Efficacy, Feasibility and Acceptability of a Mediterranean Diet Intervention on Hormonal, Metabolic and Anthropometric Measures in Overweight and Obese Women with Polycystic Ovary Syndrome: Study Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Participants and Recruitment
2.2. Data Collection
2.3. Biochemical Assessments
2.4. Anthrompometry and Body Composition
2.5. Physical Activity
2.6. Dietary Intake
2.7. Adherence to the MedDiet Intervention
2.8. Demographic Questionnarie
2.9. Dietary Protocol
2.10. Education and Counselling
2.11. Digital Messaging
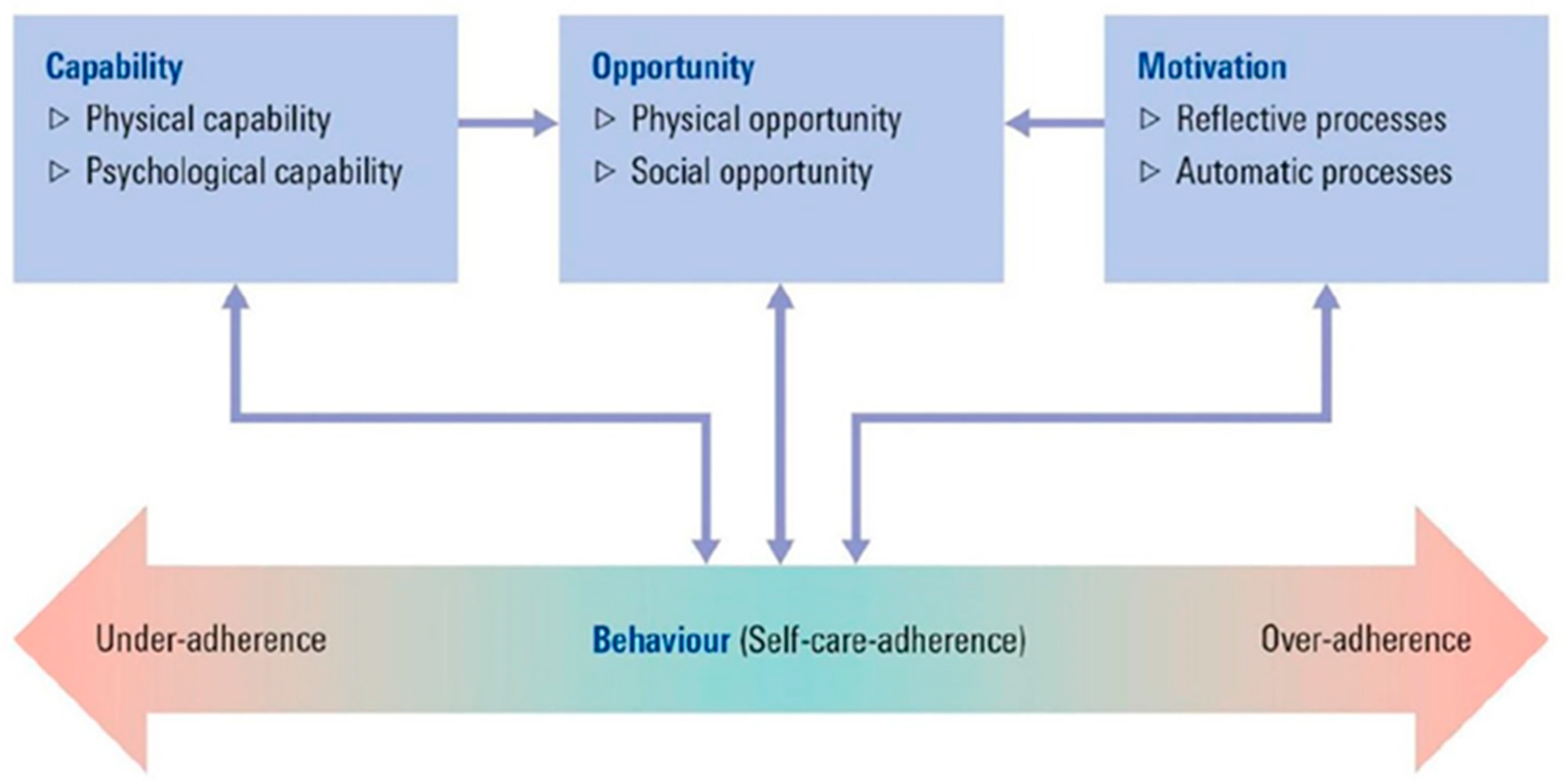
2.12. Acceptability and Feasibility of the MedDiet Intervention: Theoretical Frameworks into Practice
2.13. Participant Interveiws
3. Analyses of Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef] [Green Version]
- Asunción, M.; Calvo, R.M.; San Millán, J.L.; Sancho, J.; Avila, S.; Escobar-Morreale, H.F. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J. Clin. Endocrinol. Metab. 2000, 85, 2434–2438. [Google Scholar] [CrossRef] [Green Version]
- Diamanti-Kandarakis, E.; Kouli, C.R.; Bergiele, A.T.; Filandra, F.A.; Tsianateli, T.C.; Spina, G.G.; Zapanti, E.D.; Bartzis, M.I. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J. Clin. Endocrinol. Metab. 1999, 84, 4006–4011. [Google Scholar] [CrossRef]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- Chang, J.; Azziz, R.; Legro, R.; Dewailly, D.; Franks, S.; Tarlatzis, B.C.; Fauser, B.; Balen, A.; Bouchard, P.; Dahlgren, E.; et al. Revised 2003 consensus on diagnostic criteria and long-term healt h risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Moran, L.J.; Misso, M.L.; Wild, R.A.; Norman, R.J. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2010, 16, 347–363. [Google Scholar] [CrossRef]
- Costello, M.F.; Misso, M.L.; Balen, A.; Boyle, J.; Devoto, L.; Garad, R.M.; Hart, R.; Johnson, L.; Jordan, C.; Legro, R.S.; et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: Assessment and treatment of infertility. Hum. Reprod. Open 2019, 2019, hoy021. [Google Scholar] [CrossRef]
- Deeks, A.A.; Gibson-Helm, M.E.; Teede, H.J. Anxiety and depression in polycystic ovary syndrome: A comprehensive investigation. Fertil. Steril. 2010, 93, 2421–2423. [Google Scholar] [CrossRef]
- Damone, A.L.; Joham, A.E.; Loxton, D.; Earnest, A.; Teede, H.J.; Moran, L.J. Depression, anxiety and perceived stress in women with and without PCOS: A community-based study. Psychol. Med. 2019, 49, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Cooney, L.G.; Lee, I.; Sammel, M.D.; Dokras, A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2017, 32, 1075–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himelein, M.J.; Thatcher, S.S. Depression and body image among women with polycystic ovary syndrome. J. Health Psychol. 2006, 11, 613–625. [Google Scholar] [CrossRef]
- Pastore, L.M.; Patrie, J.T.; Morris, W.L.; Dalal, P.; Bray, M.J. Depression symptoms and body dissatisfaction association among polycystic ovary syndrome women. J. Psychosom. Res. 2011, 71, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Pirotta, S.; Barillaro, M.; Brennan, L.; Grassi, A.; Jeanes, Y.M.; Joham, A.E.; Kulkarni, J.; Couch, L.M.; Lim, S.S.; Moran, L.J. Disordered eating behaviours and eating disorders in women in Australia with and without Polycystic Ovary Syndrome: A cross-sectional study. J. Clin. Med. 2019, 8, 1682. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.S.; Norman, R.J.; Davies, M.J.; Moran, L.J. The effect of obesity on polycystic ovary syndrome: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Joham, A.E.; Paul, E.; Moran, L.J.; Loxton, D.; Jolley, D.; Lombard, C. Longitudinal weight gain in women identified with polycystic ovary syndrome: Results of an observational study in young women. Obesity 2013, 21, 1526–1532. [Google Scholar] [CrossRef]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef]
- Wekker, V.; van Dammen, L.; Koning, A.; Heida, K.Y.; Painter, R.C.; Limpens, J.; Laven, J.S.E.; Roeters van Lennep, J.E.; Roseboom, T.J.; Hoek, A. Long-term cardiometabolic disease risk in women with PCOS: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 942–960. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M.M.D.; Tarlatzis, B.C.M.D.; Rebar, R.W.M.D.; Legro, R.S.M.D.; Balen, A.H.M.D.; Lobo, R.M.D.; Carmina, E.M.D.; Chang, J.M.D.; Yildiz, B.O.M.D.; Laven, J.S.E.M.D.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28.e25–38.e25. [Google Scholar] [CrossRef]
- Moran, L.J.; Pasquali, R.; Teede, H.J.; Hoeger, K.M.; Norman, R.J. Treatment of obesity in polycystic ovary syndrome: A position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil. Steril. 2009, 92, 1966–1982. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, L.J.; Ko, H.; Misso, M.; Marsh, K.; Noakes, M.; Talbot, M.; Frearson, M.; Thondan, M.; Stepto, N.; Teede, H.J. Dietary composition in the treatment of polycystic ovary syndrome: A systematic review to inform evidence-based guidelines. Hum. Reprod. Update 2013, 19, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, J.; Tassone, E.C.; Misso, M.; Joham, A.E.; Stener-Victorin, E.; Teede, H.; Moran, L.J. Weight Management Interventions in Women with and without PCOS: A Systematic Review. Nutrients 2017, 9, 996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teede, H.J.; Misso, M.L.; Deeks, A.A.; Moran, L.J.; Stuckey, B.G.; Wong, J.L.; Norman, R.J.; Costello, M.F. Assessment and management of polycystic ovary syndrome: Summary of an evidence-based guideline. Med. J. Aust. 2011, 195, S65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, D.; Ozier, A.; Bundros, J.; Moore, J.; Kreiser, A.; Morris, M.N. Impact of Non-Diet Approaches on Attitudes, Behaviors, and Health Outcomes: A Systematic Review. J. Nutr. Educ. Behav. 2015, 47, 143.e141–155.e141. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [Green Version]
- Keys, A. Mediterranean diet and public health: Personal reflections. Am. J. Clin. Nutr. 1995, 61, 1321S–1323S. [Google Scholar] [CrossRef] [Green Version]
- Donini, L.M.; Serra-Majem, L.; Bulló, M.; Gil, Á.; Salas-Salvadó, J. The Mediterranean diet: Culture, health and science. Br. J. Nutr. 2015, 113, S1–S3. [Google Scholar] [CrossRef] [Green Version]
- Villani, A.; Sultana, J.; Doecke, J.; Mantzioris, E. Differences in the interpretation of a modernized Mediterranean diet prescribed in intervention studies for the management of type 2 diabetes: How closely does this align with a traditional Mediterranean diet? Eur. J. Nutr. 2018, 58, 1369–1380. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of olive oil on markers of inflammation and endothelial function—A systematic review and meta-analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Rejón, A.I.; Castro-Quezada, I.; Ruano-Rodríguez, C.; Ruiz-López, M.D.; Sánchez-Villegas, A.; Toledo, E.; Artacho, R.; Estruch, R.; Salas-Salvadó, J.; Covas, M.I.; et al. Effect of a Mediterranean Diet Intervention on Dietary Glycemic Load and Dietary Glycemic Index: The PREDIMED Study. J. Nutr. Metab. 2014, 2014, 985373. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, M.; Hadi, A.; Pierson, R.A.; Lujan, M.E.; Zello, G.A.; Chilibeck, P.D. Effects of Dietary Glycemic Index and Glycemic Load on Cardiometabolic and Reproductive Profiles in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Adv. Nutr. (Bethesda Md.) 2021, 12, 161–178. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Guo, Y.; Lai, Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int. J. Endocrinol. 2019, 2019, 4386401. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef] [Green Version]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The effect of the Mediterranean diet on metabolic health: A systematic review and meta-analysis of controlled trials in adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Sleiman, D.; Al-Badri, M.R.; Azar, S.T. Effect of mediterranean diet in diabetes control and cardiovascular risk modification: A systematic review. Front. Public Health 2015, 3, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendall, C.L.; Mayr, H.L.; Opie, R.S.; Bes-Rastrollo, M.; Itsiopoulos, C.; Thomas, C.J. Central obesity and the Mediterranean diet: A systematic review of intervention trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3070–3084. [Google Scholar] [CrossRef] [PubMed]
- Agnoli, C.; Sieri, S.; Ricceri, F.; Giraudo, M.T.; Masala, G.; Assedi, M.; Panico, S.; Mattiello, A.; Tumino, R.; Giurdanella, M.C.; et al. Adherence to a Mediterranean diet and long-term changes in weight and waist circumference in the EPIC-Italy cohort. Nutr. Diabetes 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Grieger, J.A.; Mishra, G.D.; Teede, H.J. The Association of a Mediterranean-Style Diet Pattern with Polycystic Ovary Syndrome Status in a Community Cohort Study. Nutrients 2015, 7, 8553–8564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniyappa, R.; Madan, R.; Varghese, R.T. Assessing Insulin Sensitivity and Resistance in Humans. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Darmouth, MA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278954/ (accessed on 30 November 2021).
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Gonzalez, M.A.; Garcia-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F.; Gomez-Gracia, E. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The predimed trial. PLoS ONE 2012, 7, e0043134. [Google Scholar] [CrossRef] [Green Version]
- Pfaeffli Dale, L.; Whittaker, R.; Jiang, Y.; Stewart, R.; Rolleston, A.; Maddison, R. Text Message and Internet Support for Coronary Heart Disease Self-Management: Results from the Text4Heart Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e237. [Google Scholar] [CrossRef] [Green Version]
- Santo, K.; Hyun, K.; de Keizer, L.; Thiagalingam, A.; Hillis, G.S.; Chalmers, J.; Redfern, J.; Chow, C.K. The effects of a lifestyle-focused text-messaging intervention on adherence to dietary guideline recommendations in patients with coronary heart disease: An analysis of the TEXT ME study. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- Zacharia, K.; Patterson, A.J.; English, C.; MacDonald-Wicks, L. Feasibility of the AusMed Diet Program: Translating the Mediterranean Diet for Older Australians. Nutrients 2020, 12, 1044. [Google Scholar] [CrossRef] [Green Version]
- Waller, K.; Furber, S.; Bauman, A.; Allman-Farinelli, M.; van den Dolder, P.; Hayes, A.; Facci, F.; Franco, L.; Webb, A.; Moses, R.; et al. Effectiveness and acceptability of a text message intervention (DTEXT) on HbA1c and self-management for people with type 2 diabetes. A randomized controlled trial. Patient Educ. Couns. 2021, 104, 1736–1744. [Google Scholar] [CrossRef]
- Chow, C.K.; Redfern, J.; Hillis, G.S.; Thakkar, J.; Santo, K.; Hackett, M.L.; Jan, S.; Graves, N.; de Keizer, L.; Barry, T.; et al. Effect of Lifestyle-Focused Text Messaging on Risk Factor Modification in Patients with Coronary Heart Disease: A Randomized Clinical Trial. JAMA 2015, 314, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Blagden, S.; Austin, C.; Richey, R.; Desai, M. NICE public health guidance update. J. Public Health 2021, 43, e107–e109. [Google Scholar] [CrossRef] [PubMed]
- Atkins, L.; Michie, S. Designing interventions to change eating behaviours. Proc. Nutr. Soc. 2015, 74, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Johnston, M. Theories and techniques of behaviour change: Developing a cumulative science of behaviour change. Health Psychol. Rev. 2012, 6, 1–6. [Google Scholar] [CrossRef]
- Michie, S.; van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Michie, S.; Carey, R.N.; Johnston, M.; Rothman, A.J.; De Bruin, M.; Kelly, M.P.; Connell, L.E. From theory-inspired to theory-based interventions: A protocol for developing and testing a methodology for linking behaviour change techniques to theoretical mechanisms of action. Ann. Behav. Med. 2018, 52, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Michie, S.; Johnston, M.; Francis, J.; Hardeman, W.; Eccles, M. From Theory to Intervention: Mapping Theoretically Derived Behavioural Determinants to Behaviour Change Techniques. Appl. Psychol. 2008, 57, 660–680. [Google Scholar] [CrossRef]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The Behavior Change Technique Taxonomy (v1) of 93 Hierarchically Clustered Techniques: Building an International Consensus for the Reporting of Behavior Change Interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef]
- Spahn, J.M.; Reeves, R.S.; Keim, K.S.; Laquatra, I.; Kellogg, M.; Jortberg, B.; Clark, N.A. State of the Evidence Regarding Behavior Change Theories and Strategies in Nutrition Counseling to Facilitate Health and Food Behavior Change. J. Am. Diet. Assoc. 2010, 110, 879–891. [Google Scholar] [CrossRef]
- Saelens, B.E.; Gehrman, C.A.; Sallis, J.F.; Calfas, K.J.; Sarkin, J.A.; Caparosa, S. Use of self-management strategies in a 2-year cognitive-behavioral intervention to promote physical activity. Behavior Ther. 2000, 31, 365–379. [Google Scholar] [CrossRef]
- Ashton, L.M.; Sharkey, T.; Whatnall, M.C.; Williams, R.L.; Bezzina, A.; Aguiar, E.J.; Collins, C.E.; Hutchesson, M.J. Effectiveness of Interventions and Behaviour Change Techniques for Improving Dietary Intake in Young Adults: A Systematic Review and Meta-Analysis of RCTs. Nutrients 2019, 11, 825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samdal, G.B.; Eide, G.E.; Barth, T.; Williams, G.; Meland, E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scannell, N.; Villani, A.; Mantzioris, E.; Swanepoel, L. Understanding the Self-Perceived Barriers and Enablers toward Adopting a Mediterranean Diet in Australia: An Application of the Theory of Planned Behaviour Framework. Int. J. Environ. Res. Public Health 2020, 17, 9321. [Google Scholar] [CrossRef]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef] [Green Version]
- Flannery, C.; McHugh, S.; Anaba, A.E.; Clifford, E.; O’Riordan, M.; Kenny, L.C.; McAuliffe, F.M.; Kearney, P.M.; Byrne, M. Enablers and barriers to physical activity in overweight and obese pregnant women: An analysis informed by the theoretical domains framework and COM-B model. BMC Pregnancy Childbirth 2018, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Pirotta, S.; Joham, A.J.; Moran, L.J.; Skouteris, H.; Lim, S.S. Implementation of evidence-based PCOS lifestyle management guidelines: Perceived barriers and facilitators by consumers using the Theoretical Domains Framework and COM-B Model. Patient Educ. Couns. 2021, 104, 2080–2088. [Google Scholar] [CrossRef]
- Timlin, D.; McCormack, J.M.; Simpson, E.E. Using the COM-B model to identify barriers and facilitators towards adoption of a diet associated with cognitive function (MIND diet). Public Health Nutr. 2021, 24, 1657–1670. [Google Scholar] [CrossRef]
- Herber, O.R.; Atkins, L.; Störk, S.; Wilm, S. Enhancing self-care adherence in patients with heart failure: A study protocol for developing a theory-based behaviour change intervention using the COM-B behaviour model (ACHIEVE study). BMJ Open 2018, 8, e025907. [Google Scholar] [CrossRef]
- Bowen, D.J.; Kreuter, M.; Spring, B.; Cofta-Woerpel, L.; Linnan, L.; Weiner, D.; Bakken, S.; Kaplan, C.P.; Squiers, L.; Fabrizio, C.; et al. How We Design Feasibility Studies. Am. J. Prev. Med. 2009, 36, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Spencer, L.; Ritchie, J.; O’Connor, W.; Morrell, G.; Ormston, R. Analysis in Practice. In Qualitative Research Practice: A Guide for Social Science Students and Researchers, 2nd ed.; Ritchie, J., Lewis, J., McNaughton, N.C., Ormston, R., Eds.; Sage: London, UK, 2014; pp. 294–343. [Google Scholar]
- Moran, L.J.; Noakes, M.; Clifton, P.; Buckley, J.; Brinkworth, G.; Thomson, R.; Norman, R.J. Predictors of Lifestyle Intervention Attrition or Weight Loss Success in Women with Polycystic Ovary Syndrome Who Are Overweight or Obese. Nutrients 2019, 11, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, L.J.; Hutchison, S.K.; Norman, R.J.; Teede, H.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2011, CD007506. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Noakes, M.; Clifton, P.M.; Tomlinson, L.; Norman, R.J. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.S.; Hutchison, S.K.; Van Ryswyk, E.; Norman, R.J.; Teede, H.J.; Moran, L.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, CD007506. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. [Google Scholar] [CrossRef] [Green Version]
- Cutillas-Tolín, A.; Arense-Gonzalo, J.J.; Mendiola, J.; Adoamnei, E.; Navarro-Lafuente, F.; Sánchez-Ferrer, M.L.; Prieto-Sánchez, M.T.; Carmona-Barnosi, A.; Vioque, J.; Torres-Cantero, A.M. Are Dietary Indices Associated with Polycystic Ovary Syndrome and Its Phenotypes? A Preliminary Study. Nutrients 2021, 13, 313. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Metabolically Healthy Obesity (MHO) vs. Metabolically Unhealthy Obesity (MUO) Phenotypes in PCOS: Association with Endocrine-Metabolic Profile, Adherence to the Mediterranean Diet, and Body Composition. Nutrients 2021, 13, 3925. [Google Scholar] [CrossRef]
- George, S.E.; Kucianski, T.; Mayr, L.H.; Moschonis, G.; Tierney, C.A.; Itsiopoulos, C. A Mediterranean Diet Model in Australia: Strategies for Translating the Traditional Mediterranean Diet into a Multicultural Setting. Nutrients 2018, 10, 465. [Google Scholar] [CrossRef] [Green Version]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [Green Version]
- Mantzioris, E.; Villani, A. Translation of a Mediterranean-Style Diet into the Australian Dietary Guidelines: A Nutritional, Ecological and Environmental Perspective. Nutrients 2019, 11, 2507. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.R.; Bryan, J.; Hodgson, J.M.; Wilson, C.; Murphy, K.J. Older Australians can adhere to a traditional Mediterranean style diet over two weeks: A pilot dietary intervention study. BMC Nutr. 2015, 1, 28. [Google Scholar] [CrossRef] [Green Version]
- Albarracín, D.; Gillette, J.C.; Earl, A.N.; Glasman, L.R.; Durantini, M.R.; Ho, M.-H. A test of major assumptions about behavior change: A comprehensive look at the effects of passive and active HIV-prevention interventions since the beginning of the epidemic. Psychol. Bull. 2005, 131, 856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noar, S.M.; Zimmerman, R.S. Health Behavior Theory and cumulative knowledge regarding health behaviors: Are we moving in the right direction? Health Educ. Res. 2005, 20, 275–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avery, J.; Ottey, S.; Morman, R.; Cree-Green, M.; Gibson-Helm, M. Polycystic ovary syndrome support groups and their role in awareness, advocacy and peer support: A systematic search and narrative review. Curr. Opin. Endocr. Metab. Res. 2020, 12, 98–104. [Google Scholar] [CrossRef]
- Percy, C.; Murray, S. The role of an online peer-to-peer health community in addressing psychosocial concerns and social support in polycystic ovary syndrome. Int. J. Web Based Communities 2010, 6, 349–361. [Google Scholar] [CrossRef]
- Oberg, E.; Gidlöf, S.; Jakson, I.; Mitsell, M.; Tollet Egnell, P.; Hirschberg, A.L. Improved menstrual function in obese women with polycystic ovary syndrome after behavioural modification intervention-A randomized controlled trial. Clin. Endocrinol. 2019, 90, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Boote, J.; Telford, R.; Cooper, C. Consumer involvement in health research: A review and research agenda. Health Policy 2002, 61, 213–236. [Google Scholar] [CrossRef]
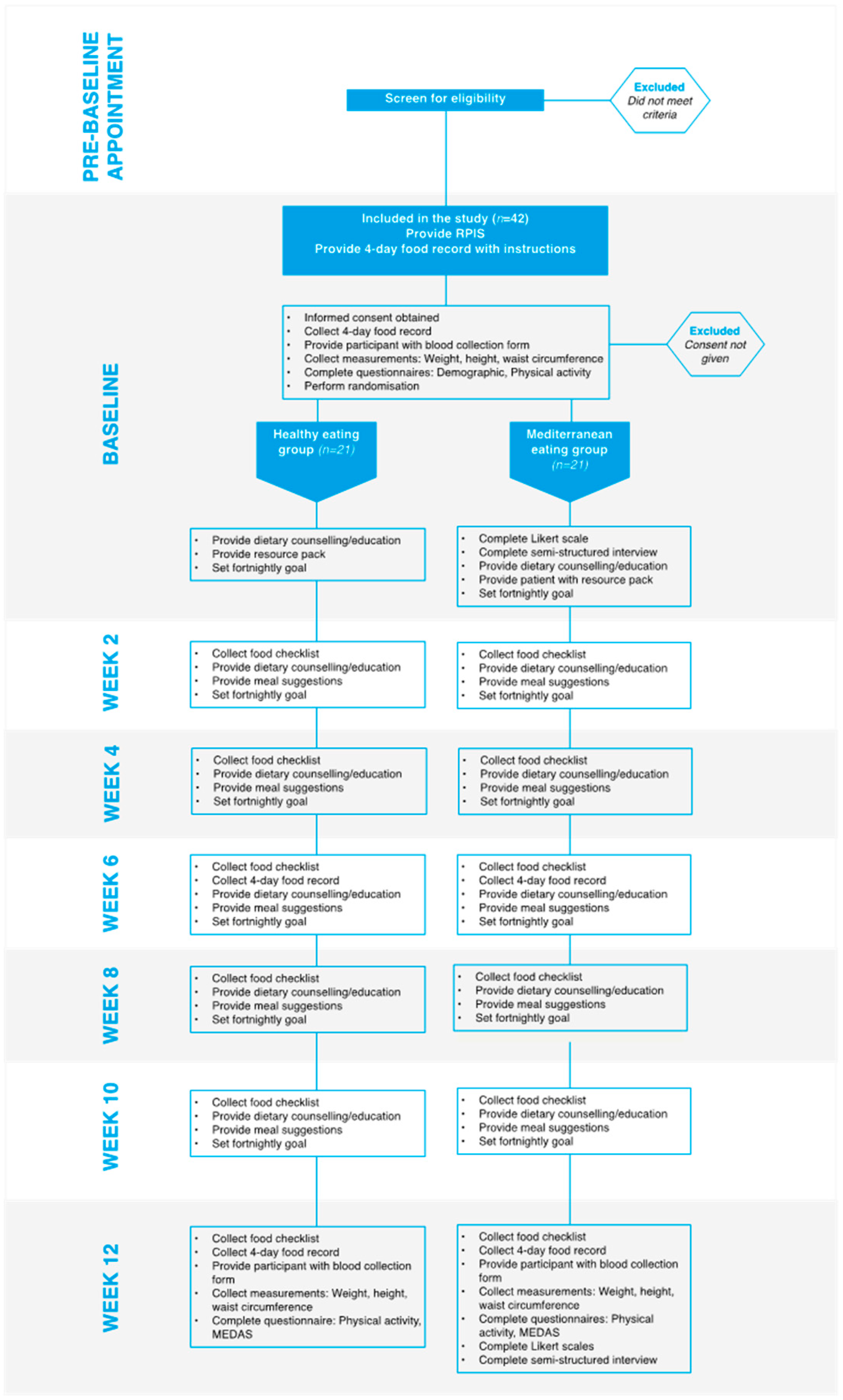
| Inclusion criteria |
|
| Exclusion criteria |
|
| Healthy Eating Dietary Approach | Mediterranean Diet Intervention | |
|---|---|---|
| Grains | Include 6 serves daily, ensuring mostly of a wholegrain variety | Include 4–6 serves daily of wholegrains |
| Vegetables | Include 5 serves of vegetables (approximately 375 g), including different types and colours each day. Can include legumes | Include 5–6 serves daily, ensuring vegetables are the central part of the dish. Include a variety of types and colours at every meal. Choose seasonal where possible |
| Fruit | Include 2 serves daily (approximately 300 g) | Include 2–3 serves daily. Choose seasonal when possible |
| Legumes | Include often. Serves are counted toward vegetables and meat/meat alternative recommendations | Include 3 serves weekly |
| Fish and Seafood | Include often | Include 2–3 serves weekly |
| Red Meat | Include lean options regularly, a maximum of 455 g per week | Limit to a maximum of 100 g per week of lean options |
| Poultry | Include often | Include 100–150 g, 1–3 times per week |
| Dairy | Include 2 ½ serves daily and select low-fat options | Include 200 g Greek yoghurt 3 times per week |
| Eggs | Include up to 7 eggs weekly | Include 4–6 eggs weekly |
| Nuts | Limit to small amounts | Include a 30 g serve 3 times per week |
| Fat | Limit to small amounts of polyunsaturated and monounsaturated fats | Include liberal amounts of extra virgin olive oil, aiming for 1–4 tablespoons daily |
| Alcohol | Limit and include 2 alcohol-free days per week | Include up to 200 mL of red wine (2 standard drinks) with meals (only for those who ordinarily consume alcohol), include 2 alcohol-free days per week |
| Discretionary foods | Limit foods high in saturated fat, salt, sugar and refined carbohydrates | Limit intake of sweets, pastries and soft drinks to special occasions only |
| Intervention Components | Behaviour Change Techniques |
|---|---|
| Individualized dietary consults | 1.1 Goal setting 1.4 Action planning 1.5 Review behaviour goal 4.1 Instructions on how to perform a behaviour |
| Resource pack(fridge magnet, shopping lists, pictorial dietary approach guidelines, health pamphlet) | 7.1 Prompts/cues 12.5 Adding objects to the environment 5.1 Information about health consequences 4.1 Instructions on how to perform a behaviour |
| Dietary education sessions | 4.1 Instructions on how to perform a behaviour 1.2 Problem solving |
| Digital messages | 7.1 Prompts/cues |
| Question | COM-B | |
|---|---|---|
| 1. | Could you describe to me what you think a Mediterranean diet is? Prompt: which foods/beverages do you think are included? | Capability (Psychological) |
| 2. | What factors or circumstances would help (make it easier) you to follow a Mediterranean Diet? | Motivation (Reflective) |
| 3. | What factors or circumstances would make it harder for you to follow a Mediterranean Diet? | Motivation (Reflective) |
| 4. | What skills do you think you will need to follow this diet? | Motivation (Reflective) |
| 5. | In what ways do you think following a Mediterranean diet could affect your health/lifestyle? | Motivation (Reflective) |
| 6. | How do you feel about following a Mediterranean diet? Prompt: is it something that you enjoy? Do you look forward to it? | Motivation (Automatic) |
| Statement | COM-B | |
|---|---|---|
| 1. | How would you rate your knowledge of what foods are part of a Mediterranean diet? | Capability (Psychological) |
| 2. | How would you rate your confidence to prepare/cook the food included in a Mediterranean diet? | Capability (Physical) |
| 3. | How would you rate your confidence toward having the time to cook/eat a Mediterranean diet? | Opportunity (Physical) |
| 4. | How would you rate your ability to afford foods that are required for a Mediterranean diet? | Opportunity (Physical) |
| 5. | How would you rate your access to the foods that are required for a Mediterranean diet? | Opportunity (Physical) |
| 6. | How would you rate the acceptability of a Mediterranean diet by your friends or family? | Motivation (Reflective) |
| 7. | How would you rate your ability to adhere to a Mediterranean diet? | Motivation (Reflective) |
| 8. | How would you rate your intention to follow a Mediterranean diet? | Motivation (Reflective) |
| Open-Ended Questions | Bowen Framework | |
|---|---|---|
| 1. | What was your view on the education resources? | Acceptability |
| 2. | Which resource/s was the most helpful? | Demand |
| 3. | Which resource/s was the least helpful? | Demand |
| 4. | Do you have any suggested improvements on the delivery of the dietary intervention and/or education resources/materials? | Acceptability |
| 5. | How did you feel about following a dietary approach independent of calorie restriction or weight loss? Prompt: did it make it easier or harder to adhere? Did you feel it would be helpful or not helpful for your health? | Acceptability |
| 6. | Any final comments that were not described in previous questions? |
| Likert Scale | Bowen | |
|---|---|---|
| 1. | I found the education resources easy to understand | Acceptability |
| 2. | I found the education resources difficult to understand | Acceptability |
| 3. | I found the education resources easy to read | Acceptability |
| 4. | I found the education resources difficult to read | Acceptability |
| 5. | I found the education resources were useful | Demand |
| 6. | I found the education resources were not useful | Demand |
| 7. | The education resources made it easier to adhere to a Mediterranean diet | Demand |
| 8. | The education resources made it difficult to adhere to a Mediterranean diet | Demand |
| 9. | I would have liked more resources | Acceptability |
| 10. | I would have liked less resources | Acceptability |
| 11. | I found the dietary consultations were useful | Demand |
| 12. | I found the dietary consultations were not useful | Demand |
| 13. | I found the text messages were helpful? | Demand |
| 14. | I found the text messages were not helpful? | Demand |
| 15. | I would have liked less text messages? | Acceptability |
| 16. | I would have liked more text messages? | Acceptability |
| 17. | The text messages were too long? | Acceptability |
| 18. | The text messages were too short? | Acceptability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scannell, N.; Moran, L.; Mantzioris, E.; Cowan, S.; Villani, A. Efficacy, Feasibility and Acceptability of a Mediterranean Diet Intervention on Hormonal, Metabolic and Anthropometric Measures in Overweight and Obese Women with Polycystic Ovary Syndrome: Study Protocol. Metabolites 2022, 12, 311. https://doi.org/10.3390/metabo12040311
Scannell N, Moran L, Mantzioris E, Cowan S, Villani A. Efficacy, Feasibility and Acceptability of a Mediterranean Diet Intervention on Hormonal, Metabolic and Anthropometric Measures in Overweight and Obese Women with Polycystic Ovary Syndrome: Study Protocol. Metabolites. 2022; 12(4):311. https://doi.org/10.3390/metabo12040311
Chicago/Turabian StyleScannell, Nicole, Lisa Moran, Evangeline Mantzioris, Stephanie Cowan, and Anthony Villani. 2022. "Efficacy, Feasibility and Acceptability of a Mediterranean Diet Intervention on Hormonal, Metabolic and Anthropometric Measures in Overweight and Obese Women with Polycystic Ovary Syndrome: Study Protocol" Metabolites 12, no. 4: 311. https://doi.org/10.3390/metabo12040311
APA StyleScannell, N., Moran, L., Mantzioris, E., Cowan, S., & Villani, A. (2022). Efficacy, Feasibility and Acceptability of a Mediterranean Diet Intervention on Hormonal, Metabolic and Anthropometric Measures in Overweight and Obese Women with Polycystic Ovary Syndrome: Study Protocol. Metabolites, 12(4), 311. https://doi.org/10.3390/metabo12040311









