Abstract
The enteroendocrine system of the gut regulates energy homeostasis through the release of hormones. Of the gut-derived hormones, GLP-1 is particularly interesting, as analogs of the hormone have proven to be highly effective for the treatment of type 2 diabetes mellitus and obesity. Observations on increased levels of GLP-1 following gastric bypass surgery have enhanced the interest in endogenous hormone secretion and highlighted the potential of endogenous secretion in therapy. The macronutrients and their digestive products stimulate the secretion of GLP-1 through various mechanisms that we have only begun to understand. From findings obtained from different experimental models, we now have strong indications for a role for both Sodium-Glucose Transporter 1 (SGLT1) and the K+ATP channel in carbohydrate-induced GLP-1 secretion. For fat, the free fatty acid receptor FFA1 and the G-protein-coupled receptor GPR119 have been linked to GLP-1 secretion. For proteins, Peptide Transporter 1 (Pept1) and the Calcium-Sensing Receptor (CaSR) are thought to mediate the secretion. However, attempts at clinical application of these mechanisms have been unsuccessful, and more work is needed before we fully understand the mechanisms of nutrient-induced GLP-1 secretion.
Keywords:
GLP-1; incretins; nutrient sensors; enteroendocrine system; L-cells; glucose homeostasis; gastric bypass; T2DM 1. Introduction
1.1. The Endocrine Intestine
Energy metabolism is a highly regulated process that depends on the signaling between various systems and organs. Essential for the signaling are the gut-derived hormones secreted by the enteroendocrine cells (EECs) of the gastrointestinal system. The EECs are scattered along the entire intestine, alongside absorptive epithelial cells and mucus-secreting cells. Although the enteroendocrine cells (EECs) account for a minor proportion of the total number of intestinal cells, the enteroendocrine system has been described as the largest endocrine organ of the body [1]. The EECs express an impressive number of hormones involved in everything from digestion, gastric motility, gastric emptying, glucose homeostasis, and appetite regulation. Nutrients and non-nutritious compounds stimulate the release of these hormones directly through sensory transporters and receptors or indirectly through their effects on cellular metabolism. Aside from the endocrine action, recent years of research have revealed a potential role for gut-derived hormones in the local activation of intestinal vagal afferent neurons [2,3,4], which could be important for regulating food intake through direct signaling with the central nervous system. This illustrates the complexity of the enteroendocrine system. Indeed, we have only begun to understand the importance of the system in energy homeostasis. Traditionally, EECs have been characterized based on their main secretory products. According to this classification, gastrin is secreted by G-cells, somatostatin by D-cells, cholecystokinin (CCK) by I-cells, secretin by S-cells, glucose-dependent insulinotropic polypeptide (GIP) by K-cells, and glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) are secreted by L-cells. It is now evident that this classification is overly simplified. Several studies have demonstrated the co-expression of hormones traditionally associated with distinct EECs [5,6,7]. Likewise, the ability of individual EECs to alter their hormonal expression profiles, depending on the signaling gradients, has been demonstrated in vitro [8]. Although there is consensus regarding the flaws of the traditional classification, it is commonly used in the literature, as a more precise alternative is missing.
1.2. The Therapeutic Potential of GLP-1 and the Nutrient-Sensing L-Cell
Of all the gut-derived hormones, GLP-1 has possibly been subject to the most interest and attention since it was first identified and described more than 30 years ago [9,10,11,12,13,14,15,16,17,18,19,20,21]. The interest in the hormone emerged from the discovery of the critical role of GLP-1 in glycemic control, where GLP-1 functions as an incretin and thus potentiates the glucose-induced insulin response [16,17,20]. Aside from the crucial role of GLP-1 in glucose homeostasis, the hormone is involved in appetite regulation [22], and it has been demonstrated to slow the rate of gastric emptying [23]. The discovery of GLP-1 receptors on intestinal and pancreatic vagal afferent neurons [2,3,4] revealed a direct route of transmission between the gut, pancreas, and brain. This gut–brain–pancreas relationship is a key element in the role of GLP-1 in regulating eating behavior and highlights the remarkable extent of action of the hormone. Through the combination of these properties, GLP-1 has become important in the pharmacological treatment of type 2 diabetes mellitus [24] and, in more recent years, obesity [25]. Initially, the application of GLP-1 as a therapeutic agent was restricted by its rapid rate of degradation, mediated by the enzyme dipeptidyl peptidase-4 (DPP4) [26]. This challenge was overcome by developing resistant, synthetic GLP-1 receptor analogs (GLP1RAs) and DDP4-inhibitors, which are now routinely used in the management of type 2 diabetes [24].
In recent years, there has been increasing interest in targeting endogenous GLP-1 secretion as an alternative or supplement to the existing GLP1RAs. This idea is based on the evidence of a correlation between increased levels of gut-derived hormones and weight loss, as well as the improved glycemic control seen after gastric bypass surgery [27,28,29,30]. The Roux-en-Y gastric bypass (RYGB) causes sustainable weight loss and rapid remission of type 2 diabetes in the majority of operated patients [27,31]. Targeting endogenous EEC secretion might therefore be expected to create bypass-like hypersecretion of gut hormones for the treatment of obesity and type 2 diabetes without surgery. There are several potential benefits to targeting endogenous GLP-1 secretion as opposed to treatments with GLP-1Ras. First, a part of the appetite- and blood glucose-lowering effect of GLP-1 is thought to involve the activation of local sensory nerves of the gut–brain axis (and probably reaching high local concentrations), whereas GLP-1Ras probably mainly act via leaks in the blood–brain barrier. Additionally, selectively targeting the mechanisms controlling L-cell secretion will result in the release of not only GLP-1 but also co-secreted hormones, namely PYY, oxyntomodulin, and perhaps also CCK, GIP, and neurotensin [5,6,7]. These hormones likewise act to inhibit appetite and are insulinotropic, thereby enhancing the insulinotropic and appetite-reducing effect. Finally, as targeting the endogenous secretion reflects normal physiology more closely, it may be possible to avoid the side effects of GLP1-RAs (nausea, vomiting, and diarrhea) affecting some patients [24].
To utilize natural GLP-1 secretion in therapy, a detailed understanding of the molecular mechanisms underlying its secretion is essential. In rats, pigs, and humans, the tissue concentration of GLP-1 peaks in the distal small intestine [32,33], whereas mice have a higher concentration in the colon [33]. It is well-established that GLP-1 is released after the ingestion of a mixed meal [34], with the different components of the food stimulating secretion through distinct mechanisms. Studies in mice have demonstrated that a mixed meal challenge induces more GLP-1 secretion compared with isocaloric glucose [35]. Thus, the presence of all macronutrients may synergistically enhance GLP-1 secretion. Even though the L-cell density is generally greater in the distal part of the intestinal system [32,33], L-cells of the proximal intestine are presumably more important for nutrient-induced GLP-1 secretion under normal circumstances, as absorption predominantly takes place in the proximal part of the intestine. However, the increase in GLP-1 observed after gastric bypass surgery can be partly attributed to increased delivery of nutrients to the distal parts of the intestine with a greater L-cell density [27,28,29,30,32]. In this short communication, we will outline the present knowledge on the nutrient-induced secretion of GLP-1, focusing on the macronutrients and their building blocks.
2. Carbohydrate-Induced GLP-1 Secretion
Carbohydrates are absorbed as monosaccharides in the small intestine, and therefore, complex carbohydrates require digestion by salivary and gastric enzymes prior to absorption. As the glucose-induced secretion of GLP-1 is one of the essential requirements of the hormone’s incretin status [16,17,20], the molecular mechanism of this has been an area of interest since the first discoveries of the hormone. In 1995, it was demonstrated that sucrose could stimulate GLP-1 secretion through both an early mechanism and a subsequent mechanism involving luminal contact [36]. In a study on the perfused rat ileum 2 years later, a Na+/glucose transporter and a Na+-independent fructose transporter were suggested as luminal mediators of monosaccharide-induced GLP-1 secretion [37]. Since these findings, studies on both primary L-cells and GLUTag cells (immortal GLP-1-secreting cells derived from a mouse colon) have proven that single L-cells respond to glucose and fructose, supporting the hypothesis of a sensory mechanism for monosaccharide-induced GLP-1 secretion [38,39,40].
In a study on GLUTag cells, low glucose concentrations (0–25 mmol/L) have an increased membrane depolarization and action potential frequency [38]. This depolarizing effect was diminished by the KATP channel opener diazoxide and the metabolic inhibitor Na+-azide, supporting the metabolism of glucose and subsequent closure of the KATP channel as potential pathways for glucose-induced GLP-1 secretion at low glucose concentrations [38] (Figure 1). In agreement with this, the KATP channel blocker tolbutamide was demonstrated to stimulate GLP-1 secretion, and both the KATP channel subunits and glucokinase could be detected in GLUTag cells through real-time PCR [38]. In the same cell line, different concentrations of fructose were found to stimulate GLP-1 secretion via closure of KATP channels [39]. In contrast to data obtained with low-concentration glucose (which support the involvement of intracellular metabolism and closure of KATP channels in glucose-induced GLP-1 secretion), a high concentration (100 mmol/L) of the non-metabolizable glucose analog methyl-α-glucopyranoside has been found to stimulate GLP-1 secretion and depolarize the GLUTag cell through an inward current [39]. This effect of methyl-α-glucopyranoside was abolished in the absence of Na+ ions and in the presence of phloridzin (an inhibitor of the Na+/glucose transporter). In further support, the Na+/glucose transporters SGLT1 and SGLT3 could be detected in the GLUTag cells by RT-PCR, supporting a role of these Na+/glucose transporters in GLP-1 secretion induced by methyl-α-glucopyranoside, at least in GLUTag cells [39]. In the same study, GLP-1 secretion stimulated by a low glucose concentration (0.5 mmol/L) was decreased (≈40%) in the presence of phloridzin, suggesting that glucose stimulates GLP-1 secretion at least partly through SGLT-mediated uptake [39] (Figure 1).
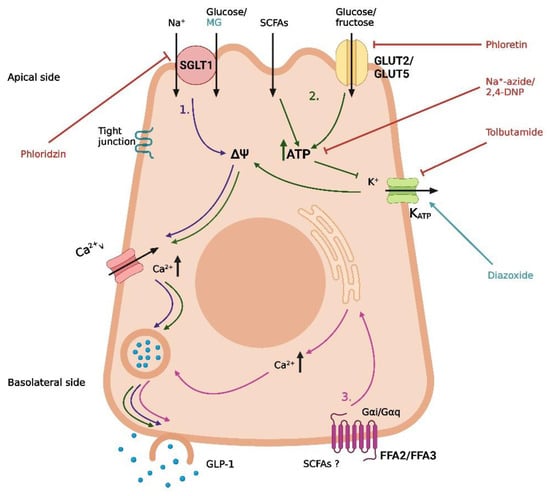
Figure 1.
Proposed mechanisms of carbohydrate-induced GLP-1 secretion in intestinal L-cells. (1) Uptake of glucose/Na+ by SGLT1, leading to depolarization and opening of voltage-gated Ca2+-channels. (2) GLUT2-mediated uptake of glucose, GLUT5-mediated uptake of fructose, and absorption of short-chain fatty acids (SCFAs), followed by intracellular metabolism, closure of KATP channels, depolarization, and opening of voltage-gated Ca2+-channels. (3) Basolateral activation of FFA2 or FFA3 by SCFAs, leading to the release of intracellular deposits of Ca2+. Methyl-α-glucopyranoside (MG) is an agonist of SGLT1, phloridzin is an SGLT-1 inhibitor, phloretin is a GLUT2 inhibitor, Na+-azide and 2,4-DNP are metabolic inhibitors, tolbutamide is a KATP channel inhibitor, and diazoxide is a KATP channel opener. The graphic was created with BioRender.com.
Supporting the GLUTag cell line studies, it has been demonstrated that primary mouse L-cells also respond to stimulation with glucose, methyl-α-glucopyranoside, and tolbutamide [40], and several in vivo studies support the findings of SGLT1 as a mediator of monosaccharide-induced GLP-1 secretion [41,42,43] (Figure 1). In a study of mice, glucose and methyl-α-glucopyranoside both stimulated GLP-1 secretion after intra-intestinal administration, an effect that was abolished when the compounds were administered in combination with phloridzin [41]. In a different study, Sglt1−/− mice had no GLP-1 response 5 min after oral administration of glucose (6 mg/g body weight), whereas a significant increase in the total systemic GLP-1 was observed for their wildtype littermates after the same treatment [42]. Similarly, in an isolated perfused rat small intestine model, SGLT1 has also been demonstrated to be crucial for glucose-induced GLP-1 secretion [43]. In this model, GLP-1 secretion was induced by both glucose and methyl-α-glucopyranoside, and again, the effect was abolished when the secretagogues were administered in combination with phloridzin or in the absence of Na+ [43]. This again supports the importance of SGLT1 in glucose-induced GLP-1 secretion in rodents. Interestingly, when administered at the same concentration (20% w/v), glucose induced a greater GLP-1 response than methyl-α-glucopyranoside, implying that an additional mechanism potentiates the glucose-induced GLP-1 response at high concentrations of glucose. The authors suggested GLUT2-mediated uptake and intracellular metabolism of glucose, leading to the opening of KATP channels, as a possible mechanism [43] (Figure 1). This hypothesis is supported by the observation that the blockage of GLUT2 decreased, but did not abolish, the glucose-induced GLP-1 secretion and that secretion could be induced by tolbutamide (causing closure of the KATP channels and depolarization) [43], similar to the results obtained in the in vitro studies referenced earlier [38,40].
Whereas there are strong indications for an essential role of SGLT-1 and KATP channels in in vitro and in vivo animal models, sufficient data from humans are missing. In one study from 2017, SGLT-1 and GLUT2 appeared to be important for the glucose-induced GLP-1 secretion in isolated human ileal mucosal explants, whereas the role of the KATP channels was less clear [44]. In the human ileal isolates, a high concentration of glucose (300 mmol/L) stimulated GLP-1 secretion, and this effect was blocked in the absence of Na+ or when glucose was co-administered with phloridzin or the GLUT2 inhibitor phloretin [44]. Regarding the role of KATP channel activity, tolbutamide (500 µmol/L) did not stimulate GLP-1 secretion, although the effect of glucose was abolished in the presence of the KATP channel opener diazoxide or the ATP synthesis inhibitor 2,4-DNP [44] (Figure 1). As Sun et al. suggested, a possible explanation for this inconsistency is that diazoxide simply counteracts the depolarization from SGLT1-mediated Na+/glucose transport and that 2,4-DNP blocks a metabolism-dependent effect of GLUT2-mediated glucose uptake, which is not related to KATP channel activity [44]. Although the study demonstrated that glucose can directly stimulate human L-cells, important limitations must be considered. As the study was conducted on isolated tissue, the cells were not polarized as under normal physiological conditions. Moreover, ileal mucosa is very unlikely to encounter the glucose concentrations of the study under normal conditions, as most glucose would be absorbed in the duodenum. Thus, whether the findings on the molecular mechanism of glucose-induced GLP-1 secretion from rodent and cell studies are directly transferrable to humans is not clear. Consequently, although the mechanisms of carbohydrate-induced GLP-1 secretion are well-described in the literature, and a consensus on the mechanism in experimental models exists, essential pieces are still missing in the puzzle of transferability.
In addition to digestible carbohydrates, microbial fermentation products of non-digestible carbohydrates have gained interest as potential GLP-1 secretagogues. An important example is short-chain fatty acids (SCFAs). In vitro and in vivo studies of rodent models have demonstrated that SCFAs may mediate GLP-1 secretion through the free fatty acid receptors FFA2 and FFA3 [45,46] (Figure 1). However, in a study on a perfused rat colon, the importance of FFA2 and FFA3 in SCFA-mediated GLP-1 secretion was questioned [47]. In this model, SCFAs affect the colonic GLP-1 secretion but not through FFA2 or FFA3, as neither agonizing nor antagonizing the receptors influences GLP-1 secretion in a perfused rat colon. Instead, SCFAs seem to increase GLP-1 secretion through intracellular metabolism, possibly followed by the closure of ATP-sensitive potassium channels [47] (Figure 1). In two comparable human studies on overweight and obese individuals, colonic infusions of SCFAs positively impacted fat oxidation, energy expenditure, and PYY levels [48,49]. In contrast, no significant increase in GLP-1 was observed. Again, therefore, it remains to be seen if the SCFA-mediated GLP-1 secretion observed in some animal models is transferrable to humans.
3. Fat-Induced GLP-1 Secretion
Fat is ingested in the form of triglycerides, phospholipids, or cholesterol, and the absorption of dietary fat is a far more complicated process than the absorption of dietary carbohydrates and proteins. In the intestinal lumen, bile salts are responsible for emulsifying the lipids, making them accessible for digestion by pancreatic lipases. The breakdown of triglycerides yields 2-monoacylglycerol (2-MAG) and free fatty acids (FFAs), whereas the breakdown of the most abundant phospholipid, phosphatidylcholine, yields lysophosphatidylcholine and FFAs. The digestive products join with bile acids to form amphiphilic micelles, which facilitate the absorption of the breakdown products. Within the enterocytes, the lipids are re-esterified and incorporated into lipoproteins termed chylomicrons, which are released through the lymphatic system [50]. It is well-established that the ingestion of dietary fat induces a significant GLP-1 response in humans [51,52]. As the pathway of absorption is complex and involves the generation of several intermediate products, various potential mediators of fat-induced GLP-1 secretion have been investigated, and some were also identified. Several studies have demonstrated that the level of lipid-induced GLP-1 secretion is reduced in the presence of the lipase inhibitor orlistat [53,54,55,56], suggesting that the enzymatic digestion of lipids is crucial for stimulating GLP-1 secretion.
One digestive product known to stimulate GLP-1 secretion is FFAs. However, important differences between FFAs of distinct types have been demonstrated with respect to stimulatory potential. In humans, the ingestion of monounsaturated fat induces a greater GLP-1 response than ingestion of polyunsaturated or saturated fat [57,58]. Similarly, chain length seems to be a determining factor for the ability of FFAs to stimulate GLP-1 secretion, although it is not possible to define the exact chain length required for inducing a GLP-1 response from the existing literature. In fetal rat cell cultures, incubation with mono-unsaturated FFAs with a chain length of 16 and 18 carbon atoms stimulated GLP-1 secretion, whereas incubation with mono-unsaturated FFAs with a chain length of 14 carbon atoms was unable to induce a response [59]. In humans, duodenal infusion with a saturated FFA with a chain length of 12 carbon atoms stimulated GLP-1 secretion, whereas infusion with a saturated FFA with a chain length of 10 carbon atoms was ineffective [60]. Although the exact chain length required for stimulation is unclear, the studies indicate that longer FFAs are more powerful intestinal stimulators than shorter FFAs. A proposed explanation for this difference is the formation of chylomicrons [60], as long FFAs are incorporated in chylomicrons and transported in the lymph following absorption, whereas short FFAs are transported in the portal system [61]. However, this hypothesis is only partly supported by observations from in vitro and in vivo studies [62,63]. In a study on GLUTag cells, primary duodenal murine cells and primary duodenal human cells, physiological concentrations of chylomicrons stimulated GLP-1 secretion [63]. In GLUTag cells, the chylomicron-induced GLP-1 secretion involved lipolysis of the chylomicrons and activation of the FFA receptor FFA1 (Figure 2), whereas the mechanism in the primary cells was unclear [63]. In a study on rats, the chylomicron inhibitor L-81 delayed the fat-induced GLP-1 response, measured in the lymph after duodenal infusion of fat alone or fat in combination with L-81 [62] (Figure 2). However, during a 6-h period, the GLP-1 output was still greater in the L-81 group when compared with a saline control group [62]. The delayed response observed when preventing chylomicron formation could indicate that chylomicrons are important for an early fat-induced GLP-1 response, whereas other mechanisms are important for the later response [62]. As fat-induced GIP secretion was completely abolished in the presence of L-81, the delayed GLP-1 response may also just reflect the absence of GIP-induced GLP-1 secretion, questioning if chylomicrons have a direct effect on GLP-1 secretion in vivo [62].
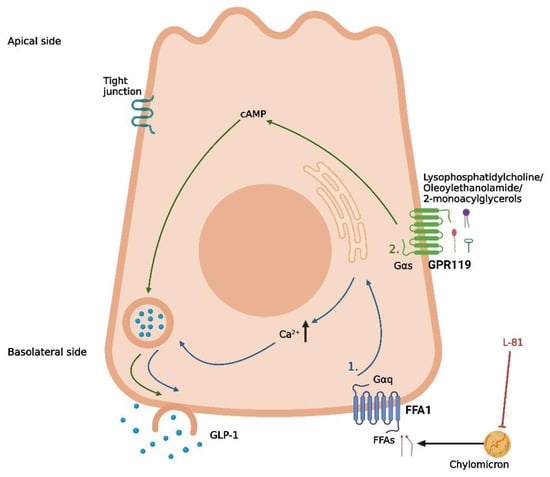
Figure 2.
Proposed mechanisms of fat-induced GLP-1 secretion in intestinal L-cells. (1) Lipolysis of absorbed chylomicrons, followed by the activation of FFA1 by free fatty acids (FFAs), leading to the release of intracellular deposits of Ca2+. (2) Basolateral activation of GPR119 by lipid derivatives (lysophosphatidylcholine, oleoylethanolamide, and 2-monoacylglycerols), leading to an increase in cAMP. L-81 is a chylomicron inhibitor. The graphic was created with BioRender.com.
Despite FFA1 being excluded as a mediator of chylomicron-induced GLP-1 secretion in primary duodenal murine cells and primary duodenal human cells [63], other models have proposed a role for FFA1 in FFA-induced GLP-1 secretion [64,65]. In a study from 2008, the co-expression of FFA1 and GLP-1 was demonstrated in enteroendocrine cells in mice [64]. In the same study, the FFA1lacZ/lacZ mice had a significantly lower GLP-1 plasma level 60 min after acute oral fat administration when compared with their wildtype littermates [64]. Supporting this, basolateral activation of FFA1 with both an endogenous ligand (linoleic acid) and synthetic agonists increased GLP-1 secretion in an isolated perfused rat small intestine model [65]. Importantly, this effect was not observed when the ligands were infused luminally, suggesting that FFAs act on FFA1 following absorption [65]. Adding to the complexity of lipid-induced GLP-1 secretion, a study from 2014 demonstrated that agonists acting on the Gq signaling pathway of FFA1, which is the pathway for FFA-induced activation of the receptor, induced a lower GLP-1 response following oral administration in mice when compared with agonists acting on both the Gq and Gs pathways [66]. In a phase I clinical trial, the FFA1 agonist TAK-875 did not have a noticeable effect on GLP-1 secretion when administered in therapeutic concentrations [67]. Instead, the improved glycemic controls in subjects receiving the drug was attributed to its effects on FFA1 in pancreatic beta cells, stimulating insulin secretion [67]. As an important side note, the development of the drug was terminated in the phase III trials due to concerns over liver toxicity not related to FFA1 [68]. Thus, the activation of FFA1 by FFAs is probably not sufficient to induce a pronounced incretin response on its own.
Following a fat-containing meal, FFAs are not the only component of dietary fat known to stimulate GLP-1 secretion. GPR119 is a well-recognized mediator of GLP-1 secretion in both rodents and humans, where the receptor acts through interactions with lipid derivatives such as lysophosphatidylcholine [69], oleoylethanolamide [70], and 2-monoacylglycerols [71,72] (Figure 2). In vivo studies on different knock-out mice strains have indicated that GPR119 is more important for GLP-1 secretion than FFA1 and that the two receptors work in synergy to create a lipid-induced GLP-1 secretion [72]. In rodents, a GRP119 agonist has been demonstrated to increase the GLP-1, GIP, and PYY levels [73], making this receptor an attractive therapeutic target. Unfortunately, the results from studies assessing the potential of GPR119 agonists in type 2 diabetes therapy have been disappointing. Many pharmaceutical companies have failed in the attempt to develop a GPR119 agonist, and no drug candidate has passed the phase II clinical trials so far, with one challenge being the required lipophilic properties of the drugs [74,75]. Thus, although attempts to uncover molecular pathways of lipid-induced GLP-1 secretion have been successful, the discoveries have yet to be translated into relevant therapy. Hopefully, this issue can be solved through focused research in the future.
4. Protein-Induced GLP-1 Secretion
Protein is absorbed as peptides or amino acids in the small intestine, and thus, the complex proteins from a diet require enzymatic digestion prior to absorption. A high-protein diet has been demonstrated to improve weight loss and weight maintenance when compared with a low-protein diet [76,77], although the underlying mechanisms are not entirely clear. Some studies indicated that dietary protein is more satiating than carbohydrates or fat [78,79,80,81], while others did not find a significant difference between the three macronutrients [82,83]. A similar discrepancy applies to the thermogenic effect and the effect on plasma GLP-1 levels when comparing proteins with carbohydrates and fat. Reports on both a higher [80] and a similar [83] thermogenic effect of protein exist. Early studies showed that ingestion of a protein meal or free amino acids induces a transient increase in plasma GLP-1 levels [51,52]. However, whether this increase is greater in magnitude than the increase induced by carbohydrates or fat is unclear, as the conclusions from different studies are inconsistent. In a randomized three-way crossover design study, no difference in plasma GLP-1 was observed in healthy or obese males after consumption of an isocaloric meal with a high proportion of either protein, carbohydrates, or fat [81]. On the contrary, other studies have demonstrated a greater GLP-1 response following a high-protein meal when compared with isocaloric meals lower in protein [83,84]. As the test meals within all reported studies were isocaloric, different energy contents of the meals cannot explain the different results. Volume differences in the meals may have influenced the GLP-1 secretion (as a meal with a higher volume would stay in the intestinal system for longer). In the one study that did not demonstrate a difference, all test meals consisted of pasta (normal or high-protein) and tomato sauce supplemented with protein, carbohydrates, or fat [81]. In contrast, the meal size varied more in the two studies demonstrating a difference [83,84]. Moreover, different types of protein may stimulate GLP-1 secretion with different potencies. As such, the source of the protein should also be considered when comparing the effect of protein on GLP-1 secretion.
As is the case for carbohydrates and fat, different digestive products of dietary protein are thought to stimulate GLP-1 secretion through different mechanisms. Peptones (protein hydrolysates of an animal source) have been demonstrated as a potent stimulator of GLP-1 secretion in murine primary colonic L-cells, STC-1 cells, and the isolated perfused rat small intestine model [85,86,87]. Peptide Transporter 1 (Pept1) and the G-protein-coupled Calcium-Sensing Receptor (CaSR) have both been suggested as potential mediators of peptone-induced GLP-1 secretion [85,87] (Figure 3). However, the importance of each of these receptors is not consistent between the different models. In murine primary colonic L-cells, the inhibition of Pept1 with 4-aminomethylbenzoic acid (4-AMBA) did not abolish the peptone-induced GLP-1 secretion, neither for low nor high peptone concentrations (5 or 50 mg/mL). In contrast, the blockage of Pept1 with the same inhibitor did abolish the GLP-1 response to luminally infused peptones (50 mg/mL) in the isolated perfused rat small intestine [87] (Figure 3). An essential difference between the two models is the preserved cellular polarization in the perfused rat intestine, which is lost in the cellular model. Thus, these results could indicate that, in a physiological setting, peptones require absorption to elicit a GLP-1 response. In a cellular model, this may not be important since the peptones can interact directly with the mediators normally located on the basolateral side of the cell. Supporting this, inhibiting the amino acid-sensing receptor CaSR with the negative allosteric modulator NPS2143 diminished the peptone-induced GLP-1 secretion in both murine colonic L-cells and after vascular stimulation in the perfused rat small intestine [85,87] (Figure 3). As the positive allosteric modulator calindol only stimulated GLP-1 secretion when infused vascularly in the perfused rat small intestine, CaSR must be located on the basolateral side of the L-cell [87] (Figure 3). Thus, peptones may stimulate GLP-1 secretion through absorption by Pept1, followed by intracellular digestion to free amino acids, finally activating CaSR on the basolateral side of the cell.
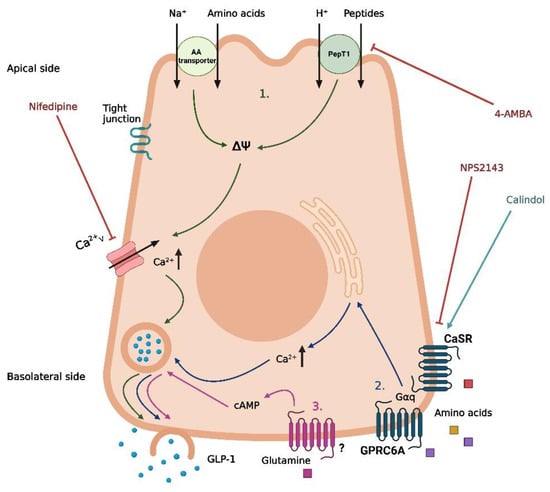
Figure 3.
Proposed mechanisms of protein-induced GLP-1 secretion in intestinal L-cells. (1) Uptake of free amino acids through coupled transport with Na+ and uptake of peptides/H+ by PepT1, leading to depolarization and opening of voltage-gated Ca2+-channels. (2) Basolateral activation of CaSR and GPRC6A by free amino acids following absorption, leading to the release of intracellular deposits of Ca2+. (3) Basolateral activation of an unknown receptor by glutamine following absorption, leading to an increase in cAMP. Nifedipine is a Ca2+ channel inhibitor, 4-aminomethylbenzoic acid (4-AMBA) is a PepT1 inhibitor, NPS2143 is a CaSR inhibitor, and calindol is a CaSR agonist. The graphic was created with BioRender.com.
Pept1 and CaSR are not the only mediators associated with protein-induced GLP-1 secretion. Whereas peptones are a non-specific mix of different peptides and free amino acids, specific peptides and free amino acids have been associated with distinct molecular pathways of GLP-1 secretion. Similar to peptones, dipeptides such as glycine-sarcosine (Gly-Sar) and glycine-glycine (Gly-Gly) have been demonstrated to stimulate GLP-1 secretion in murine primary colonic L-cells, STC-1 cells, and in the isolated perfused rat small intestine [85,87,88]. In contrast to the peptone-induced GLP-1 secretion, inhibition of Pept1 with 4-AMBA did abolish GLP-1 secretion induced by the non-metabolizable Gly-Sar in murine primary colonic L-cells [85]. Similar results were obtained for Gly-Gly in STC1 cells [88] and for Gly-Sar in the perfused rat small intestine [87]. This indicates a direct mechanism for dipeptide-induced GLP-1 secretion after uptake by Pept1, independent of intracellular digestion to free amino acids and interaction with CaSR. As Pept1 is a proton-coupled transporter, this mechanism may involve membrane depolarization and a subsequent influx of Ca2+ from voltage-gated Ca2+ channels. In support of this, GLP-1 secretion mediated by Gly-Sar and Gly-Gly was diminished by the presence of the Ca2+ channel inhibitor nifedipine in vitro [85,88] (Figure 3). However, this hypothesis was not supported by results from the perfused rat small intestine model, where GLP-1 secretion induced by Gly-Sar was not abolished in the presence of nifedipine [87]. Thus, the exact pathway coupling dipeptides and Pept1 to GLP-1 secretion remains to be clarified.
In a study on female individuals with obesity, the circulating levels of eight specific amino acids (isoleucine, leucine, lysine, methionine, phenylalanine, proline, tyrosine, and valine) were positively correlated with the plasma GLP-1 levels [89], and in experimental models, a range of specific free amino acids has been demonstrated to induce GLP-1 secretion with different potencies and through different mechanisms [90,91,92,93,94,95]. From these studies, the most important amino acids for GLP-1 secretion appear to be L-glutamine, L-tryptophan, L-phenylalanine, L-asparagine, L-arginine, and L-valine. In murine primary colonic L-cells and GLUTag-cells, glutamine-induced GLP-1 secretion involves a rise in cytosolic Ca2+ and requires the presence of Na+ in the media [90,91], suggesting Na+-coupled uptake and the subsequent opening of voltage-gated Ca2+ channels as a potential pathway for glutamine-induced GLP-1 secretion (Figure 3). However, the exact transporters involved remain to be established. An additional mechanism proposed for glutamine-induced GLP-1 secretion is an elevation of cAMP [91], although the results from GLUTag cells are somewhat conflicting. In one experimental set-up, the protein kinase A (PKA) inhibitor H89 did not affect glutamine-induced GLP-1 secretion [90], whereas monitoring the cAMP levels in another experimental design revealed that glutamine increased the cAMP levels in GLUTag cells [91]. Thus, glutamine may increase GLP-1 through a pathway downstream of increased cytosolic cAMP, independent on PKA activation (Figure 3). Importantly, glutamine has been demonstrated to increase GLP-1 and insulin levels following oral administration in humans [96], proposing a therapeutic potential of the amino acid.
As for peptone-induced GLP-1 secretion, CaSR has also been linked to the GLP-1 secretion induced by certain free amino acids [93,95] (Figure 3). In the perfused rat small intestine, inhibition of CaSR decreased the GLP-1 response to the luminal infusion of vamin (a mix of 18 different amino acids) [95]. However, inhibition of CaSR did not affect the GLP-1 response to vascular-infused arginine [95], suggesting that CaSR is only involved in the GLP-1 response to some amino acids. One amino acid where CaSR does seem to be involved in the GLP-1 response is phenylalanine. In the perfused rat small intestine, phenylalanine has been demonstrated to stimulate GLP-1 secretion following both luminal and vascular infusion [95], and as the amino acid is efficiently absorbed from the rat intestine [95], a basolateral mechanism involving CaSR is plausible. This is supported by another rodent study where intra-ileal administration of phenylalanine reduced the acute food intake in rats, an effect that was abolished when co-administered with the CaSR inhibitor NPS2143 [93]. In the same study, phenylalanine-induced GLP-1 secretion from different cell lines was also diminished in the presence of NPS2143, supporting a role for CaSR in phenylalanine-induced GLP-1 secretion [93]. Two additional G-protein coupled receptors, GPRC6A and GPR142, have also been associated with GLP-1 secretion induced by specific amino acids [92,94] (Figure 3). In the GLUTag cells, L-ornithine-induced GLP-1 secretion was significantly reduced when the cells were treated with siRNA for GPRC6A [92]. However, in the GPRC6A knock-out mice, no difference in plasma GLP-1 was observed 15 min after oral administration of L-arginine or L-ornithine when compared to the wildtype littermates [97]. Although the GLP-1 response was attenuated 30 and 60 min after administration of the amino acids, this did not significantly impact the total GLP-1 release [97]. Adding to this, GPRC6A could not be detected in cells from segments of the mice’s duodenum, jejunum, or ileum [95], again challenging the idea of GPRC6A as an important mediator of amino acid-induced GLP-1 secretion.
Altogether, unidentified mechanisms remain to be uncovered to obtain a complete understanding of protein-induced GLP-1 secretion. Glutamine and arginine have been demonstrated to induce GLP-1 secretion in humans [96,98], and recently, valine was demonstrated as a potent stimulator of GLP-1 secretion when infused luminally in the perfused rat small intestine [95]. These findings strongly emphasize the relevance of uncovering the mechanisms of protein-induced GLP-1 secretion, as these may prove valuable in future therapy.
5. Future Perspectives
Through a joined effort and various experimental models, recent research has begun to reveal the complexity of nutrient-induced GLP-1 secretion. It turns out that the digestion products of the three macronutrients stimulate secretion through widely different mechanisms, with a large number of sensors and receptors involved in the nutrient-induced GLP-1 secretion. As we dive further into the mechanisms involved, we may be able to utilize approaches involving stimulation of the secretion of endogenous gut hormones in the clinic, supporting and expanding existing therapeutic options. An important feature of new GLP-1 based therapeutics is to reduce side effects. Targeting endogenous secretion is a potential approach, and perhaps in combination with incretin enhancers like DPP4 inhibitors or somatostatin subtype 5 receptor antagonists (preventing feedback inhibition of GLP-1 secretion by somatostatin), this may be even more effective than current GLP-1-based therapies. However, we still have a lot to learn and have probably only begun to uncover the mechanistic links between eating and hormone secretion. In several of the studies on GLP-1 secretion, it is curious that the timing rather than the total hormone secretion is affected by the nutrient stimulus. The implication of this is difficult to determine. The effectiveness of gastric bypass operations (which induce a high and transient elevation in GLP-1) supports timing as the main factor, whereas the superior effect of the weekly GLP-1Ras compared with the short-acting agonists supports total hormone secretion. As stimulating the endogenous GLP-1 secretion of the L-cells is an attempt to mimic gastric surgery, the effectiveness of the strategy would rely on the timing as a determining factor.
An important challenge in the field is the sometimes-limited transferability between experimental animal models and human subjects. This has been exemplified in several studies on secretion elicited experimentally with lipids and amino acids, where attempts at clinical application have been disappointing. To obtain a better understanding of the nutrient-induced GLP-1 secretion and utilize this understanding in therapy, we need more human studies and more experimental studies with improved clinically relevant models to confirm the patterns that we are beginning to understand from in vitro and in vivo animal models. One attempt to improve preclinical models is the use of human intestinal organoids, which display a high degree of similarity with human intestinal cells [99]. Hopefully, these attempts and the following years of research will provide us with some of the missing pieces of knowledge that exist for all the macronutrients.
Author Contributions
Original draft preparation, A.P.H.; review and editing, I.M.M. and J.J.H.; preparation of figures, A.P.H.; funding acquisition, J.J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Research Council, grant number 695069 and an unrestricted grant to Jens Juul Holst from the Novo Nordisk Foundation Center for Basic Metabolic Research (Novo Nordisk Foundation, Denmark).
Acknowledgments
We thank Novo Nordisk Denmark for financial support given to Anna Pii Hjørne through the Novo Nordisk Scholarship Programme for master students enrolled at Danish Universities. Graphical abstract and figures are created with Biorender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahlman, H.; Nilsson, O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2001, 12 (Suppl. 2), S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Bucinskaite, V.; Tolessa, T.; Pedersen, J.; Rydqvist, B.; Zerihun, L.; Holst, J.J.; Hellström, P.M. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol. Motil. 2009, 21, 978-e78. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.; Parker, H.E.; Adriaenssens, A.E.; Hodgson, J.M.; Cork, S.C.; Trapp, S.; Gribble, F.M.; Reimann, F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 2014, 63, 1224–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egerod, K.L.; Petersen, N.; Timshel, P.N.; Rekling, J.C.; Wang, Y.; Liu, Q.; Schwartz, T.W.; Gautron, L. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol. Metab. 2018, 12, 62–75. [Google Scholar]
- Mortensen, K.; Christensen, L.L.; Holst, J.J.; Orskov, C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 2003, 114, 189–196. [Google Scholar] [CrossRef]
- Egerod, K.L.; Engelstoft, M.S.; Grunddal, K.V.; Nøhr, M.K.; Secher, A.; Sakata, I.; Pedersen, J.; Windeløv, J.A.; Füchtbauer, E.M.; Olsen, J.; et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 2012, 153, 5782–5795. [Google Scholar] [CrossRef] [Green Version]
- Svendsen, B.; Pedersen, J.; Albrechtsen, N.J.; Hartmann, B.; Toräng, S.; Rehfeld, J.F.; Poulsen, S.S.; Holst, J.J. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology 2015, 156, 847–857. [Google Scholar] [CrossRef]
- Beumer, J.; Artegiani, B.; Post, Y.; Reimann, F.; Gribble, F.; Nguyen, T.N.; Zeng, H.; Van den Born, M.; Van Es, J.H.; Clevers, H. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat. Cell Biol. 2018, 20, 909–916. [Google Scholar] [CrossRef]
- Lund, P.K.; Goodman, R.H.; Dee, P.C.; Habener, J.F. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc. Natl. Acad. Sci. USA 1982, 79, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Lund, P.K.; Goodman, R.H.; Montminy, M.R.; Dee, P.C.; Habener, J.F. Anglerfish islet pre-proglucagon II. Nucleotide and corresponding amino acid sequence of the cDNA. J. Biol. Chem. 1983, 258, 3280–3284. [Google Scholar] [CrossRef]
- Bell, G.I.; Santerre, R.F.; Mullenbach, G.T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 1983, 302, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.I.; Sanchez-Pescador, R.; Laybourn, P.J.; Najarian, R.C. Exon duplication and divergence in the human preproglucagon gene. Nature 1983, 304, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Mojsov, S.; Habener, J.F. Cell-specific post-translational processing of preproglucagon expressed from a metallothionein-glucagon fusion gene. J. Biol. Chem. 1986, 261, 9637–9643. [Google Scholar] [CrossRef]
- Mojsov, S.; Heinrich, G.; Wilson, I.B.; Ravazzola, M.; Orci, L.; Habener, J.F. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J. Biol. Chem. 1986, 261, 11880–11889. [Google Scholar] [CrossRef]
- Orskov, C.; Holst, J.J.; Knuhtsen, S.; Baldissera, F.G.; Poulsen, S.S.; Nielsen, O.V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 1986, 119, 1467–1475. [Google Scholar] [CrossRef]
- Holst, J.J.; Orskov, C.; Nielsen, O.V.; Schwartz, T.W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987, 211, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Mojsov, S.; Weir, G.C.; Habener, J.F. Insulinotropin: Glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J. Clin. Investig. 1987, 79, 616–619. [Google Scholar] [CrossRef] [Green Version]
- Drucker, D.J.; Philippe, J.; Mojsov, S.; Chick, W.L.; Habener, J.F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci. USA 1987, 84, 3434–3438. [Google Scholar] [CrossRef] [Green Version]
- Orskov, C.; Holst, J.J.; Poulsen, S.S.; Kirkegaard, P. Pancreatic and intestinal processing of proglucagon in man. Diabetologia 1987, 30, 874–881. [Google Scholar] [CrossRef]
- Kreymann, B.; Williams, G.; Ghatei, M.A.; Bloom, S.R. Glucagon-like peptide-1 7-36: A physiological incretin in man. Lancet 1987, 2, 1300–1304. [Google Scholar] [CrossRef]
- Orskov, C.; Bersani, M.; Johnsen, A.H.; Højrup, P.; Holst, J.J. Complete sequences of glucagon-like peptide-1 from human and pig small intestine. J. Biol. Chem. 1989, 264, 12826–12829. [Google Scholar] [CrossRef]
- Näslund, E.; Barkeling, B.; King, N.; Gutniak, M.; Blundell, J.E.; Holst, J.J.; Rössner, S.; Hellström, P.M. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int. J. Obes. 1999, 23, 304–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, A.; Raben, A.; Ersbøll, A.K.; Holst, J.J.; Astrup, A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int. J. Obes. 2001, 25, 781–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef]
- Korner, J.; Bessler, M.; Inabnet, W.; Taveras, C.; Holst, J.J. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg. Obes. Relat. Dis. 2007, 3, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Svane, M.S.; Bojsen-Møller, K.N.; Nielsen, S.; Jørgensen, N.B.; Dirksen, C.; Bendtsen, F.; Kristiansen, V.B.; Hartmann, B.; Holst, J.J.; Madsbad, S. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E505–E514. [Google Scholar] [CrossRef] [Green Version]
- Larraufie, P.; Roberts, G.P.; McGavigan, A.K.; Kay, R.G.; Li, J.; Leiter, A.; Melvin, A.; Biggs, E.K.; Ravn, P.; Davy, K.; et al. Important Role of the GLP-1 Axis for Glucose Homeostasis after Bariatric Surgery. Cell Rep. 2019, 26, 1399–1408.e6. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.; de Souza, A.L.; Batista, G.A.; Duran, L.F.T.; Fernandes, D.P.; Molina, V.B.C.; Gonçalves, R.; Giorgetti, J.S.; Chaim, E.A.; Alegre, S.M. GLP-1: 10-year follow-up after Roux-en-Y gastric bypass. Langenbecks Arch. Surg. 2022, 407, 559–568. [Google Scholar] [CrossRef]
- Sjöström, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Guedes, T.P.; Martins, S.; Costa, M.; Pereira, S.S.; Morais, T.; Santos, A.; Nora, M.; Monteiro, M.P. Detailed characterization of incretin cell distribution along the human small intestine. Surg. Obes. Relat. Dis. 2015, 11, 1323–1331. [Google Scholar] [CrossRef]
- Wewer Albrechtsen, N.J.; Kuhre, R.E.; Toräng, S.; Holst, J.J. The intestinal distribution pattern of appetite- and glucose regulatory peptides in mice, rats and pigs. BMC Res. Notes 2016, 9, 60. [Google Scholar]
- Orskov, C.; Wettergren, A.; Holst, J.J. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand. J. Gastroenterol. 1996, 31, 665–670. [Google Scholar] [PubMed]
- Ahlkvist, L.; Vikman, J.; Pacini, G.; Ahrén, B. Synergism by individual macronutrients explains the marked early GLP-1 and islet hormone responses to mixed meal challenge in mice. Regul. Pept. 2012, 178, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qualmann, C.; Nauck, M.A.; Holst, J.J.; Orskov, C.; Creutzfeldt, W. Glucagon-like peptide 1 (7-36 amide) secretion in response to luminal sucrose from the upper and lower gut. A study using alpha-glucosidase inhibition (acarbose). Scand. J. Gastroenterol. 1995, 30, 892–896. [Google Scholar] [CrossRef]
- Ritzel, U.; Fromme, A.; Ottleben, M.; Leonhardt, U.; Ramadori, G. Release of glucagon-like peptide-1 (GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol. 1997, 34, 18–21. [Google Scholar] [CrossRef]
- Reimann, F.; Gribble, F.M. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes 2002, 51, 2757–2763. [Google Scholar] [CrossRef] [Green Version]
- Gribble, F.M.; Williams, L.; Simpson, A.K.; Reimann, F. A Novel Glucose-Sensing Mechanism Contributing to Glucagon-Like Peptide-1 Secretion from the GLUTag Cell Line. Diabetes 2003, 52, 1147–1154. [Google Scholar] [CrossRef] [Green Version]
- Reimann, F.; Habib, A.M.; Tolhurst, G.; Parker, H.E.; Rogers, G.J.; Gribble, F.M. Glucose Sensing in L Cells: A Primary Cell Study. Cell Metab. 2008, 8, 532–539. [Google Scholar]
- Moriya, R.; Shirakura, T.; Ito, J.; Mashiko, S.; Seo, T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1358–E1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhre, R.E.; Frost, C.R.; Svendsen, B.; Holst, J.J. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes 2015, 64, 370–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, E.W.; de Fontgalland, D.; Rabbitt, P.; Hollington, P.; Sposato, L.; Due, S.L.; Wattchow, D.A.; Rayner, C.K.; Deane, A.M.; Young, R.L.; et al. Mechanisms Controlling Glucose-Induced GLP-1 Secretion in Human Small Intestine. Diabetes 2017, 66, 2144–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [Green Version]
- van der Beek, C.M.; Canfora, E.E.; Lenaerts, K.; Troost, F.J.; Damink, S.; Holst, J.J.; Masclee, A.A.M.; Dejong, C.H.C.; Blaak, E.E. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin. Sci. 2016, 130, 2073–2082. [Google Scholar] [CrossRef]
- Canfora, E.E.; van der Beek, C.M.; Jocken, J.W.E.; Goossens, G.H.; Holst, J.J.; Olde Damink, S.W.M.; Lenaerts, K.; Dejong, C.H.C.; Blaak, E.E. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: A randomized crossover trial. Sci. Rep. 2017, 7, 2360. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [Green Version]
- Elliott, R.M.; Morgan, L.M.; Tredger, J.A.; Deacon, S.; Wright, J.; Marks, V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: Acute post-prandial and 24-h secretion patterns. J. Endocrinol. 1993, 138, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Göke, R.; Richter, G.; Fehmann, H.C.; Arnold, R.; Göke, B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 1995, 56, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Feinle, C.; O’Donovan, D.; Doran, S.; Andrews, J.M.; Wishart, J.; Chapman, I.; Horowitz, M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G798–G807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilichiewicz, A.; O’Donovan, D.; Feinle, C.; Lei, Y.; Wishart, J.M.; Bryant, L.; Meyer, J.H.; Horowitz, M.; Jones, K.L. Effect of lipase inhibition on gastric emptying of, and the glycemic and incretin responses to, an oil/aqueous drink in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003, 88, 3829–3834. [Google Scholar] [CrossRef] [Green Version]
- Ellrichmann, M.; Kapelle, M.; Ritter, P.R.; Holst, J.J.; Herzig, K.H.; Schmidt, W.E.; Schmitz, F.; Meier, J.J. Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1-(7-36)-amide-1, cholecystokinin, and peptide YY concentrations. J. Clin. Endocrinol. Metab. 2008, 93, 3995–3998. [Google Scholar] [CrossRef] [Green Version]
- Beglinger, S.; Drewe, J.; Schirra, J.; Göke, B.; D’Amato, M.; Beglinger, C. Role of fat hydrolysis in regulating glucagon-like Peptide-1 secretion. J. Clin. Endocrinol. Metab. 2010, 95, 879–886. [Google Scholar] [CrossRef]
- Beysen, C.; Karpe, F.; Fielding, B.A.; Clark, A.; Levy, J.C.; Frayn, K.N. Interaction between specific fatty acids, GLP-1 and insulin secretion in humans. Diabetologia 2002, 45, 1533–1541. [Google Scholar]
- Thomsen, C.; Rasmussen, O.; Lousen, T.; Holst, J.J.; Fenselau, S.; Schrezenmeir, J.; Hermansen, K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am. J. Clin. Nutr. 1999, 69, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Rocca, A.S.; Brubaker, P.L. Stereospecific effects of fatty acids on proglucagon-derived peptide secretion in fetal rat intestinal cultures. Endocrinology 1995, 136, 5593–5599. [Google Scholar] [CrossRef]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Smout, A.J.; Wishart, J.; Pilichiewicz, A.N.; Rades, T.; Chapman, I.M.; Feinle-Bisset, C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R524–R533. [Google Scholar] [CrossRef] [Green Version]
- Kiyasu, J.Y.; Bloom, B.; Chaikoff, I.L. The portal transport of absorbed fatty acids. J. Biol. Chem. 1952, 199, 415–419. [Google Scholar] [CrossRef]
- Lu, W.J.; Yang, Q.; Yang, L.; Lee, D.; D’Alessio, D.; Tso, P. Chylomicron formation and secretion is required for lipid-stimulated release of incretins GLP-1 and GIP. Lipids 2012, 47, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psichas, A.; Larraufie, P.F.; Goldspink, D.A.; Gribble, F.M.; Reimann, F. Chylomicrons stimulate incretin secretion in mouse and human cells. Diabetologia 2017, 60, 2475–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edfalk, S.; Steneberg, P.; Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008, 57, 2280–2287. [Google Scholar] [CrossRef] [Green Version]
- Christensen, L.W.; Kuhre, R.E.; Janus, C.; Svendsen, B.; Holst, J.J. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiol. Rep. 2015, 3, e12551. [Google Scholar] [CrossRef] [Green Version]
- Hauge, M.; Vestmar, M.A.; Husted, A.S.; Ekberg, J.P.; Wright, M.J.; Di Salvo, J.; Weinglass, A.B.; Engelstoft, M.S.; Madsen, A.N.; Lückmann, M.; et al. GPR40 (FFAR1)—Combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol. Metab. 2015, 4, 3–14. [Google Scholar] [CrossRef]
- Leifke, E.; Naik, H.; Wu, J.; Viswanathan, P.; DeManno, D.; Kipnes, M.; Vakilynejad, M. A Multiple-Ascending-Dose Study to Evaluate Safety, Pharmacokinetics, and Pharmacodynamics of a Novel GPR40 Agonist, TAK-875, in Subjects with Type 2 Diabetes. Clin. Pharmacol. Ther. 2012, 92, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Kaku, K.; Enya, K.; Nakaya, R.; Ohira, T.; Matsuno, R. Long-term safety and efficacy of fasiglifam (TAK-875), a G-protein-coupled receptor 40 agonist, as monotherapy and combination therapy in Japanese patients with type 2 diabetes: A 52-week open-label phase III study. Diabetes Obes. Metab. 2016, 18, 925–929. [Google Scholar] [CrossRef]
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J.; Matsumoto, S.; Kamohara, M.; Hiyama, H.; Yoshida, S.; et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005, 326, 744–751. [Google Scholar] [CrossRef]
- Lauffer, L.M.; Iakoubov, R.; Brubaker, P.L. GPR119 Is Essential for Oleoylethanolamide-Induced Glucagon-Like Peptide-1 Secretion From the Intestinal Enteroendocrine L-Cell. Diabetes 2009, 58, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Hansen, K.B.; Rosenkilde, M.M.; Knop, F.K.; Wellner, N.; Diep, T.A.; Rehfeld, J.F.; Andersen, U.B.; Holst, J.J.; Hansen, H.S. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J. Clin. Endocrinol. Metab. 2011, 96, E1409–E1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekberg, J.H.; Hauge, M.; Kristensen, L.V.; Madsen, A.N.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.; Egerod, K.L.; Timshel, P.; Kowalski, T.J.; et al. GPR119, a Major Enteroendocrine Sensor of Dietary Triglyceride Metabolites Coacting in Synergy with FFA1 (GPR40). Endocrinology 2016, 157, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Mace, O.J.; Tough, I.R.; White, J.; Cock, T.A.; Warpman Berglund, U.; Schindler, M.; Cox, H.M. Gastrointestinal hormonal responses on GPR119 activation in lean and diseased rodent models of type 2 diabetes. Int. J. Obes. 2014, 38, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Ritter, K.; Buning, C.; Halland, N.; Pöverlein, C.; Schwink, L. G Protein-Coupled Receptor 119 (GPR119) Agonists for the Treatment of Diabetes: Recent Progress and Prevailing Challenges. J. Med. Chem. 2016, 59, 3579–3592. [Google Scholar] [CrossRef]
- Dahlén, A.D.; Dashi, G.; Maslov, I.; Attwood, M.M.; Jonsson, J.; Trukhan, V.; Schiöth, H.B. Trends in Antidiabetic Drug Discovery: FDA Approved Drugs, New Drugs in Clinical Trials and Global Sales. Front. Pharmacol. 2021, 12, 807548. [Google Scholar] [CrossRef]
- Skov, A.R.; Toubro, S.; Rønn, B.; Holm, L.; Astrup, A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.; Nijs, I.; van Ooijen, M.; Kovacs, E.M. High protein intake sustains weight maintenance after body weight loss in humans. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Latner, J.D.; Schwartz, M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite 1999, 33, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Poppitt, S.D.; McCormack, D.; Buffenstein, R. Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiol. Behav. 1998, 64, 279–285. [Google Scholar] [CrossRef]
- Crovetti, R.; Porrini, M.; Santangelo, A.; Testolin, G. The influence of thermic effect of food on satiety. Eur. J. Clin. Nutr. 1998, 52, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Batterham, R.L.; Heffron, H.; Kapoor, S.; Chivers, J.E.; Chandarana, K.; Herzog, H.; Le Roux, C.W.; Thomas, E.L.; Bell, J.D.; Withers, D.J. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geliebter, A.A. Effects of equicaloric loads of protein, fat, and carbohydrate on food intake in the rat and man. Physiol. Behav. 1979, 22, 267–273. [Google Scholar] [CrossRef]
- Raben, A.; Agerholm-Larsen, L.; Flint, A.; Holst, J.J.; Astrup, A. Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. Am. J. Clin. Nutr. 2003, 77, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejeune, M.P.; Westerterp, K.R.; Adam, T.C.; Luscombe-Marsh, N.D.; Westerterp-Plantenga, M.S. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am. J. Clin. Nutr. 2006, 83, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Diakogiannaki, E.; Pais, R.; Tolhurst, G.; Parker, H.E.; Horscroft, J.; Rauscher, B.; Zietek, T.; Daniel, H.; Gribble, F.M.; Reimann, F. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 2013, 56, 2688–2696. [Google Scholar] [CrossRef] [Green Version]
- Cordier-Bussat, M.; Bernard, C.; Levenez, F.; Klages, N.; Laser-Ritz, B.; Philippe, J.; Chayvialle, J.A.; Cuber, J.C. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes 1998, 47, 1038–1045. [Google Scholar] [CrossRef]
- Modvig, I.M.; Kuhre, R.E.; Holst, J.J. Peptone-mediated glucagon-like peptide-1 secretion depends on intestinal absorption and activation of basolaterally located Calcium-Sensing Receptors. Physiol. Rep. 2019, 7, e14056. [Google Scholar] [CrossRef]
- Matsumura, K.; Miki, T.; Jhomori, T.; Gonoi, T.; Seino, S. Possible role of PEPT1 in gastrointestinal hormone secretion. Biochem. Biophys. Res. Commun. 2005, 336, 1028–1032. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Leoncini, R.; De Col, A.; Tamini, S.; Cicolini, S.; Abbruzzese, L.; Cella, S.G.; Sartorio, A. The Appetite-Suppressant and GLP-1-Stimulating Effects of Whey Proteins in Obese Subjects are Associated with Increased Circulating Levels of Specific Amino Acids. Nutrients 2020, 12, 775. [Google Scholar] [CrossRef] [Green Version]
- Reimann, F.; Williams, L.; da Silva Xavier, G.; Rutter, G.A.; Gribble, F.M. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 2004, 47, 1592–1601. [Google Scholar] [CrossRef] [Green Version]
- Tolhurst, G.; Zheng, Y.; Parker, H.E.; Habib, A.M.; Reimann, F.; Gribble, F.M. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 2011, 152, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oya, M.; Kitaguchi, T.; Pais, R.; Reimann, F.; Gribble, F.; Tsuboi, T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J. Biol. Chem. 2013, 288, 4513–4521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamshah, A.; Spreckley, E.; Norton, M.; Kinsey-Jones, J.S.; Amin, A.; Ramgulam, A.; Cao, Y.; Johnson, R.; Saleh, K.; Akalestou, E.; et al. l-phenylalanine modulates gut hormone release and glucose tolerance, and suppresses food intake through the calcium-sensing receptor in rodents. Int. J. Obes. 2017, 41, 1693–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudenko, O.; Shang, J.; Munk, A.; Ekberg, J.P.; Petersen, N.; Engelstoft, M.S.; Egerod, K.L.; Hjorth, S.A.; Wu, M.; Feng, Y.; et al. The aromatic amino acid sensor GPR142 controls metabolism through balanced regulation of pancreatic and gut hormones. Mol. Metab. 2019, 19, 49–64. [Google Scholar] [CrossRef]
- Modvig, I.M.; Kuhre, R.E.; Jepsen, S.L.; Xu, S.F.S.; Engelstoft, M.S.; Egerod, K.L.; Schwartz, T.W.; Ørskov, C.; Rosenkilde, M.M.; Holst, J.J. Amino acids differ in their capacity to stimulate GLP-1 release from the perfused rat small intestine and stimulate secretion by different sensing mechanisms. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E874–E885. [Google Scholar] [CrossRef]
- Greenfield, J.R.; Farooqi, I.S.; Keogh, J.M.; Henning, E.; Habib, A.M.; Blackwood, A.; Reimann, F.; Holst, J.J.; Gribble, F.M. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am. J. Clin. Nutr. 2009, 89, 106–113. [Google Scholar] [CrossRef]
- Clemmensen, C.; Jørgensen, C.V.; Smajilovic, S.; Bräuner-Osborne, H. Robust GLP-1 secretion by basic L-amino acids does not require the GPRC6A receptor. Diabetes Obes. Metab. 2017, 19, 599–603. [Google Scholar] [CrossRef]
- Amin, A.; Neophytou, C.; Thein, S.; Martin, N.M.; Alamshah, A.; Spreckley, E.; Bloom, S.R.; Murphy, K.G. L-Arginine Increases Postprandial Circulating GLP-1 and PYY Levels in Humans. Obesity 2018, 26, 1721–1726. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).