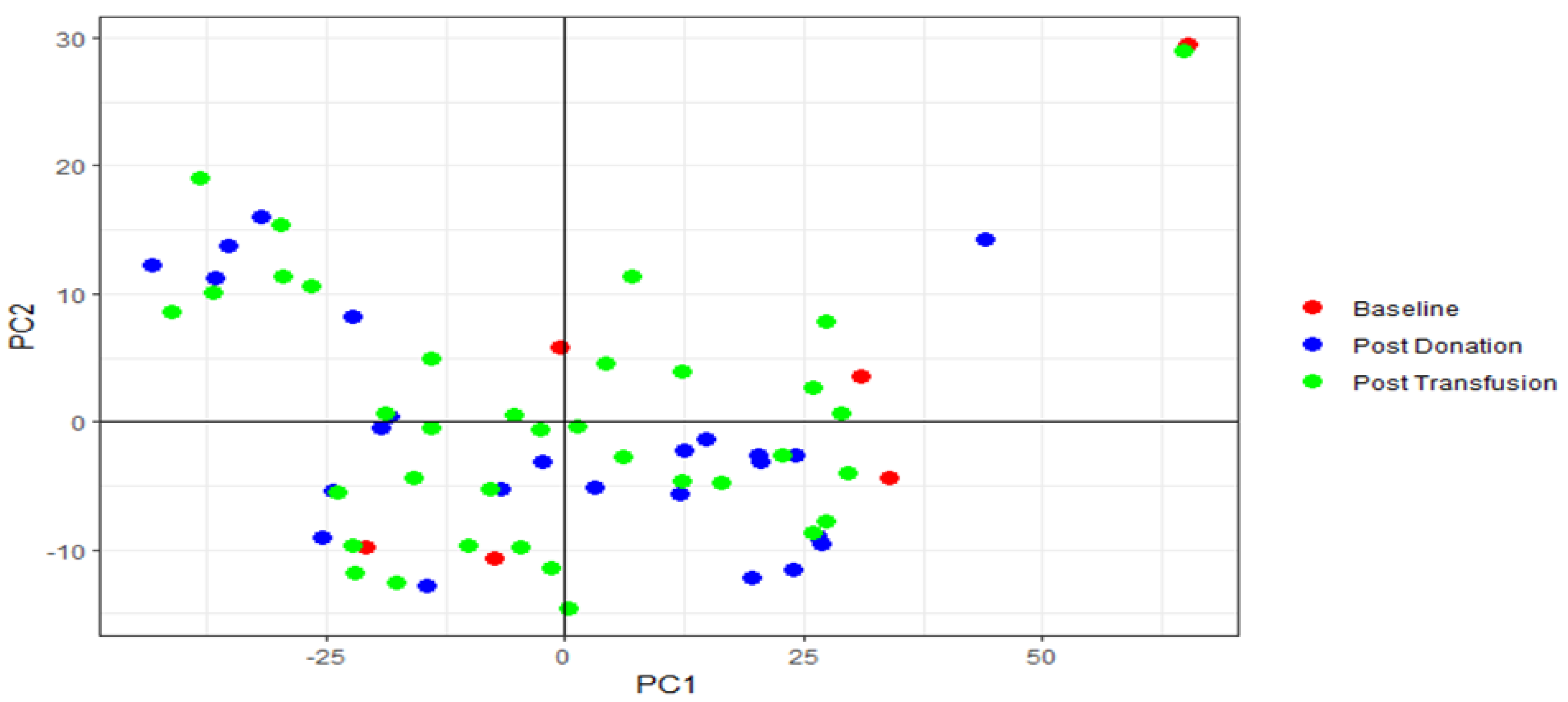
2.3. PCA Analysis
2.4. Panel Identification
2.4.1. Metabolites Altered during the Experiment
2.4.2. Selected Metabolite Panel
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Study Participants
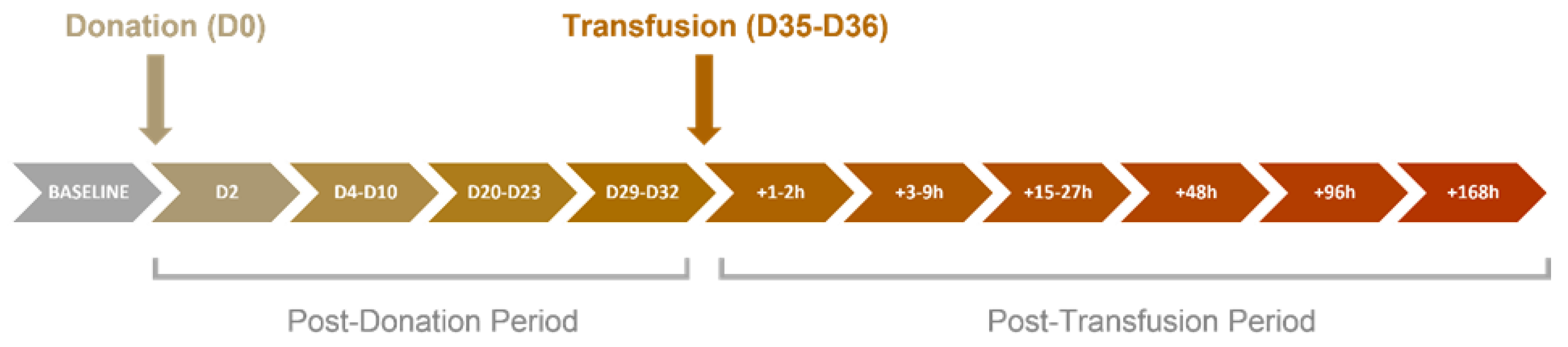
4.3. Study Design
4.4. Blood and Urine Sampling
4.5. Blood Donation and Storage procedure
4.6. Blood Transfusion
4.7. Sample Analysis
4.8. Validation of Metabolomics Method
4.9. Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectroscopy (UPLC-MS/MS)
4.10. Data Extraction and Compound Identification
4.11. Statistics and Bioinformatics
4.11.1. Volcano Plots
4.11.2. Principal Component Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkinson, T.; Kahn, M. Blood doping: Then and now. A narrative review of the history, science and efficacy of blood doping in elite sport. Blood Rev. 2020, 39, 100632. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Banfi, G. Blood transfusions in athletes. Old dogmas, new tricks. Clin. Chem. Lab. Med. 2006, 44, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Tunstall Pedoe, D. Blood doping—A literature review. Br. J. Sports Med. 1989, 23, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, N. Blood doping and related issues. Med. Sci. Sports Exerc. 1982, 14, 183–189. [Google Scholar] [CrossRef]
- Solheim, S.; Bejder, J.; Breenfeldt Andersen, A.; Mørkeberg, J.; Nordsborg, N. Autologous Blood Transfusion Enhances Exercise Performance—Strength of the Evidence and Physiological Mechanisms. Sports Med. Open 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Spriet, L.L.; Gledhill, N.; Froese, A.B.; Wilkes, D.L. Effect of graded erythrocythemia on cardiovascular and metabolic responses to exercise. J. Appl. Physiol. 1986, 61, 1942–1948. [Google Scholar] [CrossRef]
- Sawka, M.N.; Dennis, R.C.; Gonzalez, R.R.; Young, A.J.; Muza, S.R.; Martin, J.W.; Wenger, C.B.; Francesconi, R.P.; Pandolf, K.B.; Valeri, C.R. Influ- ence of polycythaemia on blood volume and ther-moregulation during exercise-heat stress. J. Appl. Physiol. 1987, 62, 912–918. [Google Scholar] [CrossRef]
- What is Prohibited? World Anti-Doping Agency. 2021. Available online: https://www.wada-ama.org/en/content/what-is-prohibited/prohibited-at-all-times/manipulation-of-blood-and-blood-components (accessed on 26 April 2021).
- Nelson, M.; Popp, H.; Sharpe, K.; Ashenden, M. Proof of homologous blood transfusion through quantification of blood group antigens. Haematologica 2003, 88, 1284–1295. [Google Scholar]
- Voss, S.; Thevis, M.; Schinkothe, T.; Schänzer, W. Detection of Homologous Blood Transfusion. Int. J. Sports Med. 2007, 28, 633–637. [Google Scholar] [CrossRef]
- Donati, F.; Stampella, A.; de la Torre, X.; Botrè, F. Investigation on the application of DNA forensic human identification techniques to detect homologous blood transfusions in doping control. Talanta 2013, 110, 28–31. [Google Scholar] [CrossRef]
- Stampella, A.; Di Marco, S.; Pirri, D.; de la Torre, X.; Botrè, F.; Donati, F. Application of DNA-based forensic analysis for the detection of homologous transfusion of whole blood and of red blood cell concentrates in doping control. Forensic Sci. Int. 2016, 265, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J. A review of the application of autologous blood transfusion. Braz. J. Med. Biol. Res. 2016, 49, e5493. [Google Scholar] [CrossRef]
- Goodnough, L.; Brecher, M. Autologous Blood Transfusion. Intern. Med. 1998, 37, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Sottas, P.; Vernec, A. Current implementation and future of the Athlete Biological Passport. Bioanalysis 2012, 4, 1645–1652. [Google Scholar] [CrossRef]
- Lobigs, L.; Sottas, P.; Bourdon, P.; Nikolovski, Z.; El-Gingo, M.; Varamenti, E.; Peeling, P.; Dawson, B.; Schumacher, Y.O. A step towards removing plasma volume variance from the Athlete’s Biological Passport: The use of biomarkers to describe vascular volumes from a simple blood test. Drug Test. Anal. 2017, 10, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Bejder, J.; Hoffmann, M.; Ashenden, M.; Nordsborg, N.; Karstoft, K.; Mørkeberg, J. Acute hyperhydration reduces athlete biological passport OFF-hr score. Scand. J. Med. Sci. Sports 2015, 26, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Bonne, T.; Lundby, C.; Lundby, A.; Sander, M.; Bejder, J.; Nordsborg, N. Altitude training causes haematological fluctuations with relevance for the Athlete Biological Passport. Drug Test. Anal. 2014, 7, 655–662. [Google Scholar] [CrossRef]
- Voss, S.C.; Al-Hamad, K.; Samsam, W.; Cherif, A.; Georgakopoulos, C.; Al Maadheed, M.; Balanos, G.; Lucas, S.; Sottas, P.; Wilson, M.; et al. A novel mixed living high training low intervention and the hematological module of the athlete biological passport. Drug Test. Anal. 2019, 12, 323–330. [Google Scholar] [CrossRef]
- Voss, S.C.; Jaganjac, M.; Al-Thani, A.M.; Grivel, J.; Raynaud, C.M.; Al-Jaber, H.; Al-Menhali, A.S.; Merenkov, Z.A.; Alsayrafi, M.; Latiff, A.; et al. Analysis of RBC-microparticles in stored whole blood bags—A promising marker to detect blood doping in sports? Drug Test. Anal. 2017, 9, 1794–1798. [Google Scholar] [CrossRef]
- Voss, S.; Yassin, M.; Grivel, J.; Al Hmissi, S.; Allahverdi, N.; Nashwan, A.; Merenkov, Z.; Abdulla, M.; Al Malki, A.; Raynaud, C.; et al. Red blood cell derived extracellular vesicles during the process of autologous blood doping. Drug Test. Anal. 2021; Epub ahead of print. [Google Scholar] [CrossRef]
- Pottgiesser, T.; Schumacher, Y.; Funke, H.; Rennert, K.; Baumstark, M.; Neunuebel, K.; Mosig, S. Gene expression in the detection of autologous blood transfusion in sports—A pilot study. Vox Sang. 2009, 96, 333–336. [Google Scholar] [CrossRef]
- Gasparello, J.; Lamberti, N.; Papi, C.; Lampronti, I.; Cosenza, L.; Fabbri, E.; Bianchi, N.; Zambon, C.; Corte, F.D.; Govoni, M.; et al. Altered erythroid-related miRNA levels as a possible novel biomarker for detection of autologous blood transfusion misuse in sport. Transfusion 2019, 59, 2709–2721. [Google Scholar] [CrossRef] [PubMed]
- Mussack, V.; Wittmann, G.; Pfaffl, M. On the trail of blood doping—microRNA fingerprints to monitor autologous blood transfusions in vivo. Am. J. Hematol. 2021, 96, 338–353. [Google Scholar] [CrossRef]
- Salamin, O.; Mignot, J.; Kuuranne, T.; Saugy, M.; Leuenberger, N. Transcriptomic biomarkers of altered erythropoiesis to detect autologous blood transfusion. Drug Test. Anal. 2017, 10, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Nikolovski, Z.; De La Torre, C.; Chiva, C.; Borràs, E.; Andreu, D.; Ventura, R.; Egura, J. Alterations of the erythrocyte membrane proteome and cytoskeleton network during storage—A possible tool to identify autologous blood transfusion. Drug Test. Anal. 2012, 4, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Salamin, O.; De Angelis, S.; Tissot, J.; Saugy, M.; Leuenberger, N. Autologous Blood Transfusion in Sports: Emerging Biomarkers. Transfus. Med. Rev. 2016, 30, 109–115. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Reisz, J.; Zhang, Y.; Gehrke, S.; Alexander, K.; Kanias, T.; Triulzi, D.J.; Donadee, C.; Barge, S.; Badlam, J.; et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019, 3, 884–896. [Google Scholar] [CrossRef]
- Bejder, J.; Gürdeniz, G.; Cuparencu, C.; Hall, F.; Gybel-Brask, M.; Andersen, A.B.; Dragsted, L.O.; Secher, N.H.; Johansson, P.I.; Nordsborg, N.B. An Untargeted Urine Metabolomics Approach for Autologous Blood Transfusion Detection. Med. Sci. Sports Exerc. 2020, 53, 236–243. [Google Scholar] [CrossRef]
- Fromme, H.; Bolte, G.; Koch, H.M.; Angerer, J.; Boehmer, S.; Drexler, H.; Mayer, R.; Liebl, B. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int. J. Hyg. Environ. Health 2007, 210, 21–33. [Google Scholar] [CrossRef]
- Preau, J.; Wong, L.; Silva, M.; Needham, L.; Calafat, A. Variability over 1 Week in the Urinary Concentrations of Metabolites of Diethyl Phthalate and Di(2-Ethylhexyl) Phthalate among Eight Adults: An Observational Study. Environ. Health Perspect. 2010, 118, 1748–1754. [Google Scholar] [CrossRef]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R Soc. Lond B Biol. Sci. 2009, 364, 2097–2113. [Google Scholar] [CrossRef]
- Basu, D.; Kulkarni, R. Overview of blood components and their preparation. Indian J. Anaesth. 2014, 58, 529. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Sigurjónsson, Ó.; Bordbar, A.; Rolfsson, Ó.; Magnusdottir, M.; Palsson, S.; Wichuk, K.; Gudmundsson, S.; Palsson, B. Metabolic fate of adenine in red blood cells during storage in SAGM solution. Transfusion 2016, 56, 2538–2547. [Google Scholar] [CrossRef]
- Howie, H.; Hay, A.; de Wolski, K.; Waterman, H.; Lebedev, J.; Fu, X.; Culp-Hill, R.; D’Alessandro, A.; Gorham, J.; Ranson, M.; et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019, 3, 2272–2285. [Google Scholar] [CrossRef]
- Roback, J.; Josephson, C.; Waller, E.; Newman, J.; Karatela, S.; Uppal, K.; Jones, D.; Zimring, J.; Dumont, L. Metabolomics of ADSOL (AS-1) Red Blood Cell Storage. Transfus. Med. Rev. 2014, 28, 41–55. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 135398635, Guanosine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Guanosine (accessed on 6 October 2021).
- Doyle, C.; Cristofaro, V.; Sullivan, M.P.; Adam, R.M. Inosine—A Multifunctional Treatment for Complications of Neurologic Injury. Cell Physiol. Biochem. 2018, 49, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Al-Khallaf, H. Isocitrate dehydrogenases in physiology and cancer: Biochemical and molecular insight. Cell Biosci. 2017, 7, 37. [Google Scholar] [CrossRef]
- Benjamins, J.A.; Hajra, A.K.; Agranoff, B.W. Lipids. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 7th ed.; George, J., Siegel, R., Albers, W., Brady, S.T., Price, D.L., Eds.; Elservier Academic Press: London, UK, 2006; p. 44. [Google Scholar]
- Riepe, F.G.; Mahler, P.; Sippell, W.G.; Partsch, C.J. Longitudinal study of plasma pregnenolone and 17-hydroxypregnenolone in full-term and preterm neonates at birth and during the early neonatal period. J. Clin. Endocrinol. Metab. 2002, 87, 4301–4306. [Google Scholar] [CrossRef]
- Ford, L.; Kennedy, A.D.; Goodman, K.D.; Pappan, K.L.; Evans, A.M.; Miller, L.A.D.; Wulff, J.E.; Wiggs, B.R.; Lennon, J.J.; Elsea, S.; et al. Precision of a Clinical Metabolomics Profiling Platform for Use in the Identification of Inbor, n Errors of Metabolism. J. Appl. Lab. Med. 2020, 5, 342–356. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- Al-Khelaifi, F.; Diboun, I.; Donati, F.; Botrè, F.; Alsayrafi, M.; Georgakopoulos, C.; Suhre, K.; Yousri, N.A.; Elrayess, M.A. A pilot study comparing the metabolic profiles of elite-level athletes from different sporting disciplines. Sports Med. Open 2018, 4, 2. [Google Scholar] [CrossRef]
- DeHaven, C.D.; Evans, J.M.; Dai, H.; Lawton, K.A. Software Techniques for Enabling High-Throughput Analysis of Metabolomic Datasets. In Metabolomics; Ute, R., Ed.; InTech: London, UK, 2012; Chapter 7. [Google Scholar] [CrossRef]
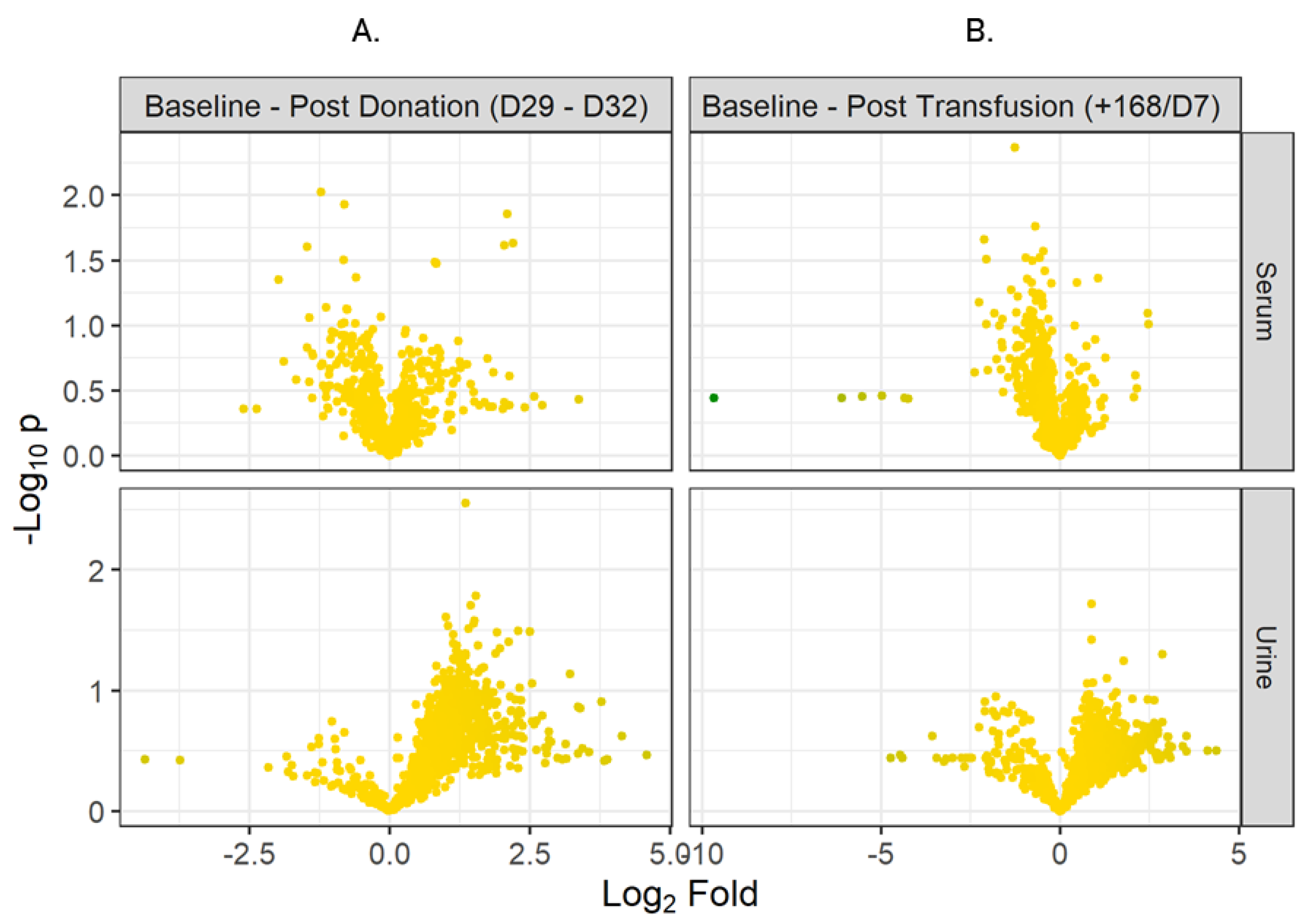



| Baseline—Post Donation (D29–D32) | p | Fold |
|---|---|---|
| Monoethyl phthalate O-beta-D-glucuronide | 0.31 | 4.12 |
| Monobutyl phthalate acyl-beta-D-glucuronide | 0.10 | 5.01 |
| Baseline—Post Transfusion (+168 h/D7) | ||
| Monoethyl phthalate O-beta-D-glucuronide | 0.35 | 3.19 |
| Monobutyl phthalate acyl-beta-D-glucuronide | 0.31 | 5.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Nesf, A.; Mohamed-Ali, N.; Acquaah, V.; Al-Jaber, M.; Al-Nesf, M.; Yassin, M.A.; Orie, N.N.; Voss, S.C.; Georgakopoulos, C.; Bhatt, R.; et al. Untargeted Metabolomics Identifies a Novel Panel of Markers for Autologous Blood Transfusion. Metabolites 2022, 12, 425. https://doi.org/10.3390/metabo12050425
Al-Nesf A, Mohamed-Ali N, Acquaah V, Al-Jaber M, Al-Nesf M, Yassin MA, Orie NN, Voss SC, Georgakopoulos C, Bhatt R, et al. Untargeted Metabolomics Identifies a Novel Panel of Markers for Autologous Blood Transfusion. Metabolites. 2022; 12(5):425. https://doi.org/10.3390/metabo12050425
Chicago/Turabian StyleAl-Nesf, Amna, Nada Mohamed-Ali, Vanessa Acquaah, Maneera Al-Jaber, Maryam Al-Nesf, Mohamed A. Yassin, Nelson N Orie, Sven Christian Voss, Costas Georgakopoulos, Rikesh Bhatt, and et al. 2022. "Untargeted Metabolomics Identifies a Novel Panel of Markers for Autologous Blood Transfusion" Metabolites 12, no. 5: 425. https://doi.org/10.3390/metabo12050425
APA StyleAl-Nesf, A., Mohamed-Ali, N., Acquaah, V., Al-Jaber, M., Al-Nesf, M., Yassin, M. A., Orie, N. N., Voss, S. C., Georgakopoulos, C., Bhatt, R., Beotra, A., Mohamed-Ali, V., & Al-Maadheed, M. (2022). Untargeted Metabolomics Identifies a Novel Panel of Markers for Autologous Blood Transfusion. Metabolites, 12(5), 425. https://doi.org/10.3390/metabo12050425








