Metabolomic Insights of the Effects of Bacterial Algicide IRI-160AA on Dinoflagellate Karlodinium veneficum
Abstract
1. Introduction
2. Results
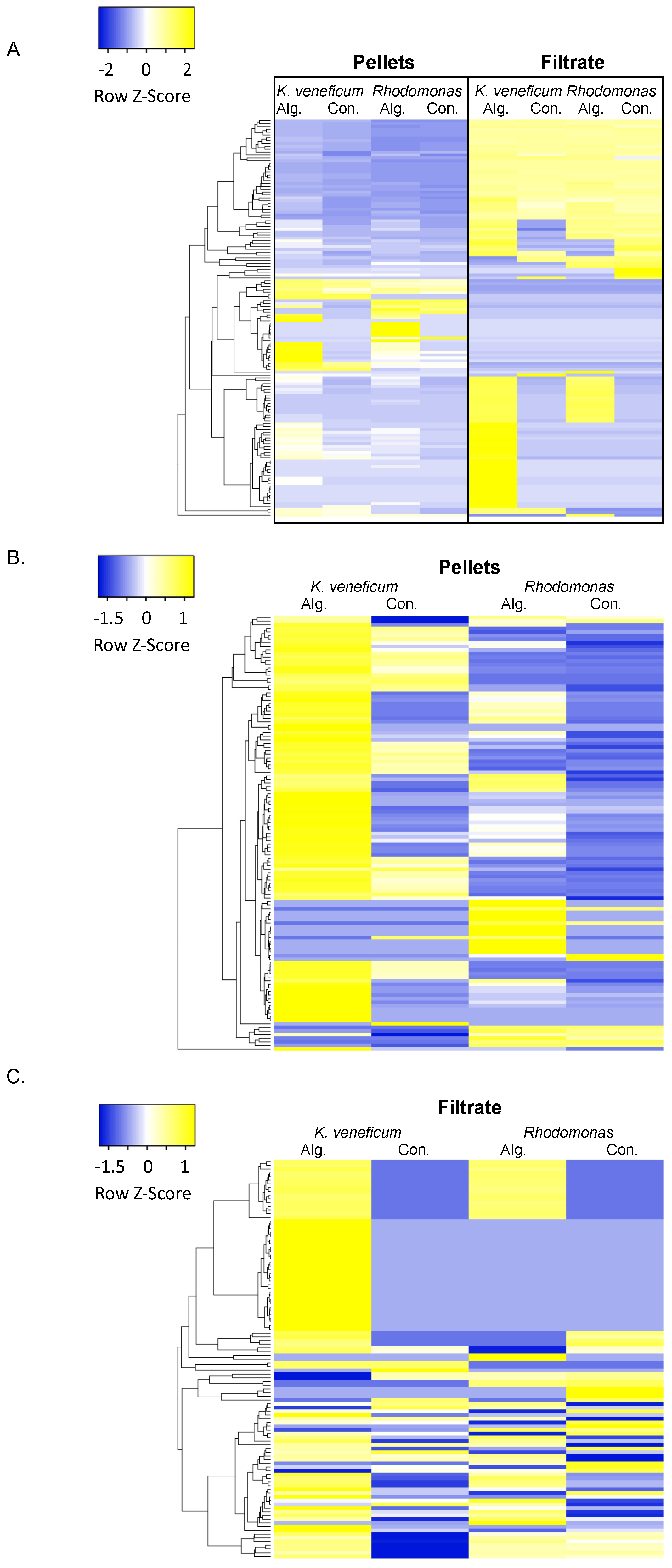
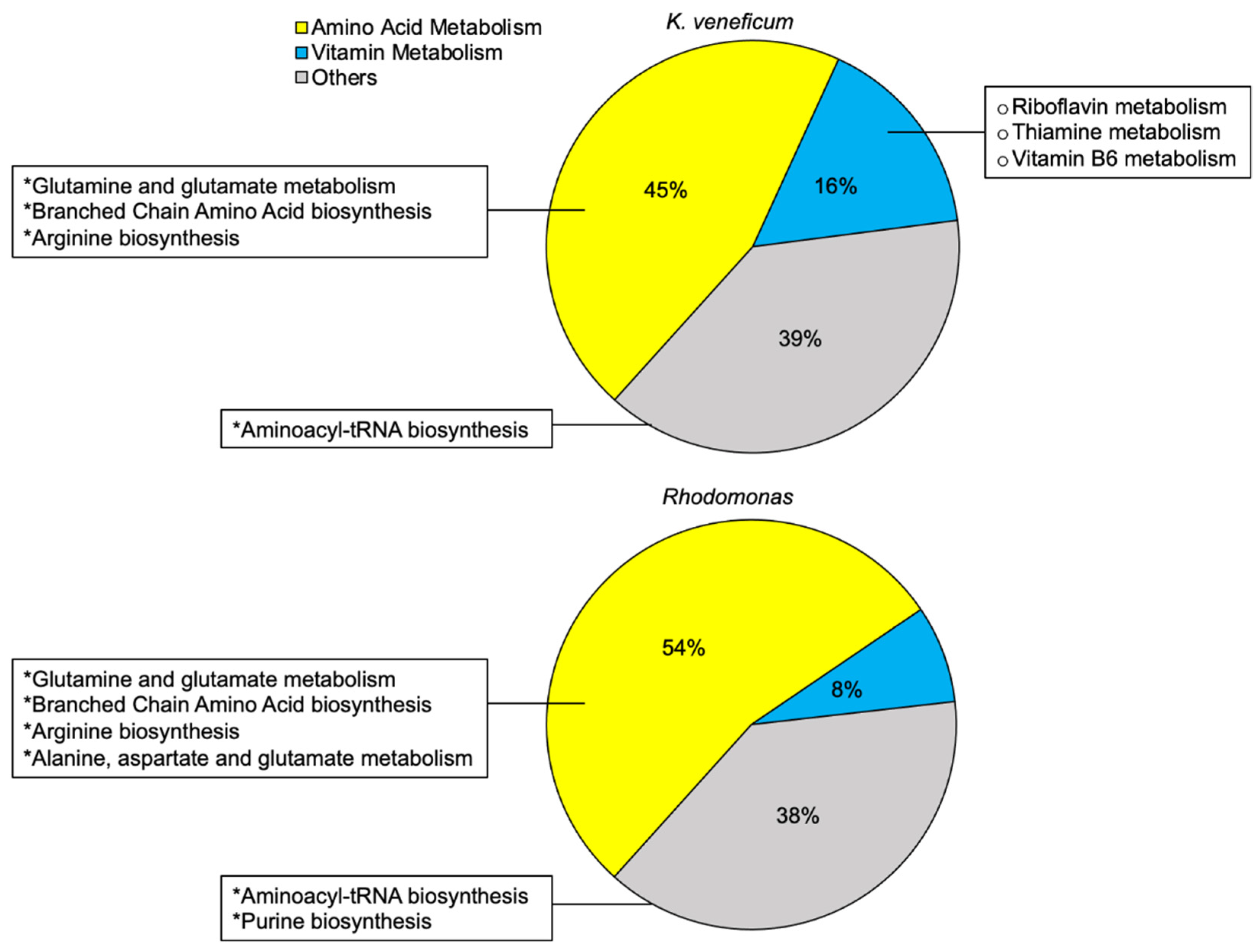
2.1. Metabolomics Profile of Cell Pellets
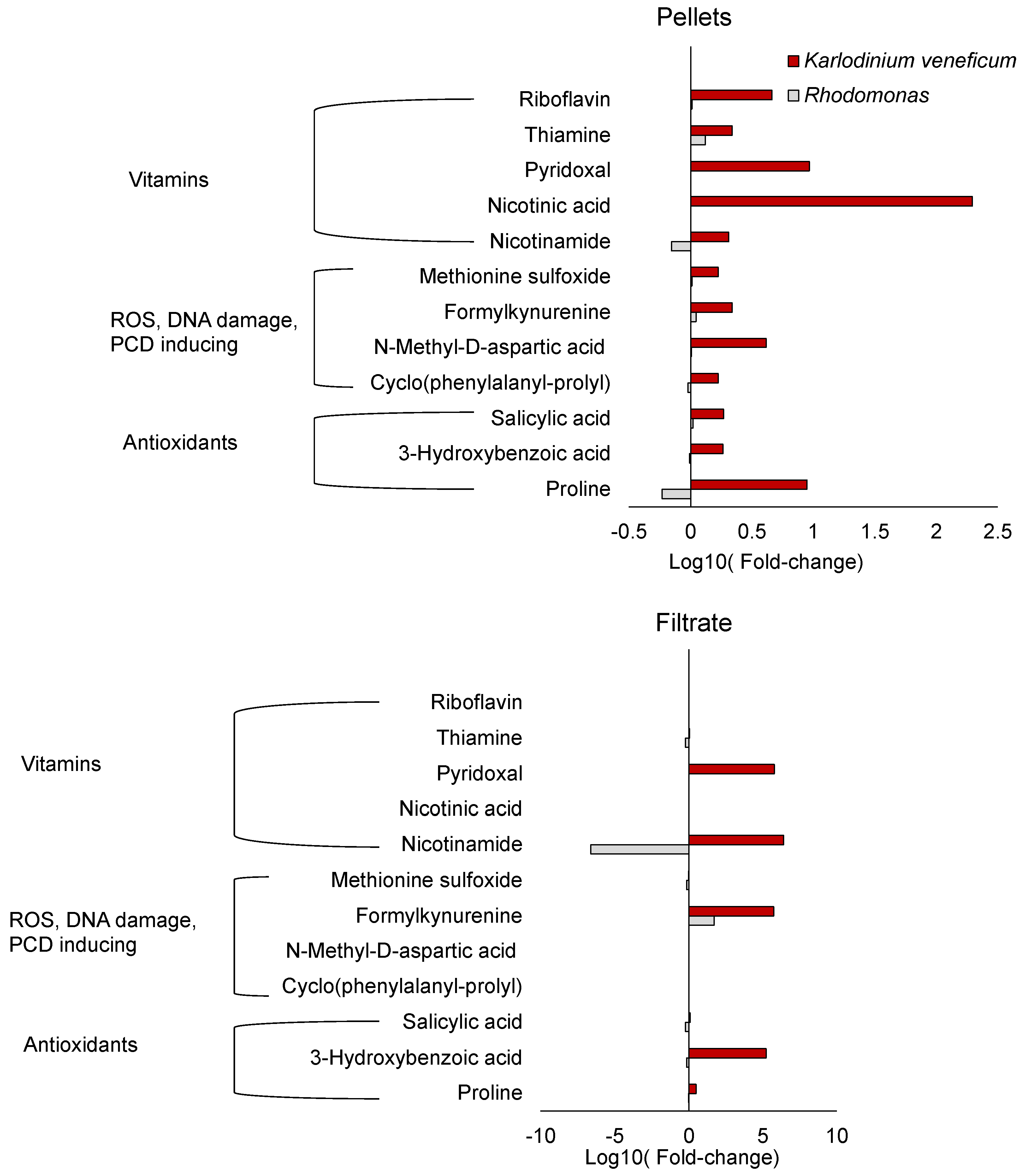
2.2. Metabolites Related to Vitamins
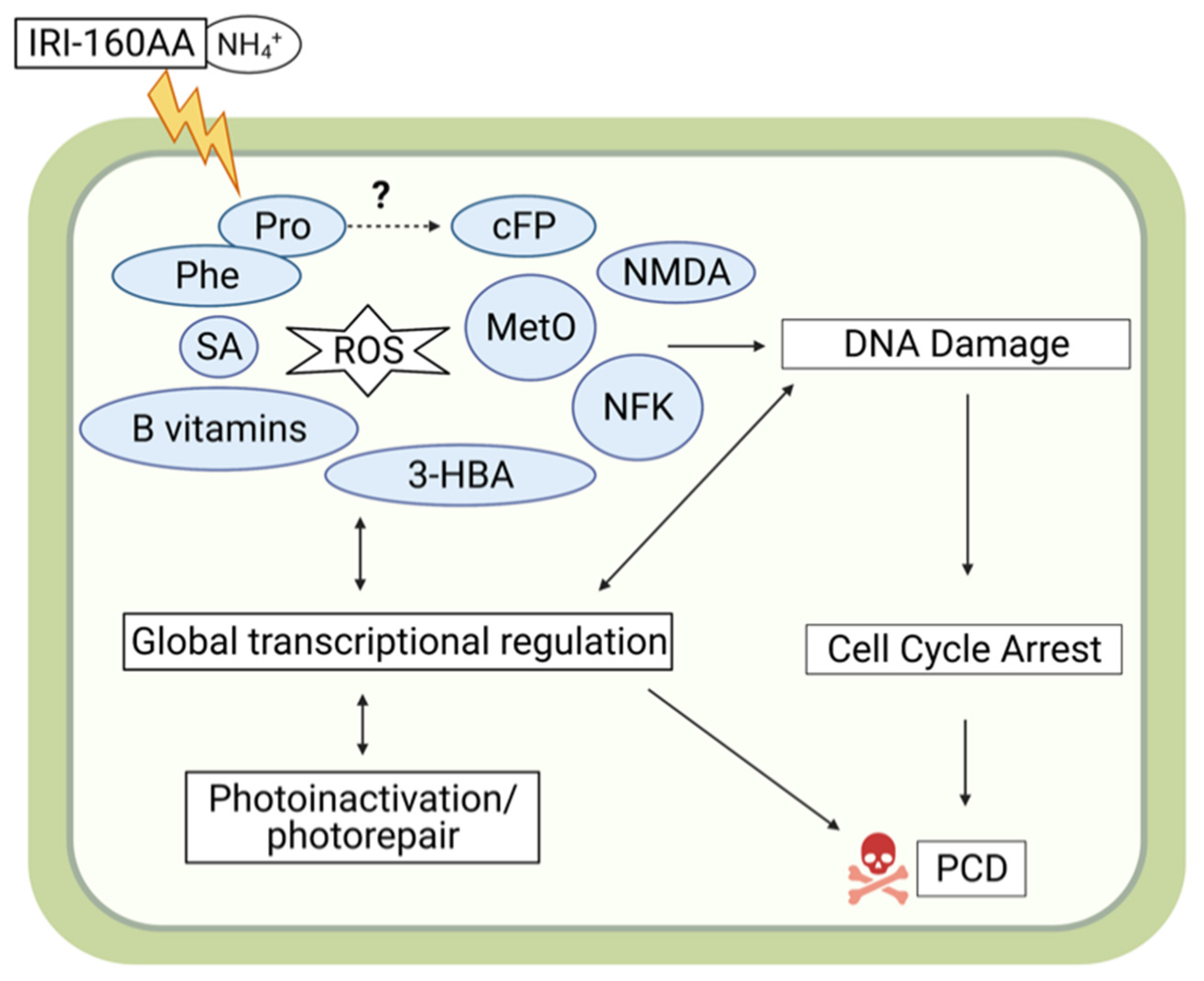
2.3. Metabolites Related to ROS Production, DNA Damage, and Programmed Cell Death
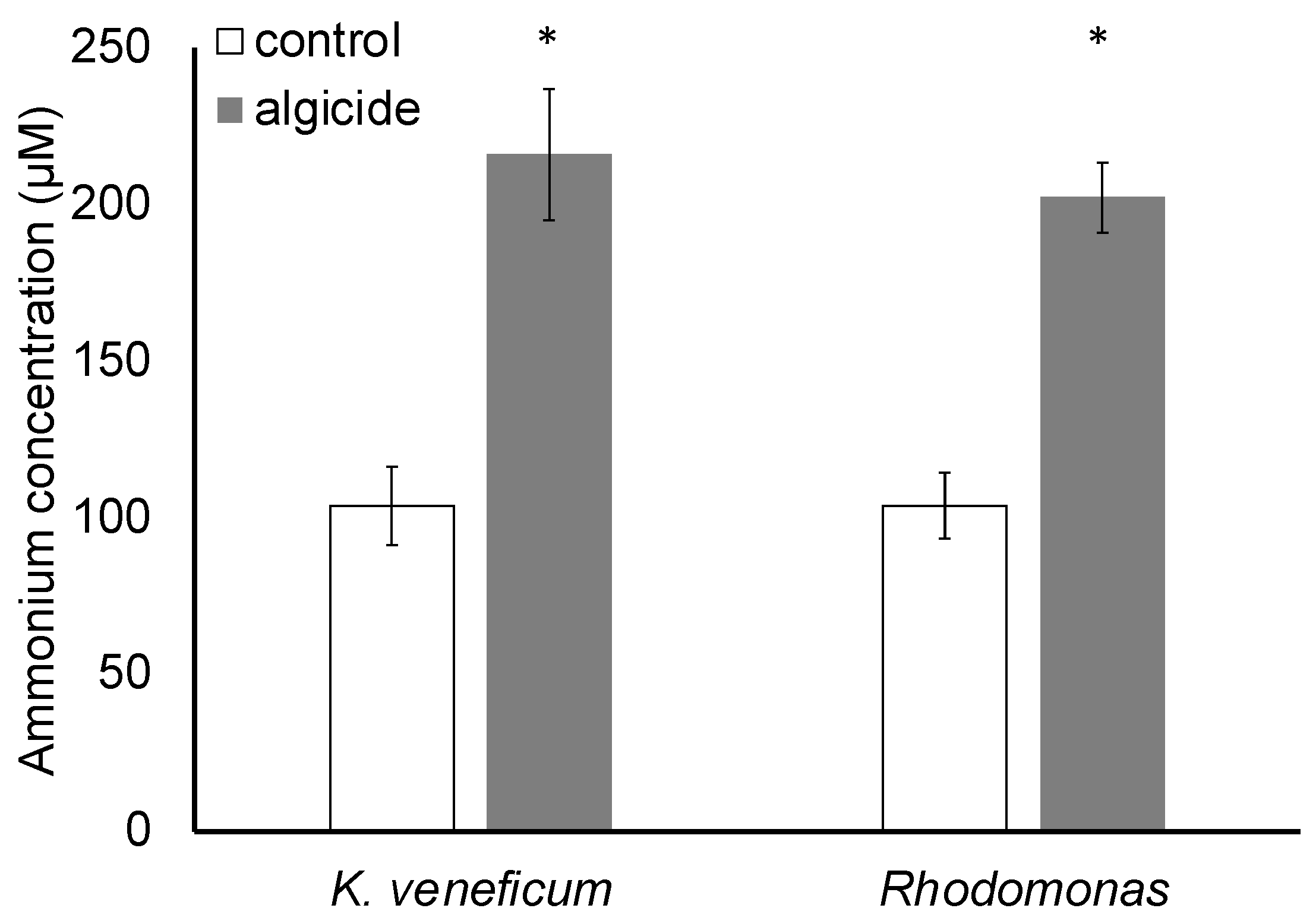
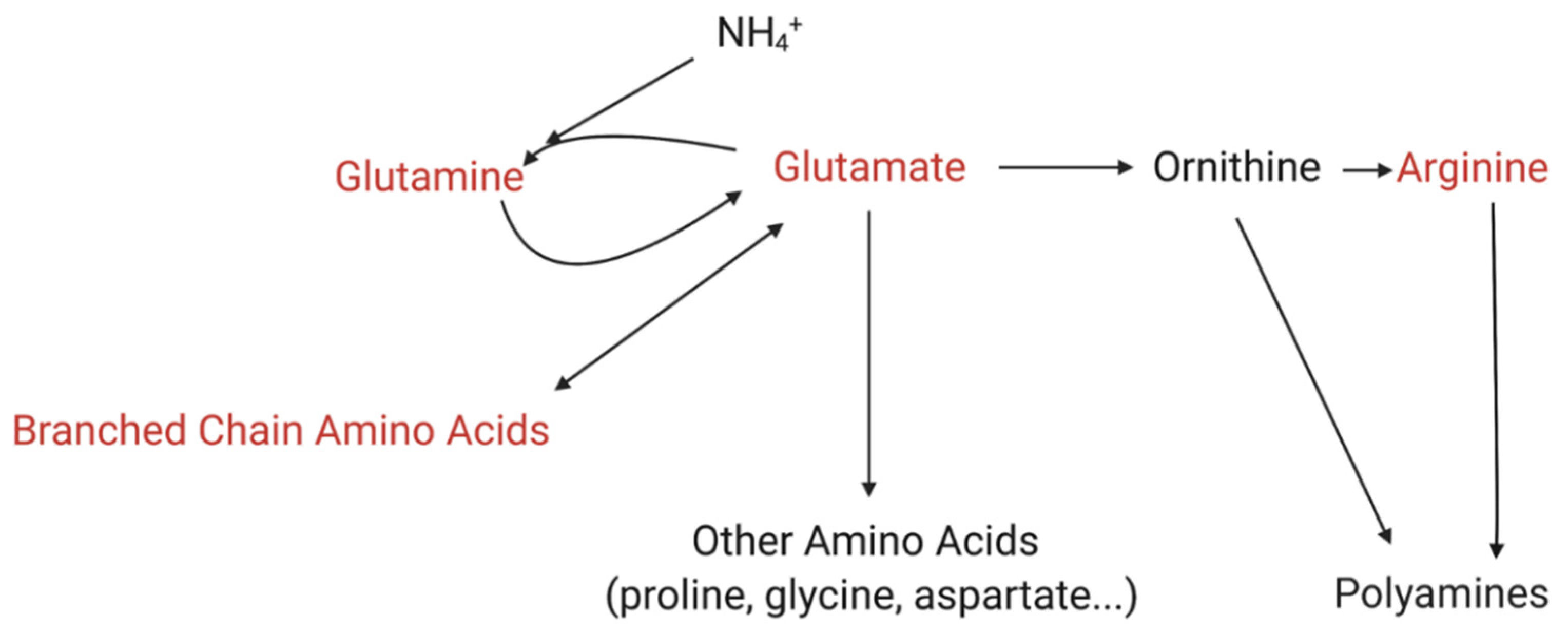
2.4. Ammonium Concentrations
3. Discussion
4. Materials and Methods
4.1. Algal Stock Culture
4.2. Algicide Preparation
4.3. Ammonium Concentration
4.4. Metabolomic Response of K. veneficum and Rhodomonas sp. to Algicide IRI-160AA
4.4.1. Culture Treatments
4.4.2. Sample Preparation for Metabolomics Analysis
4.4.3. Sample Analysis for Polar and Semi-Polar Metabolites
4.4.4. Data Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falkowski, P.G. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.R.; Amin, S.A.; Raina, J.B.; Stocker, R. Zooming in on the phycosphere: The ecological interface for phytoplankton-bacteria relationships. Nat. Microbiol. 2017, 2, 17065. [Google Scholar] [CrossRef]
- Meyer, N.; Bigalke, A.; Kaulfuß, A.; Pohnert, G. Strategies and ecological roles of algicidal bacteria. FEMS Microbiol. Rev. 2017, 41, 880–899. [Google Scholar] [CrossRef]
- Amin, S.A.; Hmelo, L.R.; Van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.S.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef]
- Bercovici, A.; Vellekoop, J. Methods in Paleopalynology and Palynostratigraphy: An Application to the K-Pg Boundary; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128032442. [Google Scholar]
- Poquita-Du, R.C.; Huang, D.; Chou, L.M.; Todd, P.A. The contribution of stress-tolerant endosymbiotic dinoflagellate Durusdinium to Pocillopora acuta survival in a highly urbanized reef system. Coral Reefs 2020, 39, 745–755. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Morse, D.; Tse, S.P.; Lo, S.C.L. Exploring dinoflagellate biology with high-throughput proteomics. Harmful Algae 2018, 75, 16–26. [Google Scholar] [CrossRef]
- Wang, D.Z. Neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef]
- Place, A.R.; Bowers, H.A.; Bachvaroff, T.R.; Adolf, J.E.; Deeds, J.R.; Sheng, J. Karlodinium veneficum—The little dinoflagellate with a big bite. Harmful Algae 2012, 14, 179–195. [Google Scholar] [CrossRef]
- Adolf, J.E.; Krupatkina, D.; Bachvaroff, T.; Place, A.R. Karlotoxin mediates grazing by Oxyrrhis marina on strains of Karlodinium veneficum. Harmful Algae 2007, 6, 400–412. [Google Scholar] [CrossRef]
- Hong, J.; Talapatra, S.; Katz, J.; Tester, P.A.; Waggett, R.J.; Place, A.R. Algal toxins alter copepod feeding behavior. PLoS ONE 2012, 7, e36845. [Google Scholar] [CrossRef]
- Peng, J.; Place, A.; Yoshida, W.; Anklin, C.; Hamann, M.T. Structure of karlotoxin-2, a toxin causing massive fish kills worldwide. Planta Med. 2008, 74, P-65. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Koch, F.; Gobler, C.J. Most harmful algal bloom species are vitamin B1 and B 12 auxotrophs. Proc. Natl. Acad. Sci. USA 2010, 107, 20756–20761. [Google Scholar] [CrossRef]
- Cruz-López, R.; Maske, H.; Yarimizu, K.; Holland, N.A. The B-vitamin mutualism between the dinoflagellate Lingulodinium polyedrum and the bacterium Dinoroseobacter shibae. Front. Mar. Sci. 2018, 5, 274. [Google Scholar] [CrossRef]
- Shi, X.; Liu, L.; Li, Y.; Xiao, Y.; Ding, G.; Lin, S.; Chen, J. Isolation of an algicidal bacterium and its effects against the harmful-algal-bloom dinoflagellate Prorocentrum donghaiense (Dinophyceae). Harmful Algae 2018, 80, 72–79. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Lei, X.; Zhang, H.; Guan, C.; Chen, Z.; Zheng, W.; Xu, H.; Tian, Y.; Yu, Z.; et al. The first evidence of deinoxanthin from Deinococcus sp. Y35 with strong algicidal effect on the toxic dinoflagellate Alexandrium tamarense. J. Hazard. Mater. 2015, 290, 87–95. [Google Scholar] [CrossRef]
- Roth, P.B.; Twiner, M.J.; Mikulski, C.M.; Barnhorst, A.B.; Doucette, G.J. Comparative analysis of two algicidal bacteria active against the red tide dinoflagellate Karenia brevis. Harmful Algae 2008, 7, 682–691. [Google Scholar] [CrossRef]
- Hare, C.E.; Demir, E.; Coyne, K.J.; Craig Cary, S.; Kirchman, D.L.; Hutchins, D.A. A bacterium that inhibits the growth of Pfiesteria piscicida and other dinoflagellates. Harmful Algae 2005, 4, 221–234. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; Astuya-Villalón, A.; Llanos-Rivera, A.; Avello-Fontalba, V.; Ulloa-Jofré, V. A critical review on control methods for harmful algal blooms. Rev. Aquac. 2019, 11, 661–684. [Google Scholar] [CrossRef]
- Pokrzywinski, K.L.; Place, A.R.; Warner, M.E.; Coyne, K.J. Investigation of the algicidal exudate produced by Shewanella sp. IRI-160 and its effect on dinoflagellates. Harmful Algae 2012, 19, 23–29. [Google Scholar] [CrossRef]
- Simons, V.E.; Coyne, K.J.; Warner, M.E.; Dolan, M.M.; Cohen, J.H. Effects of a bacteria-produced algicide on non-target marine invertebrate species. Sci. Rep. 2021, 11, 583. [Google Scholar] [CrossRef]
- Pokrzywinski, K.L.; Tilney, C.L.; Warner, M.E.; Coyne, K.J. Cell cycle arrest and biochemical changes accompanying cell death in harmful dinoflagellates following exposure to bacterial algicide IRI-160AA. Sci. Rep. 2017, 7, 45102. [Google Scholar] [CrossRef]
- Pokrzywinski, K.L.; Tilney, C.L.; Modla, S.; Caplan, J.L.; Ross, J.; Warner, M.E.; Coyne, K.J. Effects of the bacterial algicide IRI-160AA on cellular morphology of harmful dinoflagellates. Harmful Algae 2017, 62, 127–135. [Google Scholar] [CrossRef]
- Tilney, C.L.; Pokrzywinski, K.L.; Coyne, K.J.; Warner, M.E. Growth, death, and photobiology of dinoflagellates (Dinophyceae) under bacterial-algicide control. J. Appl. Phycol. 2014, 26, 2117–2127. [Google Scholar] [CrossRef]
- Ternon, E.; Wang, Y.; Coyne, K.J. Small polar molecules: A challenge in marine chemical ecology. Molecules 2019, 24, 135. [Google Scholar] [CrossRef]
- Grasso, C.R. Effects of the Bacterial Algicide IRI-160AA on the Microbial Community Composition of the Delaware Inland Bays. Master’s Thesis, University of Delaware, Newark, DE, USA, 2018. [Google Scholar]
- Wang, Y. Bacterial Algicides: Application Strategies and Cellular Response of Target Organisms. Ph.D. Dissertation, University of Delaware, Newark, DE, USA, 2021. [Google Scholar]
- Chorazyczewski, A.M.; Huang, I.S.; Abdulla, H.; Mayali, X.; Zimba, P.V. The Influence of bacteria on the growth, lipid production, and extracellular metabolite accumulation by Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 2021, 57, 931–940. [Google Scholar] [CrossRef]
- Paul, C.; Mausz, M.A.; Pohnert, G. A co-culturing/metabolomics approach to investigate chemically mediated interactions of planktonic organisms reveals influence of bacteria on diatom metabolism. Metabolomics 2013, 9, 349–359. [Google Scholar] [CrossRef]
- Kim, J.; Lyu, X.M.; Lee, J.J.L.; Zhao, G.; Chin, S.F.; Yang, L.; Chen, W.N. Metabolomics analysis of Pseudomonas chlororaphis JK12 algicidal activity under aerobic and micro-aerobic culture condition. AMB Express 2018, 8, 131. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, G.; Mao, F.; Li, W.; He, Y.; Gin, K.Y.H.; Ong, C.N. Use of an integrated metabolomics platform for mechanistic investigations of three commonly used algaecides on cyanobacterium, Microcystis aeruginosa. J. Hazard. Mater. 2019, 367, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Liu, Q.; Tang, X.; Wang, Y.; Yang, Y.; Zhang, W.; Zhao, Y.; Zhang, X. ROS changes are responsible for tributyl phosphate (TBP)-induced toxicity in the alga Phaeodactylum tricornutum. Aquat. Toxicol. 2019, 208, 168–178. [Google Scholar] [CrossRef]
- Guzzetti, E.; Salabery, E.; Ferriol, P.; Díaz, J.A.; Tejada, S.; Faggio, C.; Sureda, A. Oxidative stress induction by the invasive sponge Paraleucilla magna growing on Peyssonnelia squamaria algae. Mar. Environ. Res. 2019, 150, 104763. [Google Scholar] [CrossRef]
- Han, Q.; Zheng, Y.; Qi, Q.; Peng, J.; Song, J.; Guo, J.; Guo, J. Involvement of oxidative stress in the sensitivity of two algal species exposed to roxithromycin. Ecotoxicology 2020, 29, 625–633. [Google Scholar] [CrossRef]
- Liang, X.; Kaya, A.; Zhang, Y.; Le, D.T.; Hua, D.; Gladyshev, V.N. Characterization of methionine oxidation and methionine sulfoxide reduction using methionine-rich cysteine-free proteins. BMC Biochem. 2012, 13, 21. [Google Scholar] [CrossRef]
- Roy, S.; Nandi, A.K. Arabidopsis thaliana methionine sulfoxide reductase B8 influences stress-induced cell death and effector-triggered immunity. Plant Mol. Biol. 2017, 93, 109–120. [Google Scholar] [CrossRef]
- Soares, M.S.P.; Viau, C.M.; Saffi, J.; Costa, M.Z.; da Silva, T.M.; Oliveira, P.S.; Azambuja, J.H.; Barschak, A.G.; Braganhol, E.; Wyse, A.T.S.; et al. Acute administration of methionine and/or methionine sulfoxide impairs redox status and induces apoptosis in rat cerebral cortex. Metab. Brain Dis. 2017, 32, 1693–1703. [Google Scholar] [CrossRef]
- Dreaden Kasson, T.M.; Rexroth, S.; Barry, B.A. Light-induced oxidative stress, N-formylkynurenine, and oxygenic photosynthesis. PLoS ONE 2012, 7, e42220. [Google Scholar] [CrossRef]
- Dreaden, T.M.; Chen, J.; Rexroth, S.; Barry, B.A. N-formylkynurenine as a marker of high light stress in photosynthesis. J. Biol. Chem. 2011, 286, 22632–22641. [Google Scholar] [CrossRef]
- Kasson, T.M.D.; Barry, B.A. Reactive oxygen and oxidative stress: N-formyl kynurenine in photosystem II and non-photosynthetic proteins. Photosynth. Res. 2012, 114, 97–110. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 2106. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Popova, L.P.; Maslenkova, L.T.; Yordanova, R.Y.; Ivanova, A.P.; Krantev, A.P.; Szalai, G.; Janda, T. Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol. Biochem. 2009, 47, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Szalai, G.; Horgosi, S.; Soós, V.; Majláth, I.; Balázs, E.; Janda, T. Salicylic acid treatment of pea seeds induces its de novo synthesis. J. Plant Physiol. 2011, 168, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Gust, A.A.; Nürnberger, T. A life or death switch. Nature 2012, 486, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Czerpak, R.; Bajguz, A.; Gromek, M.; Kozłowska, G.; Nowak, I. Activity of salicylic acid on the growth and biochemism of Chlorella vulgaris Beijerinck. Acta Physiol. Plant. 2002, 24, 45–52. [Google Scholar] [CrossRef]
- Guo, P.Y.; Liu, Y.; Wen, X.; Chen, S.F. Effects of algicide on the growth of Microcystis flos-aquae and adsorption capacity to heavy metals. Int. J. Environ. Sci. Technol. 2015, 12, 2339–2348. [Google Scholar] [CrossRef][Green Version]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Chvátalová, K.; Slaninová, I.; Březinová, L.; Slanina, J. Influence of dietary phenolic acids on redox status of iron: Ferrous iron autoxidation and ferric iron reduction. Food Chem. 2008, 106, 650–660. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, S.B.; Wang, Y.C.; Li, X.Q.; Zheng, L.H.; Zhao, M.G. Neuroprotective effects of formononetin against NMDA-induced apoptosis in cortical neurons. Phyther. Res. 2013, 27, 1770–1775. [Google Scholar] [CrossRef]
- Lam, T.T.; Abler, A.S.; Kwong, J.M.K.; Tso, M.O.M. N-Methyl-D-Aspartate (NMDA)–induced apoptosis in rat retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2391–2397. [Google Scholar]
- Huang, R.M.; Yi, X.X.; Zhou, Y.; Su, X.; Peng, Y.; Gao, C.H. An update on 2,5-Diketopiperazines from marine organisms. Mar. Drugs 2014, 12, 6213–6235. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T. Amino acid–derived quorum sensing molecules controlling the virulence of Vibrios (and beyond). PLoS Pathog. 2019, 15, e1007815. [Google Scholar] [CrossRef]
- Park, N.Y.; Cho, Y.B.; Kim, O.B.; Kim, K.S. Cyclo(Phe-Pro) produced by Vibrio species passes through biological membranes by simple diffusion. Appl. Microbiol. Biotechnol. 2020, 104, 6791–6798. [Google Scholar] [CrossRef] [PubMed]
- Brauns, S.C.; Dealtry, G.; Milne, P.; Naudé, R.; Van De Venter, M. Caspase-3 activation and induction of PARP cleavage by cyclic dipeptide cyclo(Phe-Pro) in HT-29 cells. Anticancer Res. 2005, 25, 4197–4202. [Google Scholar] [PubMed]
- Lee, K.; Jeong, J.E.; Kim, I.H.; Kim, K.S.; Ju, B.G. Cyclo(phenylalanine-proline) induces DNA damage in mammalian cells via reactive oxygen species. J. Cell. Mol. Med. 2015, 19, 2851–2864. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Minami, M. Sensing bacterial-induced DNA damaging effects via natural killer group 2 Member D immune receptor: From dysbiosis to autoimmunity and carcinogenesis. Front. Immunol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Quang, D.D. Unraveling the antioxidant potential of thiamine: Thermochemical and kinetics studies in aqueous phase using DFT. Vietnam J. Chem. 2019, 57, 485–490. [Google Scholar] [CrossRef]
- Chumnantana, R.; Yokochi, N.; Yagi, T. Vitamin B6 compounds prevent the death of yeast cells due to menadione, a reactive oxygen generator. Biochim. Biophys. Acta-Gen. Subj. 2005, 1722, 84–91. [Google Scholar] [CrossRef]
- Sinthupoom, N.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Nicotinic acid and derivatives as multifunctional pharmacophores for medical applications. Eur. Food Res. Technol. 2014, 240, 1–17. [Google Scholar] [CrossRef]
- Rawat, D.; Chhonker, S.K.; Naik, R.A.; Koiri, R.K. Modulation of antioxidant enzymes, SIRT1 and NF-κB by resveratrol and nicotinamide in alcohol-aflatoxin B1-induced hepatocellular carcinoma. J. Biochem. Mol. Toxicol. 2020, 35, e22625. [Google Scholar] [CrossRef]
- Suárez, M.F.; Avila, C.; Gallardo, F.; Cantón, F.R.; García-Gutiérrez, A.; Claros, M.G.; Cánovas, F.M. Molecular and enzymatic analysis of ammonium assimilation in woody plants. J. Exp. Bot. 2002, 53, 891–904. [Google Scholar]
- Liu, N.; Li, F.; Ge, F.; Tao, N.; Zhou, Q.; Wong, M. Mechanisms of ammonium assimilation by Chlorella vulgaris F1068: Isotope fractionation and proteomic approaches. Bioresour. Technol. 2015, 190, 307–314. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming ammonium toxicity. Plant Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mustafavi, S.H.; Naghdi Badi, H.; Sękara, A.; Mehrafarin, A.; Janda, T.; Ghorbanpour, M.; Rafiee, H. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol. Plant. 2018, 40, 102. [Google Scholar] [CrossRef]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis-Angelakis, K.A. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, C.; Andersen, J.V.; Vinten, K.T.; Pinborg, L.H.; Waagepetersen, H.S.; Freude, K.K.; Aldana, B.I. Functional metabolic mapping reveals highly active branched-chain amino acid metabolism in human astrocytes, which is impaired in iPSC-derived astrocytes in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 553. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched chain amino acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, F.; Yu, C.; Li, C.; Huang, T.; Hu, H. Amino acid catabolism during nitrogen limitation in Phaeodactylum tricornutum. Front. Plant Sci. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Cruz-López, R.; Maske, H. The vitamin B1 and B12 required by the marine dinoflagellate Lingulodinium polyedrum can be provided by its associated bacterial community in culture. Front. Microbiol. 2016, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Bolch, C.J.S.; Bejoy, T.A.; Green, D.H. Bacterial associates modify growth dynamics of the dinoflagellate Gymnodinium catenatum. Front. Microbiol. 2017, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Ternon, E.; Pavaux, A.S.; Marro, S.; Thomas, O.P.; Lemée, R. Allelopathic interactions between the benthic toxic dinoflagellate Ostreopsis cf. ovata and a co-occurring diatom. Harmful Algae 2018, 75, 35–44. [Google Scholar] [CrossRef]
- Vidoudez, C.; Pohnert, G. Comparative metabolomics of the diatom Skeletonema marinoi in different growth phases. Metabolomics 2012, 8, 654–669. [Google Scholar] [CrossRef]
- Gaubert, J.; Greff, S.; Thomas, O.P.; Payri, C.E. Metabolomic variability of four macroalgal species of the genus Lobophora using diverse approaches. Phytochemistry 2019, 162, 165–172. [Google Scholar] [CrossRef]
- Jacinavicius, F.R.; Geraldes, V.; Crnkovic, C.M.; Delbaje, E.; Fiore, M.F.; Pinto, E. Effect of ultraviolet radiation on the metabolomic profiles of potentially toxic cyanobacteria. FEMS Microbiol. Ecol. 2021, 97, 243. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms I. Cyclotella nana Hustedt, and Detonula confervasea (Cleve). Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012; Volume 3, p. 1817. [Google Scholar]
- Wang, Y.; Coyne, K.J. Immobilization of algicidal bacterium Shewanella sp. IRI-160 and its application to control harmful dinoflagellates. Harmful Algae 2020, 94, 101798. [Google Scholar] [CrossRef]
- Doneanu, C.E.; Chen, W.; Mazzeo, J.R.; Corporation, W. UPLC/MS Monitoring of Water-Soluble Vitamin Bs in Cell Culture Media in Minutes. Waters Appl. Notes 2011, 1–7. Available online: https://www.waters.com/waters/library.htm?locale=en_US&lid=134636355 (accessed on 31 March 2022).
- Hsiao, J.J.; Potter, O.G.; Chu, T.W.; Yin, H. Improved LC/MS Methods for the Analysis of Metal-Sensitive Analytes Using Medronic Acid as a Mobile Phase Additive. Anal. Chem. 2018, 90, 9457–9464. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Coyne, K.J. Metabolomic Insights of the Effects of Bacterial Algicide IRI-160AA on Dinoflagellate Karlodinium veneficum. Metabolites 2022, 12, 317. https://doi.org/10.3390/metabo12040317
Wang Y, Coyne KJ. Metabolomic Insights of the Effects of Bacterial Algicide IRI-160AA on Dinoflagellate Karlodinium veneficum. Metabolites. 2022; 12(4):317. https://doi.org/10.3390/metabo12040317
Chicago/Turabian StyleWang, Yanfei, and Kathryn J. Coyne. 2022. "Metabolomic Insights of the Effects of Bacterial Algicide IRI-160AA on Dinoflagellate Karlodinium veneficum" Metabolites 12, no. 4: 317. https://doi.org/10.3390/metabo12040317
APA StyleWang, Y., & Coyne, K. J. (2022). Metabolomic Insights of the Effects of Bacterial Algicide IRI-160AA on Dinoflagellate Karlodinium veneficum. Metabolites, 12(4), 317. https://doi.org/10.3390/metabo12040317





