Interplay between Fatty Acid Binding Protein 4, Fetuin-A, Retinol Binding Protein 4 and Thyroid Function in Metabolic Dysregulation
Abstract
1. Introduction
2. FABP4 and Hypothalamic-Pituitary-Thyroid Axis—Experimental Studies
3. Fetuin-A and Hypothalamic-Pituitary-Thyroid Axis—Experimental Studies
4. RBP4 and Hypothalamic-Pituitary-Thyroid Axis—Experimental Studies
5. Adipokines and Hypothalamic-Pituitary-Thyroid Axis—Clinical Studies
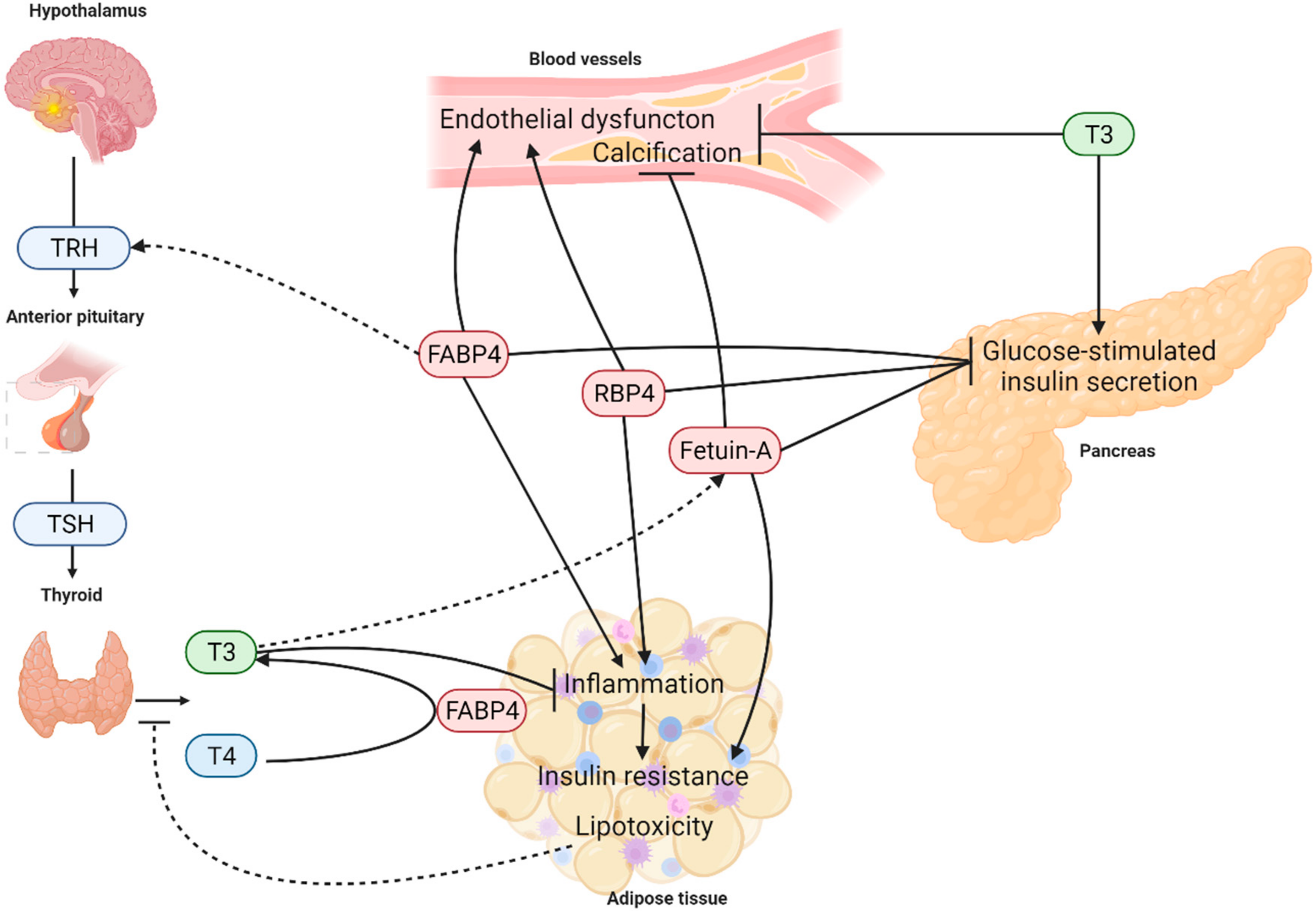
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volke, L.; Krause, K. Effect of Thyroid Hormones on Adipose Tissue Flexibility. Eur. Thyroid J. 2021, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Fátima dos Santos Teixeira, P.; dos Santos, P.B.; Pazos-Moura, C.C. The Role of Thyroid Hormone in Metabolism and Metabolic Syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820917869. [Google Scholar] [CrossRef]
- Ahmadi, N.; Ahmadi, F.; Sadiqi, M.; Ziemnicka, K.; Minczykowski, A. Thyroid Gland Dysfunction and Its Effect on the Cardiovascular System: A Comprehensive Review of the Literature. Endokrynol. Pol. 2020, 71, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Gutaj, N.; Zybek-Kocik, A.; Kloska, M.; Ziółkowska, P.; Czarnywojtek, A.; Sowiński, J.; Mańkowska-Wierzbicka, D.; Ruchała, M. Effect of Restoration of Euthyroidism on Visfatin Concentrations and Body Composition in Women. Endocr. Connect. 2021, 10, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Zybek-Kocik, A.; Sawicka-Gutaj, N.; Szczepanek-Parulska, E.; Andrusiewicz, M.; Waligórska-Stachura, J.; Białas, P.; Krauze, T.; Guzik, P.; Skrobisz, J.; Ruchała, M. The Association between Irisin and Muscle Metabolism in Different Thyroid Disorders. Clin. Endocrinol. 2018, 88, 460–467. [Google Scholar] [CrossRef]
- Zybek-Kocik, A.; Sawicka-Gutaj, N.; Domin, R.; Szczepanek-Parulska, E.; Krauze, T.; Guzik, P.; Ruchała, M. Titin and Dystrophin Serum Concentration Changes in Patients Affected by Thyroid Disorders. Endokrynol. Pol. 2021, 72, 1–7. [Google Scholar] [CrossRef]
- García-Solís, P.; García, O.P.; Hernández-Puga, G.; Sánchez-Tusie, A.A.; Sáenz-Luna, C.E.; Hernández-Montiel, H.L.; Solis-S, J.C. Thyroid Hormones and Obesity: A Known but Poorly Understood Relationship. Endokrynol. Pol. 2018, 69, 292–303. [Google Scholar] [CrossRef]
- Walczak, K.; Sieminska, L. Obesity and Thyroid Axis. Int. J. Environ. Res. Public. Health 2021, 18, 9434. [Google Scholar] [CrossRef]
- Pala, L.; Monami, M.; Ciani, S.; Dicembrini, I.; Pasqua, A.; Pezzatini, A.; Francesconi, P.; Cresci, B.; Mannucci, E.; Rotella, C.M. Adipokines as Possible New Predictors of Cardiovascular Diseases: A Case Control Study. J. Nutr. Metab. 2012, 2012, 253428. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Al-Daghri, N.M.; Vanhoutte, P.M.; Krishnaswamy, S.; Xu, A. Serum Retinol-Binding Protein 4 as a Marker for Cardiovascular Disease in Women. PLoS ONE 2012, 7, e48612. [Google Scholar] [CrossRef]
- Fan, J.; Yin, S.; Lin, D.; Liu, Y.; Chen, N.; Bai, X.; Ke, Q.; Shen, J.; You, L.; Lin, X.; et al. Association of Serum Retinol-Binding Protein 4 Levels and the Risk of Incident Type 2 Diabetes in Subjects with Prediabetes. Diabetes Care 2019, 42, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, L.; Lin, X.; Yuan, J.-M.; Koh, W.-P.; Pan, A. Retinol Binding Protein 4 and Risk of Type 2 Diabetes in Singapore Chinese Men and Women: A Nested Case-Control Study. Nutr. Metab. 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Drogan, D.; Weikert, C.; Schulze, M.B.; Fritsche, A.; Boeing, H.; Pischon, T. Fatty Acid-Binding Protein 4 and Risk of Type 2 Diabetes, Myocardial Infarction and Stroke: A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 5991–6002. [Google Scholar] [CrossRef]
- Egbuche, O.; Biggs, M.L.; Ix, J.H.; Kizer, J.R.; Lyles, M.F.; Siscovick, D.S.; Djoussé, L.; Mukamal, K.J. Fatty Acid Binding Protein-4 and Risk of Cardiovascular Disease: The Cardiovascular Health Study. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2020, 9, e014070. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Fritsche, A.; Weikert, C.; Boeing, H.; Joost, H.-G.; Häring, H.-U.; Schulze, M.B. Plasma Fetuin-A Levels and the Risk of Type 2 Diabetes. Diabetes 2008, 57, 2762–2767. [Google Scholar] [CrossRef]
- Sujana, C.; Huth, C.; Zierer, A.; Meesters, S.; Sudduth-Klinger, J.; Koenig, W.; Herder, C.; Peters, A.; Thorand, B. Association of Fetuin-A with Incident Type 2 Diabetes: Results from the MONICA/KORA Augsburg Study and a Systematic Meta-Analysis. Eur. J. Endocrinol. 2018, 178, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Roshanzamir, F.; Miraghajani, M.; Rouhani, M.H.; Mansourian, M.; Ghiasvand, R.; Safavi, S.M. The Association between Circulating Fetuin-A Levels and Type 2 Diabetes Mellitus Risk: Systematic Review and Meta-Analysis of Observational Studies. J. Endocrinol. Invest. 2018, 41, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Baxa, C.A.; Sha, R.S.; Buelt, M.K.; Smith, A.J.; Matarese, V.; Chinander, L.L.; Boundy, K.L.; Bernlohr, D.A. Human Adipocyte Lipid-Binding Protein: Purification of the Protein and Cloning of Its Complementary DNA. Biochemistry 1989, 28, 8683–8690. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lui, D.T.W.; Lam, K.S.L. Adipocyte Fatty Acid-Binding Protein, Cardiovascular Diseases and Mortality. Front. Immunol. 2021, 12, 589206. [Google Scholar] [CrossRef]
- Trojnar, M.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Leszczyńska-Gorzelak, B.; Mosiewicz, J. Associations between Fatty Acid-Binding Protein 4–A Proinflammatory Adipokine and Insulin Resistance, Gestational and Type 2 Diabetes Mellitus. Cells 2019, 8, 227. [Google Scholar] [CrossRef]
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S.; Rubinstein, M. FABP4 Attenuates PPARγ and Adipogenesis and Is Inversely Correlated with PPARγ in Adipose Tissues. Diabetes 2014, 63, 900–911. [Google Scholar] [CrossRef]
- Furuhashi, M. Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. J. Atheroscler. Thromb. 2019, 26, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, D.; Xu, J.; Chen, C.; Deng, D.; Pan, F.; Dong, L.; Li, S.; Ye, S. Circulating FABP4, Nesfatin-1, and Osteocalcin Concentrations in Women with Gestational Diabetes Mellitus: A Meta-Analysis. Lipids Health Dis. 2020, 19, 199. [Google Scholar] [CrossRef] [PubMed]
- Prentice, K.J.; Saksi, J.; Robertson, L.T.; Lee, G.Y.; Inouye, K.E.; Eguchi, K.; Lee, A.; Cakici, O.; Otterbeck, E.; Cedillo, P.; et al. A Hormone Complex of FABP4 and Nucleoside Kinases Regulates Islet Function. Nature 2021, 600, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Shu, L.; Wu, X.; Liu, Y.; Cheong, L.Y.; Liao, B.; Xiao, X.; Hoo, R.L.; Zhou, Z.; Xu, A. Fatty Acid Binding Protein 4 Promotes Autoimmune Diabetes by Recruitment and Activation of Pancreatic Islet Macrophages. JCI Insight 2021, 6, 141814. [Google Scholar] [CrossRef] [PubMed]
- Peeters, W.; de Kleijn, D.P.V.; Vink, A.; van de Weg, S.; Schoneveld, A.H.; Sze, S.K.; van der Spek, P.J.; de Vries, J.-P.P.M.; Moll, F.L.; Pasterkamp, G. Adipocyte Fatty Acid Binding Protein in Atherosclerotic Plaques Is Associated with Local Vulnerability and Is Predictive for the Occurrence of Adverse Cardiovascular Events. Eur. Heart J. 2011, 32, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Molvin, J.; Jujić, A.; Melander, O.; Pareek, M.; Råstam, L.; Lindblad, U.; Daka, B.; Leósdóttir, M.; Nilsson, P.M.; Olsen, M.H.; et al. Proteomic Exploration of Common Pathophysiological Pathways in Diabetes and Cardiovascular Disease. ESC Heart Fail. 2020, 7, 4151–4158. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; O’Leary, D.H. Carotid Intima-Media Thickness as Surrogate for and Predictor of CVD. Glob. Heart 2016, 11, 295–312. [Google Scholar] [CrossRef]
- Tan, M.; Korkmaz, H.; Aydın, H.; Kumbul Doğuç, D. FABP4 Levels in Hypothyroidism and Its Relationship with Subclinical Atherosclerosis. Turk. J. Med. Sci. 2019, 49, 1490–1497. [Google Scholar] [CrossRef]
- Papadopoulou, A.-M.; Bakogiannis, N.; Skrapari, I.; Moris, D.; Bakoyiannis, C. Thyroid Dysfunction and Atherosclerosis: A Systematic Review. Vivo Athens Greece 2020, 34, 3127–3136. [Google Scholar] [CrossRef]
- Furuhashi, M.; Yuda, S.; Muranaka, A.; Kawamukai, M.; Matsumoto, M.; Tanaka, M.; Moniwa, N.; Ohnishi, H.; Saitoh, S.; Shimamoto, K.; et al. Circulating Fatty Acid-Binding Protein 4 Concentration Predicts the Progression of Carotid Atherosclerosis in a General Population without Medication. Circ. J. Off. J. Jpn. Circ. Soc. 2018, 82, 1121–1129. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, X.; Luo, Y.; Shen, Y.; Dou, J.; Pan, X.; Bao, Y.; Jia, W. Serum Adipocyte Fatty Acid Binding Protein Levels Are Positively Associated with Subclinical Atherosclerosis in Chinese Pre- and Postmenopausal Women with Normal Glucose Tolerance. J. Clin. Endocrinol. Metab. 2014, 99, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Parra, S.; Cabré, A.; Marimon, F.; Ferré, R.; Ribalta, J.; Gonzàlez, M.; Heras, M.; Castro, A.; Masana, L. Circulating FABP4 Is a Marker of Metabolic and Cardiovascular Risk in SLE Patients. Lupus 2014, 23, 245–254. [Google Scholar] [CrossRef]
- Ibarretxe, D.; Girona, J.; Amigó, N.; Plana, N.; Ferré, R.; Guaita, S.; Mallol, R.; Heras, M.; Masana, L. Impact of Epidermal Fatty Acid Binding Protein on 2D-NMR-Assessed Atherogenic Dyslipidemia and Related Disorders. J. Clin. Lipidol. 2016, 10, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Orsag, J.; Karasek, D.; Halenka, M.; Vaverkova, H.; Spurna, J.; Kubickova, V.; Lukes, J.; Zadrazil, J. Association of Serum Adipocyte Fatty Acid-Binding Protein and Apolipoprotein B /Apolipoprotein A1 Ratio with Intima Media Thickness of Common Carotid Artery in Dyslipidemic Patients. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2019, 163, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Tso, A.W.K.; Xu, A.; Sham, P.C.; Wat, N.M.S.; Wang, Y.; Fong, C.H.Y.; Cheung, B.M.Y.; Janus, E.D.; Lam, K.S.L. Serum Adipocyte Fatty Acid Binding Protein as a New Biomarker Predicting the Development of Type 2 Diabetes: A 10-Year Prospective Study in a Chinese Cohort. Diabetes Care 2007, 30, 2667–2672. [Google Scholar] [CrossRef]
- Mastracci, T.L.; Evans-Molina, C. Pancreatic and Islet Development and Function: The Role of Thyroid Hormone. J. Endocrinol. Diabetes Obes. 2014, 2, 1044. [Google Scholar]
- Aguayo-Mazzucato, C.; Zavacki, A.M.; Marinelarena, A.; Hollister-Lock, J.; El Khattabi, I.; Marsili, A.; Weir, G.C.; Sharma, A.; Larsen, P.R.; Bonner-Weir, S. Thyroid Hormone Promotes Postnatal Rat Pancreatic β-Cell Development and Glucose-Responsive Insulin Secretion through MAFA. Diabetes 2013, 62, 1569–1580. [Google Scholar] [CrossRef]
- Verga Falzacappa, C.; Mangialardo, C.; Raffa, S.; Mancuso, A.; Piergrossi, P.; Moriggi, G.; Piro, S.; Stigliano, A.; Torrisi, M.R.; Brunetti, E.; et al. The Thyroid Hormone T3 Improves Function and Survival of Rat Pancreatic Islets during in Vitro Culture. Islets 2010, 2, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Verga Falzacappa, C.; Petrucci, E.; Patriarca, V.; Michienzi, S.; Stigliano, A.; Brunetti, E.; Toscano, V.; Misiti, S. Thyroid Hormone Receptor TRbeta1 Mediates Akt Activation by T3 in Pancreatic Beta Cells. J. Mol. Endocrinol. 2007, 38, 221–233. [Google Scholar] [CrossRef]
- Safayee, S.; Karbalaei, N.; Noorafshan, A.; Nadimi, E. Induction of Oxidative Stress, Suppression of Glucose-Induced Insulin Release, ATP Production, Glucokinase Activity, and Histomorphometric Changes in Pancreatic Islets of Hypothyroid Rat. Eur. J. Pharmacol. 2016, 791, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Faddladdeen, K.; Ali, S.S.; Bahshwan, S.; Ayuob, N. Thymoquinone Preserves Pancreatic Islets Structure Through Upregulation of Pancreatic β-Catenin in Hypothyroid Rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 2913–2924. [Google Scholar] [CrossRef]
- Nie, X.; Ma, X.; Xu, Y.; Shen, Y.; Wang, Y.; Bao, Y. Increased Serum Adipocyte Fatty Acid-Binding Protein Levels Are Associated with Decreased Sensitivity to Thyroid Hormones in the Euthyroid Population. Thyroid Off. J. Am. Thyroid Assoc. 2020, 30, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Laclaustra, M.; Moreno-Franco, B.; Lou-Bonafonte, J.M.; Mateo-Gallego, R.; Casasnovas, J.A.; Guallar-Castillon, P.; Cenarro, A.; Civeira, F. Impaired Sensitivity to Thyroid Hormones Is Associated with Diabetes and Metabolic Syndrome. Diabetes Care 2019, 42, 303–310. [Google Scholar] [CrossRef]
- Mehran, L.; Delbari, N.; Amouzegar, A.; Hasheminia, M.; Tohidi, M.; Azizi, F. Reduced Sensitivity to Thyroid Hormone Is Associated with Diabetes and Hypertension. J. Clin. Endocrinol. Metab. 2022, 107, 167–176. [Google Scholar] [CrossRef]
- Alonso, S.P.; Valdés, S.; Maldonado-Araque, C.; Lago, A.; Ocon, P.; Calle, A.; Castaño, L.; Delgado, E.; Menéndez, E.; Franch-Nadal, J.; et al. Thyroid Hormone Resistance Index and Mortality in Euthyroid Subjects: Di@bet.Es Study. Eur. J. Endocrinol. 2021, 186, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Li, J.; Wang, Z.; Wang, W.; Guan, H. Sensitivity to Thyroid Hormone Indices Are Closely Associated with NAFLD. Front. Endocrinol. 2021, 12, 766419. [Google Scholar] [CrossRef] [PubMed]
- Štěpánek, L.; Horáková, D.; Štěpánek, L.; Janout, V.; Janoutová, J.; Bouchalová, K.; Martiník, K. Free Triiodothyronine/Free Thyroxine (FT3/FT4) Ratio Is Strongly Associated with Insulin Resistance in Euthyroid and Hypothyroid Adults: A Cross-Sectional Study. Endokrynol. Pol. 2021, 72, 8–13. [Google Scholar] [CrossRef]
- Shu, L.; Hoo, R.L.C.; Wu, X.; Pan, Y.; Lee, I.P.C.; Cheong, L.Y.; Bornstein, S.R.; Rong, X.; Guo, J.; Xu, A. A-FABP Mediates Adaptive Thermogenesis by Promoting Intracellular Activation of Thyroid Hormones in Brown Adipocytes. Nat. Commun. 2017, 8, 14147. [Google Scholar] [CrossRef]
- Russo, S.C.; Salas-Lucia, F.; Bianco, A.C. Deiodinases and the Metabolic Code for Thyroid Hormone Action. Endocrinology 2021, 162, bqab059. [Google Scholar] [CrossRef]
- de Jesus, L.A.; Carvalho, S.D.; Ribeiro, M.O.; Schneider, M.; Kim, S.W.; Harney, J.W.; Larsen, P.R.; Bianco, A.C. The Type 2 Iodothyronine Deiodinase Is Essential for Adaptive Thermogenesis in Brown Adipose Tissue. J. Clin. Invest. 2001, 108, 1379–1385. [Google Scholar] [CrossRef]
- Laffitte, B.A.; Chao, L.C.; Li, J.; Walczak, R.; Hummasti, S.; Joseph, S.B.; Castrillo, A.; Wilpitz, D.C.; Mangelsdorf, D.J.; Collins, J.L.; et al. Activation of Liver X Receptor Improves Glucose Tolerance through Coordinate Regulation of Glucose Metabolism in Liver and Adipose Tissue. Proc. Natl. Acad. Sci. USA 2003, 100, 5419–5424. [Google Scholar] [CrossRef] [PubMed]
- Efanov, A.M.; Sewing, S.; Bokvist, K.; Gromada, J. Liver X Receptor Activation Stimulates Insulin Secretion via Modulation of Glucose and Lipid Metabolism in Pancreatic Beta-Cells. Diabetes 2004, 53 (Suppl. 3), S75–S78. [Google Scholar] [CrossRef] [PubMed]
- Russo-Savage, L.; Schulman, I.G. Liver X Receptors and Liver Physiology. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166121. [Google Scholar] [CrossRef]
- Christoffolete, M.A.; Doleschall, M.; Egri, P.; Liposits, Z.; Zavacki, A.M.; Bianco, A.C.; Gereben, B. Regulation of Thyroid Hormone Activation via the Liver X-Receptor/Retinoid X-Receptor Pathway. J. Endocrinol. 2010, 205, 179–186. [Google Scholar] [CrossRef]
- Miao, Y.; Wu, W.; Dai, Y.; Maneix, L.; Huang, B.; Warner, M.; Gustafsson, J.-Å. Liver X Receptor β Controls Thyroid Hormone Feedback in the Brain and Regulates Browning of Subcutaneous White Adipose Tissue. Proc. Natl. Acad. Sci. USA 2015, 112, 14006–14011. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Tuncman, G.; Görgün, C.Z.; Makowski, L.; Atsumi, G.; Vaillancourt, E.; Kono, K.; Babaev, V.R.; Fazio, S.; Linton, M.F.; et al. Treatment of Diabetes and Atherosclerosis by Inhibiting Fatty-Acid-Binding Protein AP2. Nature 2007, 447, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Burak, M.F.; Inouye, K.E.; White, A.; Lee, A.; Tuncman, G.; Calay, E.S.; Sekiya, M.; Tirosh, A.; Eguchi, K.; Birrane, G.; et al. Development of a Therapeutic Monoclonal Antibody That Targets Secreted Fatty Acid-Binding Protein AP2 to Treat Type 2 Diabetes. Sci. Transl. Med. 2015, 7, 319ra205. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wang, Y.; Wang, W.; Lv, X.; Wang, M.; Yin, H. The MAb against Adipocyte Fatty Acid-Binding Protein 2E4 Attenuates the Inflammation in the Mouse Model of High-Fat Diet-Induced Obesity via Toll-like Receptor 4 Pathway. Mol. Cell. Endocrinol. 2015, 403, 1–9. [Google Scholar] [CrossRef]
- Bosquet, A.; Girona, J.; Guaita-Esteruelas, S.; Heras, M.; Saavedra-García, P.; Martínez-Micaelo, N.; Masana, L.; Rodríguez-Calvo, R. FABP4 Inhibitor BMS309403 Decreases Saturated-Fatty-Acid-Induced Endoplasmic Reticulum Stress-Associated Inflammation in Skeletal Muscle by Reducing P38 MAPK Activation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 604–613. [Google Scholar] [CrossRef]
- Lee, M.Y.; Li, H.; Xiao, Y.; Zhou, Z.; Xu, A.; Vanhoutte, P.M. Chronic Administration of BMS309403 Improves Endothelial Function in Apolipoprotein E-Deficient Mice and in Cultured Human Endothelial Cells. Br. J. Pharmacol. 2011, 162, 1564–1576. [Google Scholar] [CrossRef]
- Hoo, R.L.C.; Lee, I.P.C.; Zhou, M.; Wong, J.Y.L.; Hui, X.; Xu, A.; Lam, K.S.L. Pharmacological Inhibition of Adipocyte Fatty Acid Binding Protein Alleviates Both Acute Liver Injury and Non-Alcoholic Steatohepatitis in Mice. J. Hepatol. 2013, 58, 358–364. [Google Scholar] [CrossRef]
- Furuhashi, M.; Mita, T.; Moniwa, N.; Hoshina, K.; Ishimura, S.; Fuseya, T.; Watanabe, Y.; Yoshida, H.; Shimamoto, K.; Miura, T. Angiotensin II Receptor Blockers Decrease Serum Concentration of Fatty Acid-Binding Protein 4 in Patients with Hypertension. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2015, 38, 252–259. [Google Scholar] [CrossRef]
- Karpisek, M.; Stejskal, D.; Kotolova, H.; Kollar, P.; Janoutova, G.; Ochmanova, R.; Cizek, L.; Horakova, D.; Yahia, R.B.; Lichnovska, R.; et al. Treatment with Atorvastatin Reduces Serum Adipocyte-Fatty Acid Binding Protein Value in Patients with Hyperlipidaemia. Eur. J. Clin. Invest. 2007, 37, 637–642. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hiramitsu, S.; Mita, T.; Omori, A.; Fuseya, T.; Ishimura, S.; Watanabe, Y.; Hoshina, K.; Matsumoto, M.; Tanaka, M.; et al. Reduction of Circulating FABP4 Level by Treatment with Omega-3 Fatty Acid Ethyl Esters. Lipids Health Dis. 2016, 15, 5. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hiramitsu, S.; Mita, T.; Fuseya, T.; Ishimura, S.; Omori, A.; Matsumoto, M.; Watanabe, Y.; Hoshina, K.; Tanaka, M.; et al. Reduction of Serum FABP4 Level by Sitagliptin, a DPP-4 Inhibitor, in Patients with Type 2 Diabetes Mellitus. J. Lipid Res. 2015, 56, 2372–2380. [Google Scholar] [CrossRef]
- Pérez-Sotelo, D.; Roca-Rivada, A.; Larrosa-García, M.; Castelao, C.; Baamonde, I.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. Visceral and Subcutaneous Adipose Tissue Express and Secrete Functional Alpha2hsglycoprotein (Fetuin a) Especially in Obesity. Endocrine 2017, 55, 435–446. [Google Scholar] [CrossRef]
- Kellermann, J.; Haupt, H.; Auerswald, E.A.; Müller-Ester, W. The Arrangement of Disulfide Loops in Human Alpha 2-HS Glycoprotein. Similarity to the Disulfide Bridge Structures of Cystatins and Kininogens. J. Biol. Chem. 1989, 264, 14121–14128. [Google Scholar] [CrossRef]
- Icer, M.A.; Yıldıran, H. Effects of Nutritional Status on Serum Fetuin-A Level. Crit. Rev. Food Sci. Nutr. 2020, 60, 1938–1946. [Google Scholar] [CrossRef]
- Nimptsch, K.; Janke, J.; Pischon, T.; Linseisen, J. Association between Dietary Factors and Plasma Fetuin-A Concentrations in the General Population. Br. J. Nutr. 2015, 114, 1278–1285. [Google Scholar] [CrossRef]
- Seo, D.Y.; Park, S.H.; Marquez, J.; Kwak, H.-B.; Kim, T.N.; Bae, J.H.; Koh, J.-H.; Han, J. Hepatokines as a Molecular Transducer of Exercise. J. Clin. Med. 2021, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Brix, J.M.; Stingl, H.; Höllerl, F.; Schernthaner, G.H.; Kopp, H.-P.; Schernthaner, G. Elevated Fetuin-A Concentrations in Morbid Obesity Decrease after Dramatic Weight Loss. J. Clin. Endocrinol. Metab. 2010, 95, 4877–4881. [Google Scholar] [CrossRef]
- Ix, J.H.; Shlipak, M.G.; Brandenburg, V.M.; Ali, S.; Ketteler, M.; Whooley, M.A. Association between Human Fetuin-A and the Metabolic Syndrome: Data from the Heart and Soul Study. Circulation 2006, 113, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, J.P.; Quiroz, R.; Nelson, K.P.; Teng, Z.; Keaney, J.F.; Sullivan, L.M.; Vasan, R.S. Prospective Relation of Circulating Adipokines to Incident Metabolic Syndrome: The Framingham Heart Study. J. Am. Heart Assoc. 2017, 6, e004974. [Google Scholar] [CrossRef]
- Ix, J.H.; Wassel, C.L.; Kanaya, A.M.; Vittinghoff, E.; Johnson, K.C.; Koster, A.; Cauley, J.A.; Harris, T.B.; Cummings, S.R.; Shlipak, M.G.; et al. Fetuin-A and Incident Diabetes Mellitus in Older Persons. JAMA 2008, 300, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kaminga, A.C.; Chen, J.; Luo, M.; Luo, J. Fetuin-A and Fetuin-B in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis and Meta-Regression. Int. J. Environ. Res. Public. Health 2020, 17, E2735. [Google Scholar] [CrossRef] [PubMed]
- Guo, V.Y.; Cao, B.; Cai, C.; Cheng, K.K.-Y.; Cheung, B.M.Y. Fetuin-A Levels and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Acta Diabetol. 2018, 55, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Birukov, A.; Polemiti, E.; Jäger, S.; Stefan, N.; Schulze, M.B. Fetuin-A and Risk of Diabetes-Related Vascular Complications: A Prospective Study. Cardiovasc. Diabetol. 2022, 21, 1–11. [Google Scholar] [CrossRef]
- Afrisham, R.; Paknejad, M.; Ilbeigi, D.; Sadegh-Nejadi, S.; Gorgani-Firuzjaee, S.; Vahidi, M. Positive Correlation between Circulating Fetuin-A and Severity of Coronary Artery Disease in Men. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Chen, Q.; Li, Q.; Li, Y.; Ling, W. Lower Plasma Fetuin-A Levels Are Associated with a Higher Mortality Risk in Patients with Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Thomas, G.R.; Pardini, A.W.; Figueira, W.F.; Caputo, J.M.; Williamson, M.K. Discovery of a High Molecular Weight Complex of Calcium, Phosphate, Fetuin, and Matrix Gamma-Carboxyglutamic Acid Protein in the Serum of Etidronate-Treated Rats. J. Biol. Chem. 2002, 277, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.M.X.; Smith, E.R.; Holt, S.G. The Role of Fetuin-A in Mineral Trafficking and Deposition. BoneKEy Rep. 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.G.; Smith, E.R. Fetuin-A-Containing Calciprotein Particles in Mineral Trafficking and Vascular Disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2016, 31, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Hokanson, J.E.; Nasir, K.; Shaw, L.J.; Kinney, G.L.; Chow, D.; Demoss, D.; Nuguri, V.; Nabavi, V.; Ratakonda, R.; et al. Progression of Coronary Artery Calcium Predicts All-Cause Mortality. JACC Cardiovasc. Imaging 2010, 3, 1229–1236. [Google Scholar] [CrossRef]
- Budoff, M.J.; Young, R.; Lopez, V.A.; Kronmal, R.A.; Nasir, K.; Blumenthal, R.S.; Detrano, R.C.; Bild, D.E.; Guerci, A.D.; Liu, K.; et al. Progression of Coronary Calcium and Incident Coronary Heart Disease Events: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2013, 61, 1231–1239. [Google Scholar] [CrossRef]
- Demer, L.L.; Tintut, Y. Vascular Calcification: Pathobiology of a Multifaceted Disease. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef]
- Ceponiene, I.; Nakanishi, R.; Osawa, K.; Kanisawa, M.; Nezarat, N.; Rahmani, S.; Kissel, K.; Kim, M.; Jayawardena, E.; Broersen, A.; et al. Coronary Artery Calcium Progression Is Associated with Coronary Plaque Volume Progression: Results From a Quantitative Semiautomated Coronary Artery Plaque Analysis. JACC Cardiovasc. Imaging 2018, 11, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Tintut, Y.; Patel, J.; Territo, M.; Saini, T.; Parhami, F.; Demer, L.L. Monocyte/Macrophage Regulation of Vascular Calcification in Vitro. Circulation 2002, 105, 650–655. [Google Scholar] [CrossRef]
- Hoshino, T.; Chow, L.A.; Hsu, J.J.; Perlowski, A.A.; Abedin, M.; Tobis, J.; Tintut, Y.; Mal, A.K.; Klug, W.S.; Demer, L.L. Mechanical Stress Analysis of a Rigid Inclusion in Distensible Material: A Model of Atherosclerotic Calcification and Plaque Vulnerability. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H802–H810. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Skepper, J.N.; McNair, R.; Kasama, T.; Gupta, K.; Weissberg, P.L.; Jahnen-Dechent, W.; Shanahan, C.M. Multifunctional Roles for Serum Protein Fetuin-a in Inhibition of Human Vascular Smooth Muscle Cell Calcification. J. Am. Soc. Nephrol. JASN 2005, 16, 2920–2930. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; Katz, R.; de Boer, I.H.; Kestenbaum, B.R.; Peralta, C.A.; Jenny, N.S.; Budoff, M.; Allison, M.A.; Criqui, M.H.; Siscovick, D.; et al. Fetuin-A Is Inversely Associated with Coronary Artery Calcification in Community-Living Persons: The Multi-Ethnic Study of Atherosclerosis. Clin. Chem. 2012, 58, 887–895. [Google Scholar] [CrossRef]
- Szeberin, Z.; Fehérvári, M.; Krepuska, M.; Apor, A.; Rimely, E.; Sarkadi, H.; Széplaki, G.; Prohászka, Z.; Kalabay, L.; Acsády, G. Serum Fetuin-A Levels Inversely Correlate with the Severity of Arterial Calcification in Patients with Chronic Lower Extremity Atherosclerosis without Renal Disease. Int. Angiol. J. Int. Union Angiol. 2011, 30, 474–480. [Google Scholar]
- Ix, J.H.; Barrett-Connor, E.; Wassel, C.L.; Cummins, K.; Bergstrom, J.; Daniels, L.B.; Laughlin, G.A. The Associations of Fetuin-A with Subclinical Cardiovascular Disease in Community-Dwelling Persons: The Rancho Bernardo Study. J. Am. Coll. Cardiol. 2011, 58, 2372–2379. [Google Scholar] [CrossRef]
- Ballestri, S.; Meschiari, E.; Baldelli, E.; Musumeci, F.E.; Romagnoli, D.; Trenti, T.; Zennaro, R.G.; Lonardo, A.; Loria, P. Relationship of Serum Fetuin-A Levels with Coronary Atherosclerotic Burden and NAFLD in Patients Undergoing Elective Coronary Angiography. Metab. Syndr. Relat. Disord. 2013, 11, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Bilgir, O.; Kebapcilar, L.; Bilgir, F.; Bozkaya, G.; Yildiz, Y.; Pinar, P.; Tastan, A. Decreased Serum Fetuin-A Levels Are Associated with Coronary Artery Diseases. Intern. Med. Tokyo Jpn. 2010, 49, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Göçer, K.; Aykan, A.Ç.; Kılınç, M.; Göçer, N.S. Association of Serum FGF-23, Klotho, Fetuin-A, Osteopontin, Osteoprotegerin and Hs-CRP Levels with Coronary Artery Disease. Scand. J. Clin. Lab. Invest. 2020, 80, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.; Moutereau, S.; Simon, T.; Gallet, R.; Probst, V.; Ferrieres, J.; Gueret, P.; Danchin, N. Usefulness of Fetuin-A and C-Reactive Protein Concentrations for Prediction of Outcome in Acute Coronary Syndromes (from the French Registry of Acute ST-Elevation Non-ST-Elevation Myocardial Infarction [FAST-MI]). Am. J. Cardiol. 2013, 111, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.; Collet, J.-P.; Moutereau, S.; Guigui, N.; Mitchell-Heggs, L.; Loric, S.; Bernard, M.; Benhamed, S.; Montalescot, G.; Randé, J.-L.D.; et al. Fetuin-A Is an Independent Predictor of Death after ST-Elevation Myocardial Infarction. Clin. Chem. 2007, 53, 1835–1840. [Google Scholar] [CrossRef]
- Xie, W.-M.; Ran, L.-S.; Jiang, J.; Chen, Y.-S.; Ji, H.-Y.; Quan, X.-Q. Association between Fetuin-A and Prognosis of CAD: A Systematic Review and Meta-Analysis. Eur. J. Clin. Invest. 2019, 49, e13091. [Google Scholar] [CrossRef] [PubMed]
- Mancio, J.; Barros, A.S.; Conceicao, G.; Pessoa-Amorim, G.; Santa, C.; Bartosch, C.; Ferreira, W.; Carvalho, M.; Ferreira, N.; Vouga, L.; et al. Epicardial Adipose Tissue Volume and Annexin A2/Fetuin-A Signalling Are Linked to Coronary Calcification in Advanced Coronary Artery Disease: Computed Tomography and Proteomic Biomarkers from the EPICHEART Study. Atherosclerosis 2020, 292, 75–83. [Google Scholar] [CrossRef]
- Naito, C.; Hashimoto, M.; Watanabe, K.; Shirai, R.; Takahashi, Y.; Kojima, M.; Watanabe, R.; Sato, K.; Iso, Y.; Matsuyama, T.-A.; et al. Facilitatory Effects of Fetuin-A on Atherosclerosis. Atherosclerosis 2016, 246, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Chen, P.-S.; Chen, P.-W.; Tsai, L.-M.; Liu, P.-Y. Fetuin A Adds Prognostic Value for Cardiovascular Outcomes among Patients with Coronary Artery Disease with Moderate Calcification. Int. J. Cardiol. 2015, 185, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nakamura, R.; Satoh, M.; Fujishita, K.; Mori, S.; Ishida, S.; Yamaguchi, T.; Inoue, K.; Nagao, T.; Ohno, Y. Thyroid Hormone Targets Matrix Gla Protein Gene Associated with Vascular Smooth Muscle Calcification. Circ. Res. 2005, 97, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Shin, J.A.; Shin, J.Y.; Lim, D.J.; Moon, S.D.; Son, H.Y.; Han, J.H. Association between Low Serum Free Thyroxine Concentrations and Coronary Artery Calcification in Healthy Euthyroid Subjects. Thyroid Off. J. Am. Thyroid Assoc. 2012, 22, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gao, C.; Wang, X.; Qi, D.; Zhang, Y.; Li, M.; Liu, W.; Hao, P. The Effect of Low FT3 Levels on Coronary Artery Calcification and MACE in Outpatients with Suspected Coronary Artery Disease. Coron. Artery Dis. 2014, 25, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Kim, J.; Han, E.J.; Park, S.E.; Park, C.-Y.; Lee, W.-Y.; Oh, K.-W.; Park, S.-W.; Rhee, E.-J. Association of Low Baseline Free Thyroxin Levels with Progression of Coronary Artery Calcification over 4 Years in Euthyroid Subjects: The Kangbuk Samsung Health Study. Clin. Endocrinol. 2016, 84, 889–895. [Google Scholar] [CrossRef]
- Peixoto de Miranda, É.J.F.; Bittencourt, M.S.; Staniak, H.L.; Pereira, A.C.; Foppa, M.; Santos, I.S.; Lotufo, P.A.; Benseñor, I.M. Thyrotrophin Levels and Coronary Artery Calcification: Cross-Sectional Results of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Clin. Endocrinol. 2017, 87, 597–604. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, B.-K.; Chang, Y.; Ryu, S.; Cho, J.; Lee, W.-Y.; Rhee, E.-J.; Kwon, M.-J.; Rampal, S.; Zhao, D.; et al. Thyroid Hormones and Coronary Artery Calcification in Euthyroid Men and Women. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Croce, L.; Sparano, C.; Petrone, L.; Sforza, A.; Maggi, M.; Chiovato, L.; Rotondi, M. Thyroid and Heart, a Clinically Relevant Relationship. J. Endocrinol. Invest. 2021, 44, 2535–2544. [Google Scholar] [CrossRef]
- Srinivas, P.R.; Wagner, A.S.; Reddy, L.V.; Deutsch, D.D.; Leon, M.A.; Goustin, A.S.; Grunberger, G. Serum Alpha 2-HS-Glycoprotein Is an Inhibitor of the Human Insulin Receptor at the Tyrosine Kinase Level. Mol. Endocrinol. Baltim. Md 1993, 7, 1445–1455. [Google Scholar] [CrossRef]
- Mathews, S.T.; Chellam, N.; Srinivas, P.R.; Cintron, V.J.; Leon, M.A.; Goustin, A.S.; Grunberger, G. Alpha2-HSG, a Specific Inhibitor of Insulin Receptor Autophosphorylation, Interacts with the Insulin Receptor. Mol. Cell. Endocrinol. 2000, 164, 87–98. [Google Scholar] [CrossRef]
- Goustin, A.S.; Derar, N.; Abou-Samra, A.B. Ahsg-Fetuin Blocks the Metabolic Arm of Insulin Action through Its Interaction with the 95-KD β-Subunit of the Insulin Receptor. Cell. Signal. 2013, 25, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Dasgupta, S.; Kundu, R.; Maitra, S.; Das, G.; Mukhopadhyay, S.; Ray, S.; Majumdar, S.S.; Bhattacharya, S. Fetuin-A Acts as an Endogenous Ligand of TLR4 to Promote Lipid-Induced Insulin Resistance. Nat. Med. 2012, 18, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Seal, S.; Mukherjee, S.; Kundu, R.; Mukherjee, S.; Ray, S.; Mukhopadhyay, S.; Majumdar, S.S.; Bhattacharya, S. Adipocyte Fetuin-A Contributes to Macrophage Migration into Adipose Tissue and Polarization of Macrophages. J. Biol. Chem. 2013, 288, 28324–28330. [Google Scholar] [CrossRef] [PubMed]
- Hennige, A.M.; Staiger, H.; Wicke, C.; Machicao, F.; Fritsche, A.; Häring, H.-U.; Stefan, N. Fetuin-A Induces Cytokine Expression and Suppresses Adiponectin Production. PLoS ONE 2008, 3, e1765. [Google Scholar] [CrossRef]
- Agarwal, S.; Chattopadhyay, M.; Mukherjee, S.; Dasgupta, S.; Mukhopadhyay, S.; Bhattacharya, S. Fetuin-A Downregulates Adiponectin through Wnt-PPARγ Pathway in Lipid Induced Inflamed Adipocyte. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 174–181. [Google Scholar] [CrossRef]
- Shen, X.; Yang, L.; Yan, S.; Zheng, H.; Liang, L.; Cai, X.; Liao, M. Fetuin A Promotes Lipotoxicity in β Cells through the TLR4 Signaling Pathway and the Role of Pioglitazone in Anti-Lipotoxicity. Mol. Cell. Endocrinol. 2015, 412, 1–11. [Google Scholar] [CrossRef]
- Mukhuty, A.; Fouzder, C.; Mukherjee, S.; Malick, C.; Mukhopadhyay, S.; Bhattacharya, S.; Kundu, R. Palmitate Induced Fetuin-A Secretion from Pancreatic β-Cells Adversely Affects Its Function and Elicits Inflammation. Biochem. Biophys. Res. Commun. 2017, 491. [Google Scholar] [CrossRef]
- Mukhuty, A.; Fouzder, C.; Kundu, R. Fetuin-A Excess Expression Amplifies Lipid Induced Apoptosis and β-Cell Damage. J. Cell. Physiol. 2022, 237, 532–550. [Google Scholar] [CrossRef]
- Mathews, S.T.; Singh, G.P.; Ranalletta, M.; Cintron, V.J.; Qiang, X.; Goustin, A.S.; Jen, K.-L.C.; Charron, M.J.; Jahnen-Dechent, W.; Grunberger, G. Improved Insulin Sensitivity and Resistance to Weight Gain in Mice Null for the Ahsg Gene. Diabetes 2002, 51, 2450–2458. [Google Scholar] [CrossRef]
- Stefan, N.; Hennige, A.M.; Staiger, H.; Machann, J.; Schick, F.; Kröber, S.M.; Machicao, F.; Fritsche, A.; Häring, H.-U. Alpha2-Heremans-Schmid Glycoprotein/Fetuin-A Is Associated with Insulin Resistance and Fat Accumulation in the Liver in Humans. Diabetes Care 2006, 29, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Emoto, M.; Yokoyama, H.; Araki, T.; Teramura, M.; Koyama, H.; Shoji, T.; Inaba, M.; Nishizawa, Y. Association of Serum Fetuin-A with Insulin Resistance in Type 2 Diabetic and Nondiabetic Subjects. Diabetes Care 2006, 29, 468. [Google Scholar] [CrossRef] [PubMed]
- Gierach, M.; Gierach, J.; Junik, R. Insulin Resistance and Thyroid Disorders. Endokrynol. Pol. 2014, 65, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, B.R.; Sola-García, A.; Cáliz-Molina, M.Á.; Lorenzo, P.I.; Cobo-Vuilleumier, N.; Capilla-González, V.; Martin-Montalvo, A. Thyroid Hormones in Diabetes, Cancer, and Aging. Aging Cell 2020, 19, e13260. [Google Scholar] [CrossRef]
- Panveloski-Costa, A.C.; Serrano-Nascimento, C.; Bargi-Souza, P.; Poyares, L.L.; de S. Viana, G.; Nunes, M.T. Beneficial Effects of Thyroid Hormone on Adipose Inflammation and Insulin Sensitivity of Obese Wistar Rats. Physiol. Rep. 2018, 6, e13550. [Google Scholar] [CrossRef]
- Panveloski-Costa, A.C.; Silva Teixeira, S.; Ribeiro, I.M.R.; Serrano-Nascimento, C.; das Neves, R.X.; Favaro, R.R.; Seelaender, M.; Antunes, V.R.; Nunes, M.T. Thyroid Hormone Reduces Inflammatory Cytokines Improving Glycaemia Control in Alloxan-Induced Diabetic Wistar Rats. Acta Physiol. Oxf. Engl. 2016, 217, 130–140. [Google Scholar] [CrossRef]
- Panveloski-Costa, A.C.; Kuwabara, W.M.T.; Munhoz, A.C.; Lucena, C.F.; Curi, R.; Carpinelli, A.R.; Nunes, M.T. The Insulin Resistance Is Reversed by Exogenous 3,5,3′triiodothyronine in Type 2 Diabetic Goto-Kakizaki Rats by an Inflammatory-Independent Pathway. Endocrine 2020, 68, 287–295. [Google Scholar] [CrossRef]
- Cabanelas, A.; Cordeiro, A.; dos Santos Almeida, N.A.; Monteiro de Paula, G.S.; Coelho, V.M.; Ortiga-Carvalho, T.M.; Pazos-Moura, C.C. Effect of Triiodothyronine on Adiponectin Expression and Leptin Release by White Adipose Tissue of Normal Rats. Horm. Metab. Res. Horm. Stoffwechs. Horm. Metab. 2010, 42, 254–260. [Google Scholar] [CrossRef]
- Seifi, S.; Tabandeh, M.R.; Nazifi, S.; Saeb, M.; Shirian, S.; Sarkoohi, P. Regulation of Adiponectin Gene Expression in Adipose Tissue by Thyroid Hormones. J. Physiol. Biochem. 2012, 68, 193–203. [Google Scholar] [CrossRef]
- Mathias, L.S.; Rodrigues, B.M.; Gonçalves, B.M.; Moretto, F.C.F.; Olimpio, R.M.C.; Deprá, I.; De Sibio, M.T.; Tilli, H.P.; Nogueira, C.R.; de Oliveira, M. Triiodothyronine Activated Extranuclear Pathways Upregulate Adiponectin and Leptin in Murine Adipocytes. Mol. Cell. Endocrinol. 2020, 503, 110690. [Google Scholar] [CrossRef]
- de Oliveira, M.; Rodrigues, B.M.; Olimpio, R.M.C.; Mathias, L.S.; De Sibio, M.T.; Moretto, F.C.F.; Graceli, J.B.; Nogueira, C.R. Adiponectin and Serine/Threonine Kinase Akt Modulation by Triiodothyronine and/or LY294002 in 3T3-L1 Adipocytes. Lipids 2019, 54, 133–140. [Google Scholar] [CrossRef]
- Aragão, C.N.; Souza, L.L.; Cabanelas, A.; Oliveira, K.J.; Pazos-Moura, C.C. Effect of Experimental Hypo- and Hyperthyroidism on Serum Adiponectin. Metabolism 2007, 56, 6–11. [Google Scholar] [CrossRef]
- Hsieh, C.-J.; Wang, P.-W. Serum Concentrations of Adiponectin in Patients with Hyperthyroidism before and after Control of Thyroid Function. Endocr. J. 2008, 55, 489–494. [Google Scholar] [CrossRef]
- Kowalska, I.; Borawski, J.; Nikołajuk, A.; Budlewski, T.; Otziomek, E.; Górska, M.; Strączkowski, M. Insulin Sensitivity, Plasma Adiponectin and SICAM-1 Concentrations in Patients with Subclinical Hypothyroidism: Response to Levothyroxine Therapy. Endocrine 2011, 40, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Siemińska, L.; Foltyn, W.; Głogowska-Szeląg, J.; Kajdaniuk, D.; Marek, B.; Nowak, M.; Walczak, K.; Kos-Kudła, B. Relationships between Adiponectin, Sex Hormone Binding Globulin and Insulin Resistance in Hyperthyroid Graves’ Disease Women. Endokrynol. Pol. 2013, 64, 26–29. [Google Scholar] [PubMed]
- Ozdemir, D.; Dagdelen, S.; Usman, A. Serum Adiponectin Levels and Changes in Glucose Metabolism before and after Treatment for Thyroid Dysfunction. Intern. Med. 2015, 54, 1849–1857. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kar, K.; Sinha, S. Variations of Adipokines and Insulin Resistance in Primary Hypothyroidism. J. Clin. Diagn. Res. JCDR 2017, 11, BC07–BC09. [Google Scholar] [CrossRef]
- Gromakova, I.A.; Zilberman, S.T.; Konovalenko, O.A. Age-Related Changes of Protein- and RNA-Synthetic Processes in Experimental Hyper- and Hypothyroidism. Biochem. Biokhimiia 2001, 66, 763–768. [Google Scholar] [CrossRef]
- Lin, K.-H.; Lee, H.-Y.; Shih, C.-H.; Yen, C.-C.; Chen, S.-L.; Yang, R.-C.; Wang, C.-S. Plasma Protein Regulation by Thyroid Hormone. J. Endocrinol. 2003, 179, 367–377. [Google Scholar] [CrossRef]
- Boone, L.R.; Lagor, W.R.; de la Llera Moya, M.; Niesen, M.I.; Rothblat, G.H.; Ness, G.C. Thyroid Hormone Enhances the Ability of Serum to Accept Cellular Cholesterol via the ABCA1 Transporter. Atherosclerosis 2011, 218, 77–82. [Google Scholar] [CrossRef][Green Version]
- Gagnon, A.; Abujrad, H.; Irobi, C.; Lochnan, H.A.; Sorisky, A. Serum Fetuin-A Levels Following Recombinant Human Thyroid-Stimulating Hormone Stimulation. Clin. Investig. Med. Med. Clin. Exp. 2013, 36, E264–E268. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasadarao, A.; Findlay, J.B. Structure-Function Studies on Human Retinol-Binding Protein Using Site-Directed Mutagenesis. Biochem. J. 1994, 300 Pt 2, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Graham, T.E.; Berndt, J.; Kralisch, S.; Kovacs, P.; Wason, C.J.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M.; et al. Serum Retinol-Binding Protein Is More Highly Expressed in Visceral than in Subcutaneous Adipose Tissue and Is a Marker of Intra-Abdominal Fat Mass. Cell Metab. 2007, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Raz, A.; Goodman, D.S. Retinol-Binding Protein: The Transport Protein for Vitamin A in Human Plasma. J. Clin. Invest. 1968, 47, 2025–2044. [Google Scholar] [CrossRef] [PubMed]
- Cappai, M.G.; Lunesu, M.G.A.; Accioni, F.; Liscia, M.; Pusceddu, M.; Burrai, L.; Nieddu, M.; Dimauro, C.; Boatto, G.; Pinna, W. Blood Serum Retinol Levels in Asinara White Donkeys Reflect Albinism-induced Metabolic Adaptation to Photoperiod at Mediterranean Latitudes. Ecol. Evol. 2016, 7, 390–398. [Google Scholar] [CrossRef]
- Graham, T.E.; Yang, Q.; Blüher, M.; Hammarstedt, A.; Ciaraldi, T.P.; Henry, R.R.; Wason, C.J.; Oberbach, A.; Jansson, P.-A.; Smith, U.; et al. Retinol-Binding Protein 4 and Insulin Resistance in Lean, Obese, and Diabetic Subjects. N. Engl. J. Med. 2006, 354, 2552–2563. [Google Scholar] [CrossRef]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum Retinol Binding Protein 4 Contributes to Insulin Resistance in Obesity and Type 2 Diabetes. Nature 2005, 436, 356–362. [Google Scholar] [CrossRef]
- Rocha, M.; Bañuls, C.; Bellod, L.; Rovira-Llopis, S.; Morillas, C.; Solá, E.; Víctor, V.M.; Hernández-Mijares, A. Association of Serum Retinol Binding Protein 4 with Atherogenic Dyslipidemia in Morbid Obese Patients. PLoS ONE 2013, 8, e78670. [Google Scholar] [CrossRef]
- Olsen, T.; Blomhoff, R. Retinol, Retinoic Acid, and Retinol-Binding Protein 4 Are Differentially Associated with Cardiovascular Disease, Type 2 Diabetes, and Obesity: An Overview of Human Studies. Adv. Nutr. Bethesda Md 2020, 11, 644–666. [Google Scholar] [CrossRef]
- Majerczyk, M.; Kocełak, P.; Choręza, P.; Arabzada, H.; Owczarek, A.J.; Bożentowicz-Wikarek, M.; Brzozowska, A.; Szybalska, A.; Puzianowska-Kuźnicka, M.; Grodzicki, T.; et al. Components of Metabolic Syndrome in Relation to Plasma Levels of Retinol Binding Protein 4 (RBP4) in a Cohort of People Aged 65 Years and Older. J. Endocrinol. Invest. 2018, 41, 1211–1219. [Google Scholar] [CrossRef]
- Park, S.E.; Kim, D.H.; Lee, J.H.; Park, J.S.; Kang, E.S.; Ahn, C.W.; Lee, H.C.; Cha, B.S. Retinol-Binding Protein-4 Is Associated with Endothelial Dysfunction in Adults with Newly Diagnosed Type 2 Diabetes Mellitus. Atherosclerosis 2009, 204, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhong, Y.; Chen, H.; Wang, D.; Wang, M.; Ou, J.-S.; Xia, M. Retinol-Binding Protein-Dependent Cholesterol Uptake Regulates Macrophage Foam Cell Formation and Promotes Atherosclerosis. Circulation 2017, 135, 1339–1354. [Google Scholar] [CrossRef]
- Farjo, K.M.; Farjo, R.A.; Halsey, S.; Moiseyev, G.; Ma, J.-X. Retinol-Binding Protein 4 Induces Inflammation in Human Endothelial Cells by an NADPH Oxidase- and Nuclear Factor Kappa B-Dependent and Retinol-Independent Mechanism. Mol. Cell. Biol. 2012, 32, 5103–5115. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Liu, Y.; Zhou, W.; Sun, R.; Xia, M. Retinol Binding Protein 4 Induces Mitochondrial Dysfunction and Vascular Oxidative Damage. Atherosclerosis 2015, 240, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Kiernan, U.A.; Shi, L.; Phillips, D.A.; Kahn, B.B.; Hu, F.B.; Manson, J.E.; Albert, C.M.; Rexrode, K.M. Plasma Retinol-Binding Protein 4 (RBP4) Levels and Risk of Coronary Heart Disease: A Prospective Analysis among Women in the Nurses’ Health Study. Circulation 2013, 127, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Cabré, A.; Lázaro, I.; Girona, J.; Manzanares, J.; Marimón, F.; Plana, N.; Heras, M.; Masana, L. Retinol-Binding Protein 4 as a Plasma Biomarker of Renal Dysfunction and Cardiovascular Disease in Type 2 Diabetes. J. Intern. Med. 2007, 262, 496–503. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, J.-W.; Yun, J.W.; Chung, I.-S.; Cho, H.C.; Song, S.-E.; Im, S.-S.; Song, D.-K. Proteomics Approach to Identify Serum Biomarkers Associated with the Progression of Diabetes in Korean Patients with Abdominal Obesity. PLoS ONE 2019, 14, e0222032. [Google Scholar] [CrossRef]
- Liu, G.; Ding, M.; Chiuve, S.E.; Rimm, E.B.; Franks, P.W.; Meigs, J.B.; Hu, F.B.; Sun, Q. Plasma Levels of Fatty Acid-Binding Protein 4, Retinol-Binding Protein 4, High-Molecular-Weight Adiponectin, and Cardiovascular Mortality Among Men with Type 2 Diabetes: A 22-Year Prospective Study. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2259–2267. [Google Scholar] [CrossRef]
- Patterson, C.C.; Blankenberg, S.; Ben-Shlomo, Y.; Heslop, L.; Bayer, A.; Lowe, G.; Zeller, T.; Gallacher, J.; Young, I.; Yarnell, J. Which Biomarkers Are Predictive Specifically for Cardiovascular or for Non-Cardiovascular Mortality in Men? Evidence from the Caerphilly Prospective Study (CaPS). Int. J. Cardiol. 2015, 201, 113–118. [Google Scholar] [CrossRef]
- Mallat, Z.; Simon, T.; Benessiano, J.; Clément, K.; Taleb, S.; Wareham, N.J.; Luben, R.; Khaw, K.-T.; Tedgui, A.; Boekholdt, S.M. Retinol-Binding Protein 4 and Prediction of Incident Coronary Events in Healthy Men and Women. J. Clin. Endocrinol. Metab. 2009, 94, 255–260. [Google Scholar] [CrossRef]
- Floriani, C.; Gencer, B.; Collet, T.-H.; Rodondi, N. Subclinical Thyroid Dysfunction and Cardiovascular Diseases: 2016 Update. Eur. Heart J. 2018, 39, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Bletsa, E.; Stampouloglou, P.K.; Tsigkou, V.; Valatsou, A.; Stefanaki, K.; Kazakou, P.; Spartalis, M.; Spartalis, E.; Oikonomou, E.; et al. Thyroid Disorders and Cardiovascular Manifestations: An Update. Endocrine 2022, 75, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Coceani, M.; Iervasi, G.; Pingitore, A.; Carpeggiani, C.; L’Abbate, A. Thyroid Hormone and Coronary Artery Disease: From Clinical Correlations to Prognostic Implications. Clin. Cardiol. 2009, 32, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Auer, J.; Berent, R.; Weber, T.; Lassnig, E.; Eber, B. Thyroid Function Is Associated with Presence and Severity of Coronary Atherosclerosis. Clin. Cardiol. 2003, 26, 569–573. [Google Scholar] [CrossRef]
- Sun, H.-X.; Ji, H.-H.; Chen, X.-L.; Wang, L.; Wang, Y.; Shen, X.-Y.; Lu, X.; Gao, W.; Wang, L.-S. Serum Retinol-Binding Protein 4 Is Associated with the Presence and Severity of Coronary Artery Disease in Patients with Subclinical Hypothyroidism. Aging 2019, 11, 4510–4520. [Google Scholar] [CrossRef] [PubMed]
- Azo Najeeb, H.; Ahmad Qasim, B.; Ahmad Mohammed, A. Parental History of Coronary Artery Disease among Adults with Hypothyroidism: Case Controlled Study. Ann. Med. Surg. 2020, 60, 92–101. [Google Scholar] [CrossRef]
- Huang, R.; Bai, X.; Li, X.; Wang, X.; Zhao, L. Retinol-Binding Protein 4 Activates STRA6, Provoking Pancreatic β-Cell Dysfunction in Type 2 Diabetes. Diabetes 2021, 70, 449–463. [Google Scholar] [CrossRef]
- Norseen, J.; Hosooka, T.; Hammarstedt, A.; Yore, M.M.; Kant, S.; Aryal, P.; Kiernan, U.A.; Phillips, D.A.; Maruyama, H.; Kraus, B.J.; et al. Retinol-Binding Protein 4 Inhibits Insulin Signaling in Adipocytes by Inducing Proinflammatory Cytokines in Macrophages through a c-Jun N-Terminal Kinase- and Toll-like Receptor 4-Dependent and Retinol-Independent Mechanism. Mol. Cell. Biol. 2012, 32, 2010–2019. [Google Scholar] [CrossRef]
- Moraes-Vieira, P.M.; Yore, M.M.; Dwyer, P.M.; Syed, I.; Aryal, P.; Kahn, B.B. RBP4 Activates Antigen-Presenting Cells, Leading to Adipose Tissue Inflammation and Systemic Insulin Resistance. Cell Metab. 2014, 19, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Vieira, P.M.; Yore, M.M.; Sontheimer-Phelps, A.; Castoldi, A.; Norseen, J.; Aryal, P.; Simonyté Sjödin, K.; Kahn, B.B. Retinol Binding Protein 4 Primes the NLRP3 Inflammasome by Signaling through Toll-like Receptors 2 and 4. Proc. Natl. Acad. Sci. USA 2020, 117, 31309–31318. [Google Scholar] [CrossRef]
- Berry, D.C.; Jacobs, H.; Marwarha, G.; Gely-Pernot, A.; O’Byrne, S.M.; DeSantis, D.; Klopfenstein, M.; Feret, B.; Dennefeld, C.; Blaner, W.S.; et al. The STRA6 Receptor Is Essential for Retinol-Binding Protein-Induced Insulin Resistance but Not for Maintaining Vitamin A Homeostasis in Tissues Other Than the Eye. J. Biol. Chem. 2013, 288, 24528–24539. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Jin, H.; Majumdar, A.; Noy, N. Signaling by Vitamin A and Retinol-Binding Protein Regulates Gene Expression to Inhibit Insulin Responses. Proc. Natl. Acad. Sci. USA 2011, 108, 4340–4345. [Google Scholar] [CrossRef]
- Huang, R.; Yin, S.; Ye, Y.; Chen, N.; Luo, S.; Xia, M.; Zhao, L. Circulating Retinol-Binding Protein 4 Is Inversely Associated with Pancreatic β-Cell Function Across the Spectrum of Glycemia. Diabetes Care 2020, 43, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Chang, X.; Xia, M.; Bian, H.; Zhang, L.; Lin, H.; Chen, G.; Zeng, M.; Gao, X. Serum Retinol Binding Protein 4 Is Negatively Related to Beta Cell Function in Chinese Women with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study. Lipids Health Dis. 2013, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Broch, M.; Vendrell, J.; Ricart, W.; Richart, C.; Fernández-Real, J.-M. Circulating Retinol-Binding Protein-4, Insulin Sensitivity, Insulin Secretion, and Insulin Disposition Index in Obese and Nonobese Subjects. Diabetes Care 2007, 30, 1802–1806. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Bao, Y.; Wu, H.; Lu, J.; Xiang, K.; Jia, W. Serum Retinol-Binding Protein 4 Is Associated with Insulin Secretion in Chinese People with Normal Glucose Tolerance. J. Diabetes 2009, 1, 125–130. [Google Scholar] [CrossRef]
- Ribel-Madsen, R.; Friedrichsen, M.; Vaag, A.; Poulsen, P. Retinol-Binding Protein 4 in Twins: Regulatory Mechanisms and Impact of Circulating and Tissue Expression Levels on Insulin Secretion and Action. Diabetes 2009, 58, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ram, J.; Snehalatha, C.; Selvam, S.; Nanditha, A.; Shetty, A.S.; Godsland, I.F.; Johnston, D.G.; Ramachandran, A. Retinol Binding Protein-4 Predicts Incident Diabetes in Asian Indian Men with Prediabetes. BioFactors Oxf. Engl. 2015, 41, 160–165. [Google Scholar] [CrossRef]
- Jin, C.; Lin, L.; Han, N.; Zhao, Z.; Liu, Z.; Luo, S.; Xu, X.; Liu, J.; Wang, H. Plasma Retinol-Binding Protein 4 in the First and Second Trimester and Risk of Gestational Diabetes Mellitus in Chinese Women: A Nested Case-Control Study. Nutr. Metab. 2020, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Zemany, L.; Bhanot, S.; Peroni, O.D.; Murray, S.F.; Moraes-Vieira, P.M.; Castoldi, A.; Manchem, P.; Guo, S.; Monia, B.P.; Kahn, B.B. Transthyretin Antisense Oligonucleotides Lower Circulating RBP4 Levels and Improve Insulin Sensitivity in Obese Mice. Diabetes 2015, 64, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Mody, N.; Graham, T.E.; Tsuji, Y.; Yang, Q.; Kahn, B.B. Decreased Clearance of Serum Retinol-Binding Protein and Elevated Levels of Transthyretin in Insulin-Resistant Ob/Ob Mice. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E785–E793. [Google Scholar] [CrossRef][Green Version]
- Pandey, G.K.; Balasubramanyam, J.; Balakumar, M.; Deepa, M.; Anjana, R.M.; Abhijit, S.; Kaviya, A.; Velmurugan, K.; Miranda, P.; Balasubramanyam, M.; et al. Altered Circulating Levels of Retinol Binding Protein 4 and Transthyretin in Relation to Insulin Resistance, Obesity, and Glucose Intolerance in Asian Indians. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2015, 21, 861–869. [Google Scholar] [CrossRef]
- Kwanbunjan, K.; Panprathip, P.; Phosat, C.; Chumpathat, N.; Wechjakwen, N.; Puduang, S.; Auyyuenyong, R.; Henkel, I.; Schweigert, F.J. Association of Retinol Binding Protein 4 and Transthyretin with Triglyceride Levels and Insulin Resistance in Rural Thais with High Type 2 Diabetes Risk. BMC Endocr. Disord. 2018, 18, 26. [Google Scholar] [CrossRef]
- Bartalena, L.; Robbins, J. Thyroid Hormone Transport Proteins. Clin. Lab. Med. 1993, 13, 583–598. [Google Scholar] [CrossRef]
- Kim, N.; Priefer, R. Retinol Binding Protein 4 Antagonists and Protein Synthesis Inhibitors: Potential for Therapeutic Development. Eur. J. Med. Chem. 2021, 226, 113856. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Carbone, A.; D’Angelo, A.; Querci, F.; Fogari, E.; Cicero, A.F.; Maffioli, P. A Randomized, Double-Blind, Placebo-Controlled Trial Evaluating Sitagliptin Action on Insulin Resistance Parameters and β-Cell Function. Expert Opin. Pharmacother. 2012, 13, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Z.; Ning, H.; Sun, H.; Ji, X. Sitagliptin Down-Regulates Retinol-Binding Protein 4 and Reduces Insulin Resistance in Gestational Diabetes Mellitus: A Randomized and Double-Blind Trial. Metab. Brain Dis. 2017, 32, 773–778. [Google Scholar] [CrossRef]
- Steinhoff, K.G.; Krause, K.; Linder, N.; Rullmann, M.; Volke, L.; Gebhardt, C.; Busse, H.; Stumvoll, M.; Blüher, M.; Sabri, O.; et al. Effects of Hyperthyroidism on Adipose Tissue Activity and Distribution in Adults. Thyroid Off. J. Am. Thyroid Assoc. 2021, 31, 519–527. [Google Scholar] [CrossRef]
- Tseng, F.-Y.; Chen, P.-L.; Chen, Y.-T.; Chi, Y.-C.; Shih, S.-R.; Wang, C.-Y.; Chen, C.-L.; Yang, W.-S. Association between Serum Levels of Adipocyte Fatty Acid-Binding Protein and Free Thyroxine. Medicine 2015, 94, e1798. [Google Scholar] [CrossRef]
- Solini, A.; Dardano, A.; Santini, E.; Polini, A.; Monzani, F. Adipocytokines Mark Insulin Sensitivity in Euthyroid Hashimoto’s Patients. Acta Diabetol. 2013, 50, 73–80. [Google Scholar] [CrossRef]
- Schovanek, J.; Krupka, M.; Cibickova, L.; Karhanova, M.; Reddy, S.; Kucerova, V.; Frysak, Z.; Karasek, D. Adipocytokines in Graves’ Orbitopathy and the Effect of High-Dose Corticosteroids. Adipocyte 2021, 10, 456–462. [Google Scholar] [CrossRef]
- Tseng, F.-Y.; Chen, Y.-T.; Chi, Y.-C.; Chen, P.-L.; Yang, W.-S. Serum Levels of Fetuin-A Are Negatively Associated with Log Transformation Levels of Thyroid-Stimulating Hormone in Patients with Hyperthyroidism or Euthyroidism: An Observational Study at a Medical Center in Taiwan. Medicine 2018, 97, e13254. [Google Scholar] [CrossRef] [PubMed]
- Pamuk, B.O.; Yilmaz, H.; Topcuoglu, T.; Bilgir, O.; Çalan, O.; Pamuk, G.; Ertugrul, D.T. Fetuin-A Levels in Hyperthyroidism. Clin. Sao Paulo Braz. 2013, 68, 379–383. [Google Scholar] [CrossRef]
- Bakiner, O.; Bozkirli, E.; Ertugrul, D.; Sezgin, N.; Ertorer, E. Plasma Fetuin-A Levels Are Reduced in Patients with Hypothyroidism. Eur. J. Endocrinol. 2014, 170, 411–418. [Google Scholar] [CrossRef]
- Bilgir, O.; Bilgir, F.; Calan, M.; Calan, O.G.; Yuksel, A. Comparison of Pre- and Post-Levothyroxine High-Sensitivity c-Reactive Protein and Fetuin-a Levels in Subclinical Hypothyroidism. Clin. Sao Paulo Braz. 2015, 70, 97–101. [Google Scholar] [CrossRef]
- Muratli, S.; Uzunlulu, M.; Gonenli, G.; Oguz, A.; Isbilen, B. Fetuin A as a New Marker of Inflammation in Hashimoto Thyroiditis. Minerva Endocrinol. 2015, 40, 9–14. [Google Scholar] [PubMed]
- Deng, X.R.; Ding, L.; Wang, T.G.; Xu, M.; Lu, J.L.; Li, M.; Zhao, Z.Y.; Chen, Y.H.; Bi, Y.F.; Xu, Y.P.; et al. Serum Fetuin-A Levels and Thyroid Function InMiddle-Aged and Elderly Chinese. Biomed. Environ. Sci. BES 2017, 30, 455–459. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, Y.J.; Park, Y.J.; Kim, K.W.; Lee, E.J.; Lim, S.; Park, D.J.; Kim, S.E.; Park, K.S.; Jang, H.C.; et al. Retinol Binding Protein-4 Elevation Is Associated with Serum Thyroid-Stimulating Hormone Level Independently of Obesity in Elderly Subjects with Normal Glucose Tolerance. J. Clin. Endocrinol. Metab. 2008, 93, 2313–2318. [Google Scholar] [CrossRef][Green Version]
- Kokkinos, S.; Papazoglou, D.; Zisimopoulos, A.; Papanas, N.; Tiaka, E.; Antonoglou, C.; Maltezos, E. Retinol Binding Protein-4 and Adiponectin Levels in Thyroid Overt and Subclinical Dysfunction. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2016, 124, 87–92. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Mok, J.-O.; Kang, S.-K.; Jang, S.-Y.; Jung, C.-H.; Kim, C.-H. The Relationship between Serum Adipocytokines and Graves’ Ophthalmopathy: A Hospital-Based Study. Endocr. J. 2016, 63, 425–430. [Google Scholar] [CrossRef]
| Adipokine | Thyroid Disorder | Adipokine Concentration | p Value 1 | Correlated HPT-axis Parameters | Correlation Coefficient | p Value | Reference |
|---|---|---|---|---|---|---|---|
| FABP4 | Hypothyroidism | ↑ | 0.0140 | TSH | 0.201 b | 0.039 | [29] |
| Hyperthyroidism | ↑ | 0.016 * | fT4 fT3 | 0.27 d 0.28 d | 0.037 0.031 | [188] | |
| ↑ | 0.038 | fT4 | 2.51 c | 0.018 | [189] | ||
| Hashimoto thyroiditis | ↑ | <0.001 | fT4 | 0.131 b | <0.001 | [190] | |
| Graves orbitopathy | no change | NA | [191] | ||||
| Fetuin-A | Hypothyroidism | ↓ | 0.0001 | TSH | −0.61 a | 0.001 | [194] |
| ↑ | 0.019 | NA | [195] | ||||
| ↓ | 0.001 | NI | [196] | ||||
| Hyperthyroidism | ↑ | <0.0001 * | TSH fT4 | −0.553 b 0.473 b | 0.0001 0.002 | [193] | |
| ↑ | 0.018 * | NI | [188] | ||||
| ↑ | 0.010 | logTSH | 53.79c | 0.010 | [192] | ||
| RBP4 | Hypothyroidism | ↑ | <0.0001 | TSH | 0.241 a | 0.001 | [198] |
| ↑ | <0.001 | TSH | 0.5 a | <0.001 | [199] | ||
| ↑ | 0.03 | TSH | 0.389 b | 0.03 | [166] | ||
| ↑ | <0.001 | TSH T3 | 0.257 b −0.247 b | <0.001 <0.001 | [165] | ||
| Hyperthyroidism | no change | [199] | |||||
| ↓ | 0.048 * | NI | [188] | ||||
| Hashimoto thyroiditis | ↑ | <0.001 | fT3 fT4 | 0.077 b 0.093 b | 0.007 0.003 | [190] | |
| Graves’ orbitopathy | no difference GO vs. no GO | NI | [200] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadej, D.; Szczepanek-Parulska, E.; Ruchała, M. Interplay between Fatty Acid Binding Protein 4, Fetuin-A, Retinol Binding Protein 4 and Thyroid Function in Metabolic Dysregulation. Metabolites 2022, 12, 300. https://doi.org/10.3390/metabo12040300
Dadej D, Szczepanek-Parulska E, Ruchała M. Interplay between Fatty Acid Binding Protein 4, Fetuin-A, Retinol Binding Protein 4 and Thyroid Function in Metabolic Dysregulation. Metabolites. 2022; 12(4):300. https://doi.org/10.3390/metabo12040300
Chicago/Turabian StyleDadej, Daniela, Ewelina Szczepanek-Parulska, and Marek Ruchała. 2022. "Interplay between Fatty Acid Binding Protein 4, Fetuin-A, Retinol Binding Protein 4 and Thyroid Function in Metabolic Dysregulation" Metabolites 12, no. 4: 300. https://doi.org/10.3390/metabo12040300
APA StyleDadej, D., Szczepanek-Parulska, E., & Ruchała, M. (2022). Interplay between Fatty Acid Binding Protein 4, Fetuin-A, Retinol Binding Protein 4 and Thyroid Function in Metabolic Dysregulation. Metabolites, 12(4), 300. https://doi.org/10.3390/metabo12040300






