Abstract
The behavior and physiology of most organisms are temporally coordinated and aligned with geophysical time by a complex interplay between the master and peripheral clocks. Disruption of such rhythmic physiological activities that are hierarchically organized has been linked to a greater risk of developing diseases ranging from cancer to metabolic syndrome. Herein, we summarize the molecular clockwork that is employed by intestinal epithelial cells to anticipate environmental changes such as rhythmic food intake and potentially dangerous environmental stress. We also discuss recent discoveries contributing to our understanding of how a proper rhythm of intestinal stem cells may achieve coherence for the maintenance of tissue integrity. Emerging evidence indicates that the circadian oscillations in the composition of the microbiota may operate as an important metronome for the proper preservation of intestinal physiology and more. Furthermore, in this review, we outline how epigenetic clocks that are based on DNA methylation levels may extensively rewire the clock-controlled functions of the intestinal epithelium that are believed to become arrhythmic during aging.
1. Introduction
In 1943, The German theoretical biologist Adolf Meyer-Abich introduced the holobiont, a concept that refers to symbiotic associations throughout a significant portion of an organism’s lifetime (from the Greek word holos, meaning whole or entire). This concept is now applied to the studies of host–microbiota interactions [1]. Although speculative, it is now conceivable that the adaptation of gut microbial communities to their nutrient-rich environment had contributed to the host’s overall fitness over millions of years of coevolution. Recent progress in high-throughput sequencing has given us a detailed view of the exquisitely balanced architecture of the microbiota. Among environmental cues that may affect the composition of the gut microbiota from the earliest days of life, daily alternations in light may coincide with changes in air temperature, food availability and humidity, suggesting a potential advantage for anticipating and adapting to such changing environmental conditions that operate as zeitgebers [2]. The circadian clock is an endogenous, time-tracking system that generates self-sustaining oscillations in a hierarchical manner (from the Latin circa diem, which means about a day). A plethora of physiological functions from several subordinate organs are entrained by the circadian system to a phase and a time period that is close to the 24 h solar day. Among internal timekeeping processes that were acquired early in evolution, several photic and non-photic cues influence biological behavior and the organismal physiological state on a daily basis through a coordinated communication between the peripheral clocks and central pacemaker located in the hypothalamic suprachiasmatic nucleus (Figure 1). Specifically, the latter is specialized in relaying temporal signals to the peripheral clocks through the neuronal and humoral pathways for maintenance of a neurological and metabolic synchrony across the sleep–wake cycle. This interorgan communication in response to the light/dark cycle is able to orchestrate a complex sequence of events that includes a fascinating regulation of the stability of several molecular timekeeping mechanisms, including the stability of the gut microbiota composition. A dysregulation of all natural phenomena that exhibit a diurnal rhythm over the course of the day has been linked to a myriad of pathological processes. The purpose of this review is to discuss recent advances that have contributed to our understanding of how specific functions of intestinal epithelial cells oscillate within a period of 24 h. Notably, we discuss how daily food ingestion may rewire several functions of intestinal epithelial cells and their subsequent impact on the regulation of biological processes at a systemic level [3,4]. Among signals that communicate this information from intestinal epithelial cells to the rest of the body, it is becoming more and more clear that the epithelial interaction with metabolites from the gut microbiota acts as a relay for maintenance of intestinal homeostasis and other systemic physiological processes, including cholesterol metabolism and bile acid synthesis.
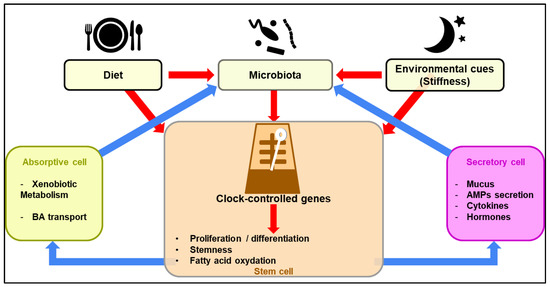
Figure 1.
Diagrammatic overview of the regulation of clock-controlled function of intestinal epithelial cells upon sensing of environmental cues. Diurnal fluctuations in the gut microbiota composition may regulate the ability of stem cells to proliferate and differentiate through a complex metabolic reprogramming (red arrows). This leads to a concerted action by intestinal epithelial cells on the gut microbiota through both positive and negative feedback loops (blue arrows).
2. The Clock Machinery of Intestinal Epithelial Cells Is a Relay for Shaping Circadian Homeostasis
Daily fluctuation observed at the cellular level implies a complex interaction of factors that are intrinsic to intestinal epithelial cells. Even at a local level, many aspects of biological behavior and physiology of intestinal epithelial cells are temporally controlled, including drug detoxification, barrier function, the bile acid enterohepatic cycle, antimicrobial defense and intestinal peristalsis (Figure 2). As another example, time-of-day variation in the renewal of the epithelium coincides with diurnal changes in the composition of the gut microbiota. Such a fluctuation observed at the cellular level implies a complex interaction of factors that are either extrinsic or intrinsic to intestinal epithelial cells. At the molecular level, the circadian clock network consists of transcription–translation feedback loops (TTFLs). Bmal1 binds to Clock in the nucleus, and the activated heterodimer induces the expression of several genes containing E-box in the promoter site. Some of these genes are Per1, Per2, Per3, Cry1, Cry2, Nr1d1 (that is encoding for Rev-erb alpha), Ror alpha and Dbp. Per and Cry are then able to translocate to the nucleus and regulate the Bmal1 activity. Another level of molecular complexity is that Rev-erb alpha and Ror alpha are involved in the repression of Bmal1 activity. A third level of regulation is governed by Dbp and the bZIP transcription factor NFIL3 (nuclear factor interleukin-3-regulated), which regulates Per expression by activation or inhibition. These clock genes, once expressed, regulate the expression of other genes that directly control biological processes; these are termed the clock-controlled genes (CCGs) [5]. Bmal1 expression is controlled by a positive and a negative loop in intestinal epithelial cells induced by NFIL3 and SIRT1, respectively. Interestingly, NFIL3 has been discovered to confer a protection against obesity by diminishing the STAT3 pathway and consequently diminishing fatty acid synthesis. NFIL3 expression is controlled by Rev-erb alpha. Contrarily to NFIL3, SIRT1 induces insulin resistance and inhibits catabolism in response to a high activation of Bmal1/Clock dimer [6,7]. This was demonstrated in studies using Bmal1-deficient mice floxed with VillinCRE as an intestinal epithelial-specific deficient model of the circadian clock. In this work, Bmal1 deficiency diminished Mrp2 mRNA and protein levels, activated by dbp and repressed by E4bp4. Furthermore, Bmal1 directly activated the transcription of dbp and Rev-erb alpha and negatively regulated E4bp4 and Mrp2 by Rev-erb alpha [8]. For a more complete view of these notions on additional peripheral clocks, we refer the reader to an excellent review by Koronowski and Sassone-Corsi [9]. In some instances, such an impairment of circadian processes can subsequently increase susceptibility to disease such as metabolic syndromes and obesity through numerous transcription/translation oscillation loops and epigenetic changes at one time of the day relative to another [10]. Several questions now arise regarding how either light deprivation or ambient light pollution may negatively impact peripheral tissues that are not directly sensitive to light, such as the intestinal epithelial barrier.
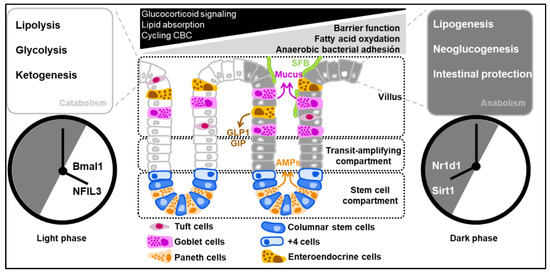
Figure 2.
Diurnal oscillations of the clock-controlled genes regulate the catabolism and anabolism of intestinal epithelial cells. Clock-controlled genes are regulated by a complex interplay of positive and negative loops within the framework of the solar day. As an example, Period 2 is thought to be repressed by the bZIP transcription factor NFIL3 that is negatively regulated when the expression of Nr1d1 (the gene that codifies Rev-erb alpha) reaches its zenith.
3. The Clock Machinery Intrinsically Coordinates the Proliferation of Intestinal Stem Cells and the Fate of Their Daughter Cells
Under normal conditions, diurnal fluctuations in the proliferation of intestinal stem cells have been noticed within the crypt for some time [11]. The sensitivity of intestinal epithelial cells to oxidative damage has been linked to circadian oscillations [12]. From a molecular point of view, Per2 reduces cell cycle progression and cellular proliferation downstream of the beta-catenin pathway in intestinal epithelial cells [13]. Per2 gene silencing was found to enhance cell proliferation and reduce cellular apoptosis in isolated epithelial cells [14]. Furthermore, it is worth noting that attenuation of Bmal1 function resulted in downregulation of genes in the canonical Wnt pathway [15]. The amplitude of the cyclic variation is also enhanced in terminally differentiated intestinal epithelial cells that have irreversibly lost their ability to proliferate when compared to the dividing intestinal stem cells and Paneth cells that reside at the crypt base [16]. Such control of the self-renewal capacity is maintained by several Paneth-cell-secreted Wnt ligands that indirectly influence the gut microbiota composition. This implies a bidirectional interplay, as the clock-controlled expression of several regulators of the cell cycle (including the orphan nuclear receptor retinoic-acid-related orphan receptor alpha (ROR alpha)) orchestrates mutualistic interactions with the microbiota [17]. Studies on stem cells demonstrated that the expression of several circadian genes is repressed by an intrinsic program that is induced during their differentiation process [18,19]. Besides those studies in mammals, other studies in Drosophila sp. revealed that intestinal stem cells do not necessarily require clock genes during differentiation. In one such study, a generated GFP-per reporter gene demonstrated a high heterogeneity of the activity of the circadian clock machinery among discrete subsets of intestinal epithelial cells, and it was not present in enteroendocrine cells [20]. Nevertheless, the use of the murine fibroblast cell line NIH3T3 cells corroborated the concept that circadian clocks, mediated by a highly robust synchronized expression and/or repression of circadian genes between two oscillators, contribute to the dividing capacity of stem cells [21]. Although challenging, further work is required to properly understand how environmental cues are involved in the metabolic reprogramming of stem cells to meet their functional bioenergetic needs at any time of the day.
4. Cyclic Variations in the Functionality of Secretory Intestinal Epithelial Cells and Interactions with the Gut Microbiome
Over the past twenty years, it has been documented that immune reactions fluctuate in magnitude to anticipate the daily time window of greater likelihood of infection. Interestingly, it has recently emerged that the abundance of several discrete subsets of bacteria is submitted to circadian oscillations [22]. The gut is inhabited by a large number of microorganisms with variable needs and behaviors, and it is now becoming clear that circadian rhythms regulate the composition of the intestinal microbiota through several mechanisms to be discussed below. Preliminary studies showed that gut hormones, glucocorticoids and serotonin are secreted by intestinal epithelial cells in a circadian manner [23,24]. Emerging work from the Weizmann Institute of Science has provided evidence that the dominant takeover of a dysbiotic microbiota is properly regulated by the epithelial secretion of antimicrobial peptides (namely, angiogenin-4, Intelectin 1 and Resistin-like molecule β/FIZZ2) downstream of IL-18 signaling [25]. There is growing appreciation, as a result of transcriptomic and epigenetic studies, of the importance of the diurnal fluctuations of bacterial attachment to the epithelial surface as instrumental for temporally modulating the functional outcome of oscillating transcriptional and epigenetic programs as a way to anticipate potential threats, including bacterial infection [26]. Furthermore, the dysbiotic microenvironment increased susceptibility to the intestinal colonization of pathobionts, such as Salmonella. Recently, a study revealed that bacterial attachment triggers a STAT3-dependent antimicrobial response to daytime feeding changes in intestinal epithelial cells [27]. Mechanistically, the authors identified that the attachment of SFB is needed for optimal induction of a STAT3-dependent gene program in intestinal epithelial cells during the feeding period. Consequently, a greater susceptibility to Salmonella infection was noticed as a consequence of the adherence of SFB that concomitantly leads to an overproduction of Reg3 gamma by intestinal epithelial cells. However, vancomycin treatment lowers the production of Reg3 gamma in models of colitis, leading to greater fungal fitness. The authors provided convincing evidence that such a protective effect of fungi on the severity of colitis is caused by an increase in the abundance of Proteobacteria, especially Enterobacteriaceae [28]. When considering this aspect, future investigation will be needed to determine the extent to which the clock-mediated regulation of antimicrobial peptide secretion by intestinal epithelial cells is similarly important for responses against other microorganisms, such as fungi and enteric viruses. Establishing all of the consequences for organismal output will be key to further chronopharmacological investigation to improve the efficacy of immune surveillance, including those that also contribute to defense against cancers.
5. Epithelium Integrity Is Intrinsically Coordinated by the Clock Machinery of Intestinal Epithelial Cells
Besides defense response to oxidative stress, the colonic permeability of ions, nutrients, and water is a mere output function of the circadian system in intestinal epithelial cells. Specifically, the expression of occludin and claudin-1 exhibits daily variations that are consistent with the nighttime nadir of cortisol. A recent study provided evidence that the key clock genes Period1 (Per1) and Per2 were expressed in antiphase with the aforementioned tight-junction proteins. It should be noted that the expression of occludin and claudin-1 is constantly lowered in the colon of mice bearing a mutation in the Per2-encoding gene, which were more resistant to colonic injury induced by dextran sodium sulfate (DSS) than wild-type mice [29]. Per2 acts as an antisense oscillator and directly contributes to the repression of clock-controlled target genes through several mechanisms. Notably, the nucleocytoplasmic shuttling of Cry1/2 is regulated by Per2, which rhythmically interacts with several RNA-binding proteins that may control the expression of tight-junction proteins [30]. However, how the circadian clock transcriptionally regulates their expression has not been clarified in enough detail. The loss of the oscillation of Per2 enhanced expression of several genes involved in epithelial–mesenchymal transition [31]. Such data are of interest, given that the loss of oscillation coincided with lowered levels of genes that are associated with progenitor/epithelial cell function, such as the α6 integrin that mediates the adhesion of epithelial cells to laminin [32]. The authors linked this observation to a mechanical control that mainly depends on the extracellular matrix. The extracellular matrix was described as one of the main actors that communicate with surrounding cells to control their clock biology. Experiments using murine organoids demonstrated that the extracellular matrix then promotes contractile movements in these cells in vitro. This contractile behavior was dependent on the increase in the percentage of oxygen at the surface of intestinal epithelial cells [33]. However, it is currently not clear how the clock machinery in intestinal epithelial cells is molecularly influenced by the stiffness of the extracellular matrix to provide protection against fragility and shedding. Answering this question will require further detailed studies making use of synchronized organoids and mouse models with specific defects of several clock genes in intestinal epithelial cells. Among molecules of interest is PPAR-gamma, loss of which heightens epithelial oxygenation and results in the loss of barrier function through actin disassembly from the cytoskeleton [34]. Although it has not been addressed experimentally, it makes sense to consider the metabolic reprogramming of intestinal stem cells through fatty acid oxidation as instrumental in the divergence of their proliferative capacity when compared to their daughter cells, in which full activation of PPAR-gamma is attained (Figure 2). Furthermore, it is important to keep in mind that the amplitude of clock gene expression is enhanced in soft 3D microenvironments compared to stiff 2D environments [35].
6. The Circadian Clock Machinery of Intestinal Epithelial Cells Regulates Their Response to Oxidative Stress
Circadian rhythmicity is reinforced by several nutrients, energy and redox level signals to adapt intestinal physiology to specific needs within the framework of the solar day. Below, we review recent findings on the daily fluctuations in the expression or activity levels that have been measured for many enzymes that protect the intestinal epithelium from oxidative stress [36]. Specifically, intestinal epithelial cells express several genes of the clock machinery, deletion of which has an impact on cellular response to oxidative stress, including xenobiotic/drug detoxification [36]. This was elegantly demonstrated in studies using a model of clock machinery deficiency in intestinal epithelial cells. Loss of the aryl hydrocarbon receptor nuclear translocator-like protein 1 (Arntl, also referred to as Bmal1 for brain and muscle ARNT-like 1) in intestinal epithelial cells lowered the expression of the multidrug-resistance-associated protein 2 (Mrp2), which is required to limit methotrexate absorption by blocking entrance into enterocytes. In everted gut sac experiments, the mucosal-to-serosal transport and intestinal accumulation of methotrexate was enhanced as a consequence of loss of Bmal1 in intestinal epithelial cells [8]. This is of particular importance, as methotrexate is an inhibitor of dihydrofolate reductase, and its anti-inflammatory action relies on the production of reactive oxygen species (ROS) [37]. When reaching elevated intracellular levels of ROS, one may anticipate some cellular damage, including lipid peroxidation. It is conceivable that continuous oxidative stress throughout the day leads to pathological conditions, including leaky gut and fibrosis. However, some bacteria, such as Lactobacillus paracasei, transfer lactate to the epithelial cells, in which acetyl-coA synthesis occurs in the presence of oxygen, favoring the tricarboxylic acid cycle (TCA) and fatty acid synthesis [38]. Dissecting how the clock machinery properly regulates the interplay between innate immunity and redox signaling will contribute to the understanding of downstream defense responses when oxidative stress excessively occurs in intestinal epithelial cells. It has been demonstrated in stem cells and epithelial cells from other organs that Bmal1 can negatively regulate the expression of the mitochondria Ucp1 protein to reduce the amount of ATP generated through an oxidation of fuels [15] and ROS generation [39]. Among several possible explanations, the potential contribution of peroxisome-proliferator-activated receptor delta (PPAR-delta), which is activated upon fasting, remains to be determined [40]. Consistent with this concept, the formation of ketone bodies is induced by long-term fasting and depends on changes in the microbiome. Specifically, increased ketone bodies were observed in conventionalized mice compared to germ-free animals [41]. In addition, the use of the Arntl-floxed villin-Cre mice revealed that the epithelial expression of Bmal1 contributes to obesity development, body weight gain and related abnormalities, such as hyperlipidemia, through decreased lipid absorption. Among the underlying mechanisms, the epithelial loss of Bmal1 led to a reduced expression of the Dgat2-encoding gene, which codifies the enzyme of triacylglycerol synthesis [42]. In this sense, it is unclear how circadian regulation of fatty acid oxidation may be correlated with epithelial renewal capacities of stem cells and whether epigenetic chromatin-modifying enzymes may contribute to this fairly dynamic process at steady state. However, natural protective mechanisms against lipid peroxidation and nitric oxide production are intrinsically controlled by the expression of PPAR-gamma in intestinal epithelial cells. Another possibility involves the orchestration of local and systemic lipid metabolism by a signaling circuit linking the epithelial clock to sensing of the gut-specific microbial components by innate immune cells [6].
7. Short-Chain Fatty-Acid-Producing Bacteria Impose a Metabolic Choice to Be Made by Intestinal Epithelial Cells
Some gut bacteria are beneficial due to the end products of their metabolism that they provide to the intestinal epithelial cell. Notably, dietary fibers are metabolized in short chain fatty acids (SCFA) by some anaerobic bacteria. The end products of their metabolism subsequently induce a metabolic reprogramming of colonocytes. One intriguing example among others is that PPAR-gamma activation by butyrate promotes aerobic glycolysis, together with fatty acid oxidation [43]. Epithelial loss of PPAR-gamma enhances nitric oxide production to a similar extent as that observed in response to interferon-gamma. Specifically, inducible nitric oxide synthase (iNOS) was inhibited by PPAR-gamma in response to butyrate to prevent dysbiosis that may predispose to colitis. The pantetheinase VNN1 that is regulated by PPAR-gamma is able to reduce the Warburg effect produced under cellular stress conditions, contributing to metabolic homeostasis. This was demonstrated by Giessner and colleagues when pantetheinase, an inducer of Vnn1 expression, was administered to mice, inducing an increase in the mitochondrial function in tumors [44]. In contrast, colonocytes cannot rely on mitochondrial fatty oxidation under inflammatory conditions but rather on anaerobic glycolysis, as observed in cancer cells. Of particular interest, the cellular metabolic choice of colonocytes was restricted by interferon-gamma, creating an environment in which oxygen supply is excessive, leading to an expansion of facultative anaerobic bacteria, such as Enterobacteriaceae. Conversely, the inhibition of PPAR-gamma contributes to a deregulation of glucose metabolism and induces an increase in glucose uptake [45]. To some extent, this is similar to the metabolic reprogramming that is needed to support cell fate and function of myeloid cells [46]. Of equal importance is the epithelial function of the pentanoate receptor GPR41 (also called free fatty acid receptor 3), which promotes inflammation [47], whereas pentanoate administration suppresses the generation of small-intestinal Th17 cells in germ-free mice mono-colonized with segmented filamentous bacteria (SFB) [48]. Furthermore, the G-protein-coupled receptor GPR43 facilitates inflammasome activation in colonocytes when treated with acetate [49]. It is conceivable that other acetate-producing bacteria may help epithelial cells generate acetyl-coenzyme A (acetyl-coA), which is required under intracellular anaerobic conditions to promote fatty acid biosynthesis. Another level of complexity that must be considered is the influence of changes in the gut microbiota composition that may lead to the generation of bacterial fermentation products that have the capacity to upregulate the expression of other genes involved in mitochondrial fatty acid oxidation, such as PPAR-alpha, CD36 and carnitine palmitoyltransferase I [50]. Furthermore, it remains to be determined whether metabolites other than SCFA may also impose a choice to be made by intestinal stem cells and their daughter cells. Among such metabolites, phytate and inositol trisphosphate have been reported to control the expression of intestinal epithelial histone deacetylase 3 (HDAC3) [51], which is recruited rhythmically to chromatin and produces diurnal oscillations in histone acetylation and therefore in metabolic gene expression, including CD36 [52]. Thus, HDAC3 represents a converging epigenetic sensor of distinct metabolites that calibrates rhythmic host responses to diverse microbial signals. Furthermore, the activity of PPAR-alpha is undoubtedly influenced by the daily fluctuation in the abundance of Gram-negative bacteria through the epithelial activation of the rate-limiting enzyme Cyp11a1 in cortisol synthesis. Prior studies using genetically modified mouse models of epithelial deficiency in either PPAR-alpha or glucocorticoid receptor confirmed microbiota-dependent mediation of several clock genes to enable lipolysis and lower insulin levels after a long period of antibiotic treatment [17]. However, it remains unclear whether such oscillating phenomena may reflect an epithelial detachment of bacteria at a specific time of day, as observed when SFB is able to adhere to the epithelium to modulate antimicrobial peptide expression during the sleep-to-wake transition [27].
8. Systemic Influence of Epithelial Clock Machinery on the Synthesis of Bile Acids and on Gut Motility
Bile acids are synthesized from cholesterol through the activation of cholesterol 7alpha-hydroxylase (CYP7A1) in the liver. After being conjugated with either glycine or taurine, they are released to the intestinal lumen to promote the epithelial absorption of long-chain fatty acids [53]. The circadian clock can change the expression of some genes that control the synthesis of bile acids, such as the farnesoid X receptor (FXR). FXR activation controls bile acid synthesis in the liver and bile acid secretion in organs by regulating the expression of bile acid transporters in both the liver and enterocytes [54]. Some colonic bacteria are then required to specifically deconjugate conjugated primary bile salts and transform them into secondary bile acids during the fasting period. The deconjugation is catalyzed when the bile salt hydrolase (BSH) is synthesised by a discrete subset of bacteria, including Lactobacillus, Clostridium, Bacteroides, Enterococcus and Bifidobacterium [55]. In response to vancomycin, postprandial fecal concentrations of secondary bile salts and of fibroblast growth factor 19 (FGF19) were significantly reduced, suggesting reduced BSH activity. Interestingly, a compensatory increase in Gram-negative bacteria coincided with a marked reduction in the abundance of Gram-positive bacteria in response to vancomycin. In agreement with the possibility that vancomycin may enhance bile acid synthesis, an increased level of the primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA) was inversely correlated with the abundance of SCFA-producing bacteria [56]. Consequently, peripheral insulin sensitivity was significantly lowered, despite the absence of changes in the plasma level of insulinotropic hormones. Intriguingly, some studies demonstrated that glucagon secretion is reduced in mice that are deficient in carnitine palmitoyltransferase (Cpt1a), a transporter of fatty acids to the mitochondria [57]. Of equal importance, vancomycin decreases the abundance of Gram-positive bacteria while changing gut motility [58]. This effect on intestinal motility is likely dependent on the bacterial sensing capacity of Toll-like receptor 4 (Tlr4) at the surface of intestinal epithelial cells [59]. Further work is required to determine whether the decreased insulin sensitivity is a consequence of the vancomycin-induced decrease in the levels of lithocholic acid (LCA) and deoxycholic acid (DCA) that are sensed by the bile acid membrane receptor TGR5 at the surface of colonic epithelial cells for maintenance of systemic insulin sensitivity. This is of particular importance to clarify the sequence of events leading from changes in the gut microbiota composition to a reduced number of enteroendocrine cells in response to dietary lards [60]. Among the bacteria found more abundantly in response to a high-fat diet, Bilophila wadsworthia is a sulfite-reducing pathobiont that subsequently affects the epithelial integrity in Interleukin-10-deficient mice [61]. Additional work is awaited to understand how the metabolism of bile acids is influenced by other bacteria, such as Parabacteroides distasonis and Ruminoclostridium [62]. P. distasonis produces secondary bile acids and succinate, which are absorbed by the intestinal cell to produce de novo glucose in response to FXR activation [63].
9. Aging
Aging is characterized by a progressive decrease in a wide variety of physiological functions. Some studies have demonstrated that cyclicity and activity of clock genes showed significant age-related alterations, becoming fragmented and diminished in elder individuals. This evidence suggests that there is a progressive degeneration of the structure and cyclicity of the circadian timing that induces a break of the control of the rest and sleep phases in the elderly [64]. Recently, epigenetic events were discovered as a consequence of hypoxia. Specifically, hypoxia-inducible factor 1 alpha (HIF1α) modifies the epithelial expression of the HDAC3 that controls epithelium integrity [65]. These pieces of evidence open the road for additional investigations on how the oscillations of the gut microbiome may occur. This conundrum is particularly difficult to address, as it will require a more detailed understanding of how arrhythmic bacterial adhesion may precisely manipulate the functionality of different cells at a specific time of the day. We can further estimate that the epigenetic events induced by environmental factors during aging are cause a metabolic alteration in intestinal epithelial cells as a consequence of the clock asynchrony. However, further investigations are required to better understand how alterations in the gut microbiome may confer a greater risk of degenerative diseases and cancer later in life as a potential consequence of epigenetic events.
10. Perspectives
A wealth of recent studies unveiled that intestinal epithelial cells play an essential role in relaying signals both to and from the intestinal microbiota during either fasting or dietary intake time windows. One may speculate that the diurnal fluctuations in the composition of the gut microbiome help to assist host defense and metabolic homeostasis. From an evolutionary perspective, this concept implies the need for specific molecular passwords that are released at a time when there is a specific need for either anabolism or catabolism. A deregulation of the axis induced by diet, antibiotics or other dysbiosis-promoting factors is challenging for the host and may contribute to disease development upon failure to properly compensate for the functionality of the gut microbiome. Notably, an alteration of the bile acid sensing and transport by intestinal epithelial cells may influence liver metabolism through PPAR-alpha-dependent mechanisms that are beginning to be understood. As an excellent example, it was described how a high-fat diet promotes a dysbiosis environment that alters the clock machinery of peripheral organs, leading to increased permeability and insulin resistance [66]. Furthermore, a high-fat diet can induce a disruption of the circadian oscillation of some important genera with BSH activity, such as Lactobacilli [67]. The importance of the circadian regulation of feeding time for the microbiota was demonstrated by the application to db/db mice of an intermittent fasting of 24 h; this condition was compared with ad libitum fed diabetic mice. During the intermittent fasting, microbiota composition of db/db mice was abruptly changed, increasing the abundance of Lachnospiraceae, Lactobacillus, Oscillospira, Ruminococcus and Clostridiales at the fecal level. In contrast, the abundance of Akkermansia and Bacteroides decreased in obese mice with intermittent fasting [68]. It is now evident that epithelial cells play a key role to control alterations of the gut microbiome. However, it remains difficult to mechanistically characterize the advantages or disadvantages that each oscillating bacterium may induce individually or collectively at either a steady state or upon metabolic stresses. Additional studies are required to optimize the design of drug dosing regimens for individuals with a disrupted circadian clock who may experience a lowered drug detoxification, leading to defects in ROS scavenging and/or antioxidant signaling.
Author Contributions
Conceptualization, M.C.; resources, M.C.; writing—original draft preparation, J.J.M.-G. and M.C.; writing—review and editing, J.J.M.-G., L.H., P.L. and M.C.; visualization, P.L. and M.C.; funding acquisition, D.R., A.L., P.L. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by funding to D.R., A.M., P.L. and M.C. from the Institut National de la Santé et de la Recherche Médicale through the French transversal program on microbiota (Grant No. R21091EK/RSE21091EKA). J.J.M.G. was a recipient of a fellowship funded by the Fondation pour La Recherche Médicale.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
BSH: Bile-Salt hydrolase; CA: Cholic acid; CCG: Clock controlled genes; CDCA: Chenodeoxycholic acid; Cpt1a: Carnitine palmitoyltransferase 1a; DCA: Deoxycholic acid; DSS: Dextran Sodium Sulfate; FXR: Farnesoid X receptor; FGF19: Fibroblast Growth Factor 19; GFP: Green fluorescent protein; GIP: glucose-dependent insulinotropic polypeptide; GLP-1: glucagon-like peptide-1; HDAC3: Histone deacetylase 3; IEC: intestinal epithelial cells; iNOS: Inducible nitric oxide synthase; mRNA: messenger ribonucleic acid; IL-18: Interleukin 18; NFIL3: Nuclear Factor, Interleukin 3 Regulated; Per1: Period 1; PPAR: Peroxisome proliferator-activated receptor; LCA: Lithocholic acid; RORalpha: RAR-related orphan receptor alpha; ROS: reactive oxygen species; SCFA: Short-Chain fatty acids; SFB: Segmented filamentous bacteria; SIRT1: Sirtuin 1; STAT3: Signal transducer and activator of transcription 3; TCA: Tricarboxylic acid cycle; TGR5: G-protein-coupled bile acid receptor; Tlr4: Toll-like receptor 4; TTFLs: transcription-translation feedback loops; Vnn-1: Vanin 1; Wnt: Wingless signal transduction pathway.
References
- van de Guchte, M.; Blottière, H.M.; Doré, J. Humans as Holobionts: Implications for Prevention and Therapy. Microbiome 2018, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Tognini, P.; Thaiss, C.A.; Elinav, E.; Sassone-Corsi, P. Circadian Coordination of Antimicrobial Responses. Cell Host Microbe 2017, 22, 185–192. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut Microbial Metabolites as Multi-Kingdom Intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Debnath, N.; Kumar, R.; Kumar, A.; Mehta, P.K.; Yadav, A.K. Gut-Microbiota Derived Bioactive Metabolites and Their Functions in Host Physiology. Biotechnol. Genet. Eng. Rev. 2021, 37, 105–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, L. Circadian Clock Regulates Inflammation and the Development of Neurodegeneration. Front. Cell. Infect. Microbiol. 2021, 11, 69554. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, Z.; Yu, X.; Ruhn, K.A.; Kubo, M.; Hooper, L.V. The Intestinal Microbiota Regulates Body Composition through NFIL3 and the Circadian Clock. Science 2017, 357, 912–916. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Zhang, Y.; Zhang, F.; Xia, Y.; Liu, J.; Huang, R.; Wang, Y.; Hu, Y.; Wu, J.; Dai, C.; et al. CLOCK/BMAL1 Regulates Circadian Change of Mouse Hepatic Insulin Sensitivity by SIRT1. Hepatology 2014, 59, 2196–2206. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, T.; Zhou, C.; Xu, H.; Guo, L.; Chen, M.; Wu, B. The Circadian Clock Gene Bmal1 Controls Intestinal Exporter MRP2 and Drug Disposition. Theranostics 2019, 9, 2754–2767. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Sassone-Corsi, P. Communicating Clocks Shape Circadian Homeostasis. Science 2021, 371, eabd0951. [Google Scholar] [CrossRef]
- Masri, S.; Sassone-Corsi, P. The Emerging Link between Cancer, Metabolism, and Circadian Rhythms. Nat. Med. 2018, 24, 1795–1803. [Google Scholar] [CrossRef]
- Neal, J.; Potten, C. Circadian Rhythms in the Epithelial Cells and the Pericryptal Fibroblast Sheath in Three Different Sites in the Murine Intestinal Tract. Cell Tissue Kinet. 1981, 14, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Karpowicz, P.; Zhang, Y.; Hogenesch, J.B.; Emery, P.; Perrimon, N. The Circadian Clock Gates the Intestinal Stem Cell Regenerative State. Cell Rep. 2013, 3, 996–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wood, P.A.; Ansell, C.M.; Ohmori, M.; Oh, E.Y.; Xiong, Y.; Berger, F.G.; Peña, M.M.O.; Hrushesky, W.J.M. β-Catenin Induces β-TrCP-Mediated PER2 Degradation Altering Circadian Clock Gene Expression in Intestinal Mucosa of ApcMin/+ Mice. J. Biochem. 2009, 145, 289–297. [Google Scholar] [CrossRef]
- Gao, J.; Xu, Q.; Wang, M.; Ouyang, J.; Tian, W.; Feng, D.; Liang, Y.; Jiang, B.; Loor, J.J. Ruminal Epithelial Cell Proliferation and Short-Chain Fatty Acid Transporters in Vitro Are Associated with Abundance of Period Circadian Regulator 2 (PER2). J. Dairy Sci. 2020, 103, 12091–12103. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chatterjee, S.; Li, L.; Kim, J.M.; Lee, J.; Yechoor, V.K.; Minze, L.J.; Hsueh, W.; Ma, K. The Clock Gene, Brain and Muscle Arnt--like 1, Regulates Adipogenesis via Wnt Signaling Pathway. FASEB J. 2012, 26, 3453–3463. [Google Scholar] [CrossRef] [PubMed]
- Matsu-ura, T.; Dovzhenok, A.; Aihara, E.; Rood, J.; Le, H.; Ren, Y.; Rosselot, A.E.; Zhang, T.; Lee, C.; Obrietan, K.; et al. Intercellular Coupling of the Cell Cycle and Circadian Clock in Adult Stem Cell Culture. Mol. Cell 2016, 64, 900–912. [Google Scholar] [CrossRef] [Green Version]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in Intestinal Epithelium Is Orchestrated by the Circadian Clock and Microbiota Cues Transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef] [Green Version]
- Yagita, K.; Horie, K.; Koinuma, S.; Nakamura, W.; Yamanaka, I.; Urasaki, A.; Shigeyoshi, Y.; Kawakami, K.; Shimada, S.; Takeda, J.; et al. Development of the Circadian Oscillator during Differentiation of Mouse Embryonic Stem Cells in Vitro. Proc. Natl. Acad. Sci. USA 2010, 107, 3846–3851. [Google Scholar] [CrossRef] [Green Version]
- Weger, M.; Diotel, N.; Dorsemans, A.C.; Dickmeis, T.; Weger, B.D. Stem Cells and the Circadian Clock. Dev. Biol. 2017, 431, 111–123. [Google Scholar] [CrossRef]
- Parasram, K.; Bernardon, N.; Hammoud, M.; Chang, H.; He, L.; Perrimon, N.; Karpowicz, P. Intestinal Stem Cells Exhibit Conditional Circadian Clock Function. Stem Cell Rep. 2018, 11, 1287–1301. [Google Scholar] [CrossRef] [Green Version]
- Bieler, J.; Cannavo, R.; Gustafson, K.; Gobet, C.; Gatfield, D.; Naef, F. Robust Synchronization of Coupled Circadian and Cell Cycle Oscillators in Single Mammalian Cells. Mol. Syst. Biol. 2014, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Lozano, M.; Mingomataj, E.L.; Wu, W.K.; Ridout, S.A.; Brubaker, P.L. Circadian Secretion of the Intestinal Hormone, Glucagon-like Peptide-1, by the Rodent L-Cell. Diabetes 2014, 63, 3674–3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, B.R.; de Souza, M.J.; Williams, N.I. Characterization of the Diurnal Rhythm of Peptide YY and Its Association with Energy Balance Parameters in Normal-Weight Premenopausal Women. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 2015, 163, 1428–1443. [Google Scholar] [CrossRef] [Green Version]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.F.; Behrendt, C.L.; Ruhn, K.A.; Lee, S.; Raj, P.; Takahashi, J.S.; Hooper, L.V. The Microbiota Coordinates Diurnal Rhythms in Innate Immunity with the Circadian Clock. Cell 2021, 184, 4154–4167.e12. [Google Scholar] [CrossRef]
- Sovran, B.; Planchais, J.; Jegou, S.; Straube, M.; Lamas, B.; Natividad, J.M.; Agus, A.; Dupraz, L.; Glodt, J.; da Costa, G.; et al. Enterobacteriaceae Are Essential for the Modulation of Colitis Severity by Fungi. Microbiome 2018, 6, 152. [Google Scholar] [CrossRef]
- Oh-oka, K.; Kono, H.; Ishimaru, K.; Miyake, K.; Kubota, T.; Ogawa, H.; Okumura, K.; Shibata, S.; Nakao, A. Expressions of Tight Junction Proteins Occludin and Claudin-1 Are under the Circadian Control in the Mouse Large Intestine: Implications in Intestinal Permeability and Susceptibility to Colitis. PLoS ONE 2014, 9, e98016. [Google Scholar] [CrossRef]
- Yang, H.; Rao, J.N.; Wang, J.Y. Posttranscriptional Regulation of Intestinal Epithelial Tight Junction Barrier by RNA-Binding Proteins and MicroRNAs. Tissue Barriers 2014, 2, e28320. [Google Scholar] [CrossRef] [Green Version]
- Hwang-Verslues, W.W.; Chang, P.H.; Jeng, Y.M.; Kuo, W.H.; Chiang, P.H.; Chang, Y.C.; Hsieh, T.H.; Su, F.Y.; Lin, L.C.; Abbondante, S.; et al. Loss of Corepressor PER2 under Hypoxia Up-Regulates OCT1-Mediated EMT Gene Expression and Enhances Tumor Malignancy. Proc. Natl. Acad. Sci. USA 2013, 110, 12331–12336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, N.; Williams, J.; Pekovic-Vaughan, V.; Wang, P.; Olabi, S.; McConnell, J.; Gossan, N.; Hughes, A.; Cheung, J.; Streuli, C.H.; et al. Cellular Mechano-Environment Regulates the Mammary Circadian Clock. Nat. Commun. 2017, 8, 14287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimarco, R.L.; Su, J.; Yan, K.S.; Dewi, R.; Kuo, C.J.; Heilshorn, S.C. Engineering of Three-Dimensional Microenvironments to Promote Contractile Behavior in Primary Intestinal Organoids. Integr. Biol. 2014, 6, 127–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.; Liu, L.; MacLean, A.L.; Wong, C.W.; Zhao, W.; Nie, Q. A Mathematical Model of Mechanotransduction Reveals How Mechanical Memory Regulates Mesenchymal Stem Cell Fate Decisions. BMC Syst. Biol. 2017, 11, 55. [Google Scholar] [CrossRef]
- Williams, J.; Yang, N.; Wood, A.; Zindy, E.; Meng, Q.-J.; Streuli, C.H. Epithelial and Stromal Circadian Clocks Are Inversely Regulated by Their Mechano-Matrix Environment. J. Cell Sci. 2018, 131, jcs208223. [Google Scholar] [CrossRef] [Green Version]
- Sládek, M.; Rybová, M.; Jindráková, Z.; Zemanová, Z.; Polidarová, L.; Mrnka, L.; O’Neill, J.; Pácha, J.; Sumová, A. Insight Into the Circadian Clock Within Rat Colonic Epithelial Cells. Gastroenterology 2007, 133, 1240–1249. [Google Scholar] [CrossRef]
- Kaundal, U.; Khullar, A.; Leishangthem, B.; Jain, S.; Dhooria, A.; Saikia, B.; Dhir, V. The Effect of Methotrexate on Neutrophil Reactive Oxygen Species and CD177 Expression in Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2021, 33, 479–486. [Google Scholar]
- Araújo, J.R.; Tazi, A.; Burlen-Defranoux, O.; Vichier-Guerre, S.; Nigro, G.; Licandro, H.; Demignot, S.; Sansonetti, P.J. Fermentation Products of Commensal Bacteria Alter Enterocyte Lipid Metabolism. Cell Host Microbe 2020, 27, 358–375.e7. [Google Scholar] [CrossRef]
- Jia, P.; Wu, X.; Pan, T.; Xu, S.; Hu, J.; Ding, X. Uncoupling Protein 1 Inhibits Mitochondrial Reactive Oxygen Species Generation and Alleviates Acute Kidney Injury. EBioMedicine 2019, 49, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Mihaylova, M.M.; Cheng, C.W.; Cao, A.Q.; Tripathi, S.; Mana, M.D.; Bauer-Rowe, K.E.; Abu-Remaileh, M.; Clavain, L.; Erdemir, A.; Lewis, C.A.; et al. Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell 2018, 22, 769–778.e4. [Google Scholar] [CrossRef] [Green Version]
- Crawford, P.A.; Crowley, J.R.; Sambandam, N.; Muegge, B.D.; Costello, E.K.; Hamady, M.; Knight, R.; Gordon, J.I. Regulation of Myocardial Ketone Body Metabolism by the Gut Microbiota during Nutrient Deprivation. Prod. Natl. Acad. Sci. USA 2009, 106, 11276–11281. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Wang, Z.; Zhang, T.; Chen, X.; Xu, H.; Wang, F.; Guo, L.; Chen, M.; Liu, K.; Wu, B. Deficiency of Intestinal Bmal1 Prevents Obesity Induced by High-Fat Feeding. Nat. Commun. 2021, 12, 5323. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-Activated PPAR-γ Signaling Inhibits Dysbiotic Enterobacteriaceae Expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef]
- Giessner, C.; Millet, V.; Mostert, K.J.; Gensollen, T.; Manh, T.P.V.; Garibal, M.; Dieme, B.; Attaf-Bouabdallah, N.; Chasson, L.; Brouilly, N.; et al. Vnn1 Pantetheinase Limits the Warburg Effect and Sarcoma Growth by Rescuing Mitochondrial Activity. Life Sci. Alliance 2018, 1, e201800073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.; Audrey Nguyen, M.T.; Yoshizaki, T.; Favelyukis, S.; Patsouris, D.; Imamura, T.; Verma, I.M.; Olefsky, J.M. Suppression of PPAR-Gamma Attenuates Insulin-Stimulated Glucose Uptake by Affecting Both GLUT1 and GLUT4 in 3T3-L1 Adipocytes. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charo, I.F. Macrophage Polarization and Insulin Resistance: PPARγ in Control. Cell Metab. 2007, 6, 96–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The Short-Chain Fatty Acid Pentanoate Suppresses Autoimmunity by Modulating the Metabolic-Epigenetic Crosstalk in Lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef] [Green Version]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Rawls, J.F. Feeling the Burn: Intestinal Epithelial Cells Modify Their Lipid Metabolism in Response to Bacterial Fermentation Products. Cell Host Microbe 2020, 27, 314–316. [Google Scholar] [CrossRef]
- Wu, S.E.; Hashimoto-Hill, S.; Woo, V.; Eshleman, E.M.; Whitt, J.; Engleman, L.; Karns, R.; Denson, L.A.; Haslam, D.B.; Alenghat, T. Microbiota-Derived Metabolite Promotes HDAC3 Activity in the Gut. Nature 2020, 586, 108–112. [Google Scholar] [CrossRef]
- Kuang, Z.; Wang, Y.; Li, Y.; Ye, C.; Ruhn, K.A.; Behrendt, C.L.; Olson, E.N.; Hooper, L.V. The Intestinal Microbiota Programs Diurnal Rhythms in Host Metabolism through Histone Deacetylase 3. Science 2019, 365, 1428–1434. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile Acids: Regulation of Synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Yang, J.; Xiang, D.; Li, G.; Liu, D.; Zhang, C. Circadian Rhythms and Bile Acid Homeostasis: A Comprehensive Review. Chronobiol. Int. 2020, 37, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.; Kootte, R.S.; van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of Oral Vancomycin on Gut Microbiota, Bile Acid Metabolism, and Insulin Sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef]
- Briant, L.J.B.; Dodd, M.S.; Chibalina, M.V.; Rorsman, N.J.G.; Johnson, P.R.V.; Carmeliet, P.; Rorsman, P.; Knudsen, J.G. CPT1a-Dependent Long-Chain Fatty Acid Oxidation Contributes to Maintaining Glucagon Secretion from Pancreatic Islets. Cell Rep. 2018, 23, 3300–3311. [Google Scholar] [CrossRef]
- Hung, L.Y.; Parathan, P.; Boonma, P.; Wu, Q.; Wang, Y.; Ann Luna, R.; Bornstein, J.C.; Savidge, T.C.; Foong, J.P. Antibiotic Exposure Postweaning Disrupts the Neurochemistry and Function of Enteric Neurons Mediating Colonic Activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G1042–G1053. [Google Scholar] [CrossRef]
- Anitha, M.; Vijay-Kumar, M.; Sitaraman, S.V.; Gewirtz, A.T.; Srinivasan, S. Gut Microbial Products Regulate Murine Gastrointestinal Motility via Toll-like Receptor 4 Signaling. Gastroenterology 2012, 143, 1006–1016. [Google Scholar] [CrossRef] [Green Version]
- Just, S.; Mondot, S.; Ecker, J.; Wegner, K.; Rath, E.; Gau, L.; Streidl, T.; Hery-Arnaud, G.; Schmidt, S.; Lesker, T.R.; et al. The Gut Microbiota Drives the Impact of Bile Acids and Fat Source in Diet on Mouse Metabolism. Microbiome 2018, 6, 134. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-Fat-Induced Taurocholic Acid Promotes Pathobiont Expansion and Colitis in Il10-/- Mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, R.S.; Gaffney, M.; Hopkins, S.; Kelley, T.; Gonzalez, A.; Bowers, S.J.; Vitaterna, M.H.; Turek, F.W.; Foxx, C.L.; Lowry, C.A.; et al. Ruminiclostridium 5, Parabacteroides Distasonis, and Bile Acid Profile Are Modulated by Prebiotic Diet and Associate with Facilitated Sleep/Clock Realignment after Chronic Disruption of Rhythms. Brain Behav. Immun. 2021, 97, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Bäckhed, F. From Association to Causality: The Role of the Gut Microbiota and Its Functional Products on Host Metabolism. Mol. Cell 2020, 78, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Liu, R.-Y.; Wang, Q.-S.; van Someren, J.W.; Xu, H.; Zhou, J.-N. Age-Associated Difference in Circadian Sleep-Wake and Rest-Activity Rhythms. Physiol. Behav. 2002, 76, 597–603. [Google Scholar] [CrossRef]
- Whitt, J.; Woo, V.; Lee, P.; Moncivaiz, J.; Haberman, Y.; Denson, L.; Tso, P.; Alenghat, T. Disruption of Epithelial HDAC3 in Intestine Prevents Diet-Induced Obesity in Mice. Gastroenterology 2018, 155, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Garidou, L.; Pomié, C.; Klopp, P.; Waget, A.; Charpentier, J.; Aloulou, M.; Giry, A.; Serino, M.; Stenman, L.; Lahtinen, S.; et al. The Gut Microbiota Regulates Intestinal CD4 T Cells Expressing RORγt and Controls Metabolic Disease. Cell Metab. 2015, 22, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [Green Version]
- Beli, E.; Yan, Y.; Moldovan, L.; Vieira, C.P.; Gao, R.; Duan, Y.; Prasad, R.; Bhatwadekar, A.; White, F.A.; Townsend, S.D.; et al. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in Db/Db Mice. Diabetes 2018, 67, 1867–1879. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).