Serum Metabolites Associated with Blood Pressure in Chronic Kidney Disease Patients
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Cohorts
4.2. Blood Pressure
4.3. High-Throughput Metabolomics and Assessment
4.4. Feature Reduction
| Algorithm 1 Correlation Clustering Algorithm |
| Require: V, E+, E− H ← ∅ while V ≠ ∅ do C ← ∅ V’ ← ∅ Pick a random pivot i ∈ V C ← {i} for j ∈ V and j ≠ i do if j, i ∈ E+ then Add j to C end if if j, i ∈ E− then Add j to V’ end if end for V ← V’ Add C to H end while return H |
| Algorithm 2 Pearson Correlation Threshold Check |
| Require: i, j, t P ← pearson(i, j) Q ← |P| return Q > t |
4.5. Statistical Analysis
4.6. Deep Learning Analysis
4.7. Metabolites Identification
4.8. Study Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourne, R.R.A.; GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar]
- Hamrahian, S.M.; Falkner, B. Hypertension in chronic kidney disease. Adv. Exp. Med. Biol. 2017, 956, 307–325. [Google Scholar] [PubMed]
- McQueen, R.B.; Farahbakhshian, S.; Bell, K.F.; Nair, K.V.; Saseen, J.J. Economic burden of comorbid chronic kidney disease and diabetes. J. Med. Econ. 2017, 20, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Anderson, A.; Charleston, J.; Chen, Z.; Ford, V.; Makos, G.; O’Connor, A.; Perumal, K.; Rahman, M.; Steigerwalt, S.; et al. Hypertension awareness, treatment, and control in adults with ckd: Results from the chronic renal insufficiency cohort (cric) study. Am. J. Kidney Dis. 2010, 55, 441–451. [Google Scholar] [CrossRef]
- Hamrahian, S.M. Management of hypertension in patients with chronic kidney disease. Curr. Hypertens. Rep. 2017, 19, 43. [Google Scholar] [CrossRef]
- Townsend, R.R.; Taler, S.J. Management of hypertension in chronic kidney disease. Nat. Rev. Nephrol. 2015, 11, 555–563. [Google Scholar] [CrossRef]
- Hocher, B.; Adamski, J. Metabolomics for clinical use and research in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 269–284. [Google Scholar] [CrossRef]
- Chen, D.Q.; Cao, G.; Chen, H.; Argyopoulos, C.P.; Yu, H.; Su, W.; Chen, L.; Samuels, D.C.; Zhuang, S.; Bayliss, G.P.; et al. Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat. Commun. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, H.; Chen, D.; Zhao, Y.Y. Machine learning distilled metabolite biomarkers for early stage renal injury. Metabolomics 2019, 16, 4. [Google Scholar] [CrossRef]
- Poll, B.G.; Cheema, M.U.; Pluznick, J.L. Gut microbial metabolites and blood pressure regulation: Focus on scfas and tmao. Physiology 2020, 35, 275–284. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, H.; Pan, X.; Xue, H.; Wan, Y.; Wang, T.; Tian, Z.; Hou, E.; Lanza, I.R.; Liu, P.; et al. Urinary metabolites associated with blood pressure on a low- or high-sodium diet. Theranostics 2018, 8, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, L.; Dai, X.; Chen, H.; Zhang, C.; Wang, P.; Wu, Q.; Zeng, L.; Yan, H. Plasma metabolites mediate the association of coarse grain intake with blood pressure in hypertension-free adults. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Ailon, N.; Charikar, M.; Newman, A. Aggregating inconsistent information: Ranking and clustering. J. ACM 2008, 55, 1–27. [Google Scholar] [CrossRef]
- Klag, M.J.; Whelton, P.K.; Randall, B.L.; Neaton, J.D.; Brancati, F.L.; Ford, C.E.; Shulman, N.B.; Stamler, J. Blood pressure and end-stage renal disease in men. N. Engl. J. Med. 1996, 334, 13–18. [Google Scholar] [CrossRef]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of hypertension in chronic kidney disease (vol 79, 365, 2019). Drugs 2020, 80, 1381. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mandal, J.; Yang, T.; Cheng, X.; Yeo, J.Y.; McCarthy, C.G.; Wenceslau, C.F.; Koch, L.G.; Hill, J.W.; Vijay-Kumar, M.; et al. Metabolites and hypertension: Insights into hypertension as a metabolic disorder 2019 harriet dustan award. Hypertension 2020, 75, 1386–1396. [Google Scholar] [CrossRef]
- Holmes, E.; Loo, R.L.; Stamler, J.; Bictash, M.; Yap, I.K.S.; Chan, Q.; Ebbels, T.; De Iorio, M.; Brown, I.J.; Veselkov, K.A.; et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008, 453, 396–400. [Google Scholar] [CrossRef]
- Hao, Y.C.; Wang, Y.; Xi, L.; Li, G.Q.; Zhao, F.; Qi, Y.; Liu, J.; Zhao, D. A nested case-control study of association between metabolome and hypertension risk. Biomed Res. Int. 2016, 2016, 7646979. [Google Scholar] [CrossRef]
- Haijes, H.A.; de Sain-van der Velden, M.G.M.; Prinsen, H.; Willems, A.P.; van der Ham, M.; Gerrits, J.; Couse, M.H.; Friedman, J.M.; van Karnebeek, C.D.M.; Selby, K.A.; et al. Aspartylglycosamine is a biomarker for ngly1-cddg, a congenital disorder of deglycosylation. Mol. Genet. Metab. 2019, 127, 368–372. [Google Scholar] [CrossRef]
- Asahina, M.; Fujinawa, R.; Fujihira, H.; Masahara-Negishi, Y.; Andou, T.; Tozawa, R.; Suzuki, T. Jf1/b6f1 ngly1(-/-) mouse as an isogenic animal model of ngly1 deficiency. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2021, 97, 89–102. [Google Scholar] [CrossRef]
- Antunes, N.; Martinusso, C.A.; Takiya, C.M.; da Silva, A.J.; de Ornellas, J.F.; Elias, P.R.; Leite, M., Jr.; Cardoso, L.R. Fructose-1,6 diphosphate as a protective agent for experimental ischemic acute renal failure. Kidney Int. 2006, 69, 68–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Wu, Y.; Yin, F.; Feng, Q.; Dong, X.; Zhang, R.; Yin, Z.; Luo, L. Fructose 1, 6-diphosphate prevents alcohol-induced liver injury through inhibiting oxidative stress and promoting alcohol metabolism in mice. Eur. J. Pharmacol. 2017, 815, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.K.; Warren, E.T.; Cohly, H.H.; Sauls, D.J.; Skelton, T.N. Influence of fructose-1,6-diphosphate on endotoxin-induced lung injuries in sheep. J. Surg. Res. 2007, 138, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.; Zhang, F.; Niu, W.; Ehringer, W.; Chiang, B.; Shi, X.; Gray, L.A., Jr. Fructose-1,6-diphosphate and a glucose-free solution enhances functional recovery in hypothermic heart preservation. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2000, 19, 277–285. [Google Scholar] [CrossRef]
- Horjus, D.L.; Oudman, I.; van Montfrans, G.A.; Brewster, L.M. Creatine and creatine analogues in hypertension and cardiovascular disease. Cochrane Database Syst. Rev. 2011, 2011, Cd005184. [Google Scholar] [CrossRef]
- ten Hove, M.; Lygate, C.A.; Fischer, A.; Schneider, J.E.; Sang, A.E.; Hulbert, K.; Sebag-Montefiore, L.; Watkins, H.; Clarke, K.; Isbrandt, D.; et al. Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-n-methyltransferase-knockout mice. Circulation 2005, 111, 2477–2485. [Google Scholar] [CrossRef]
- Xu, L.; Wang, C.Y.; Lv, L.; Liu, K.X.; Sun, H.J.; Han, G.Z. Pharmacokinetics of phosphocreatine and its active metabolite creatine in the mouse plasma and myocardium. Pharmacol. Rep. PR 2014, 66, 908–914. [Google Scholar] [CrossRef]
- Shopit, A.; Niu, M.; Wang, H.; Tang, Z.; Li, X.; Tesfaldet, T.; Ai, J.; Ahmad, N.; Al-Azab, M.; Tang, Z. Protection of diabetes-induced kidney injury by phosphocreatine via the regulation of erk/nrf2/ho-1 signaling pathway. Life Sci. 2020, 242, 117248. [Google Scholar] [CrossRef]
- Greco, A.V.; Mingrone, G.; Capristo, E.; Benedetti, G.; De Gaetano, A.; Gasbarrini, G. The metabolic effect of dodecanedioic acid infusion in non-insulin-dependent diabetic patients. Nutrition 1998, 14, 351–357. [Google Scholar] [CrossRef]
- Mingrone, G.; Castagneto-Gissey, L.; Mace, K. Use of dicarboxylic acids in type 2 diabetes. Br. J. Clin. Pharmacol. 2013, 75, 671–676. [Google Scholar] [CrossRef]
- Diaz, J.; Ni, W.; Thompson, J.; King, A.; Fink, G.D.; Watts, S.W. 5-hydroxytryptamine lowers blood pressure in normotensive and hypertensive rats. J. Pharmacol. Exp. Ther. 2008, 325, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Seitz, B.M.; Demireva, E.Y.; Xie, H.; Fink, G.D.; Krieger-Burke, T.; Burke, W.M.; Watts, S.W. 5-ht does not lower blood pressure in the 5-ht(7) knockout rat. Physiol. Genom. 2019, 51, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Don-Doncow, N.; Zhang, Y.; Matuskova, H.; Meissner, A. The emerging alliance of sphingosine-1-phosphate signalling and immune cells: From basic mechanisms to implications in hypertension. Br. J. Pharmacol. 2019, 176, 1989–2001. [Google Scholar] [CrossRef]
- Del Gaudio, I.; Rubinelli, L.; Sasset, L.; Wadsack, C.; Hla, T.; Di Lorenzo, A. Endothelial spns2 and apom regulation of vascular tone and hypertension via sphingosine-1-phosphate. J. Am. Heart Assoc. 2021, 10, e021261. [Google Scholar] [CrossRef] [PubMed]
- Cantalupo, A.; Gargiulo, A.; Dautaj, E.; Liu, C.; Zhang, Y.; Hla, T.; Di Lorenzo, A. S1pr1 (sphingosine-1-phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension 2017, 70, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Intapad, S. Sphingosine-1-phosphate signaling in blood pressure regulation. Am. J. Physiology. Ren. Physiol. 2019, 317, F638–F640. [Google Scholar] [CrossRef]
- Ding, D.; Xu, S.; Zhang, H.; Zhao, W.; Zhang, X.; Jiang, Y.; Wang, P.; Dai, Z.; Zhang, J. 3-methyladenine and dexmedetomidine reverse lipopolysaccharide-induced acute lung injury through the inhibition of inflammation and autophagy. Exp. Ther. Med. 2018, 15, 3516–3522. [Google Scholar] [CrossRef]
- Li, M.N.; Qian, S.H.; Yao, Z.Y.; Ming, S.P.; Shi, X.J.; Kang, P.F.; Zhang, N.R.; Wang, X.J.; Gao, D.S.; Gao, Q.; et al. Correlation of serum n-acetylneuraminic acid with the risk and prognosis of acute coronary syndrome: A prospective cohort study. BMC Cardiovasc. Disord. 2020, 20, 404. [Google Scholar] [CrossRef]
- Mohammad, J.; Scanni, R.; Bestmann, L.; Hulter, H.N.; Krapf, R. A controlled increase in dietary phosphate elevates bp in healthy human subjects. J. Am. Soc. Nephrol. 2018, 29, 2089–2098. [Google Scholar] [CrossRef]
- Elliott, P.; Kesteloot, H.; Appel, L.J.; Dyer, A.R.; Ueshima, H.; Chan, Q.; Brown, I.J.; Zhao, L.; Stamler, J.; Group, I.C.R. Dietary phosphorus and blood pressure: International study of macro- and micro-nutrients and blood pressure. Hypertension 2008, 51, 669–675. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Foundation, N.K. K/doqi clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 39, S1–S266. [Google Scholar]
- Altmann, A.; Tolosi, L.; Sander, O.; Lengauer, T. Permutation importance: A corrected feature importance measure. Bioinformatics 2010, 26, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Chen, W.C.; Lai, P.T.; Lin, C.H.; Lee, C.C. Comparing deep neural network and other machine learning algorithms for stroke prediction in a large-scale population-based electronic medical claims database. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; pp. 3110–3113. [Google Scholar]
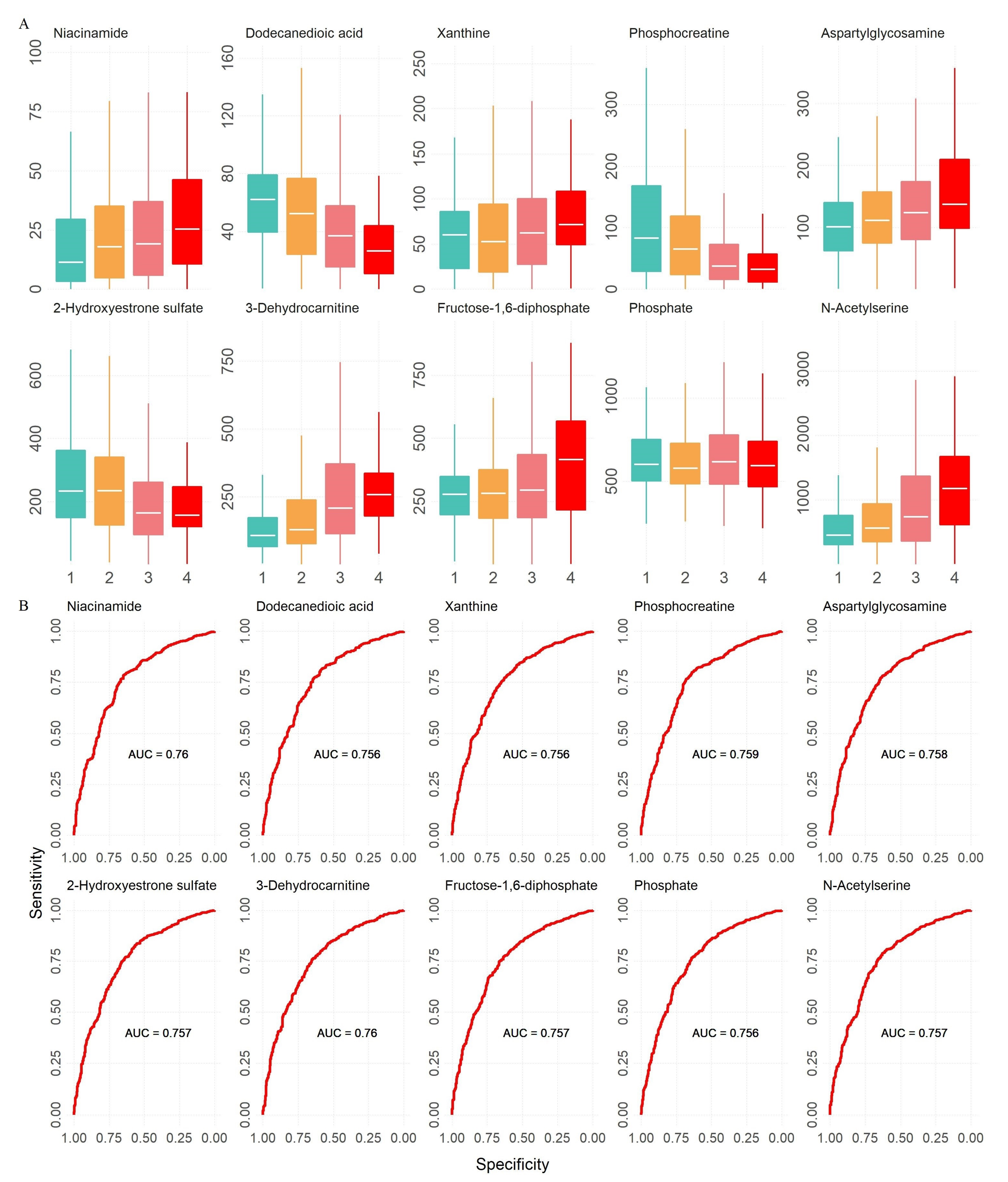
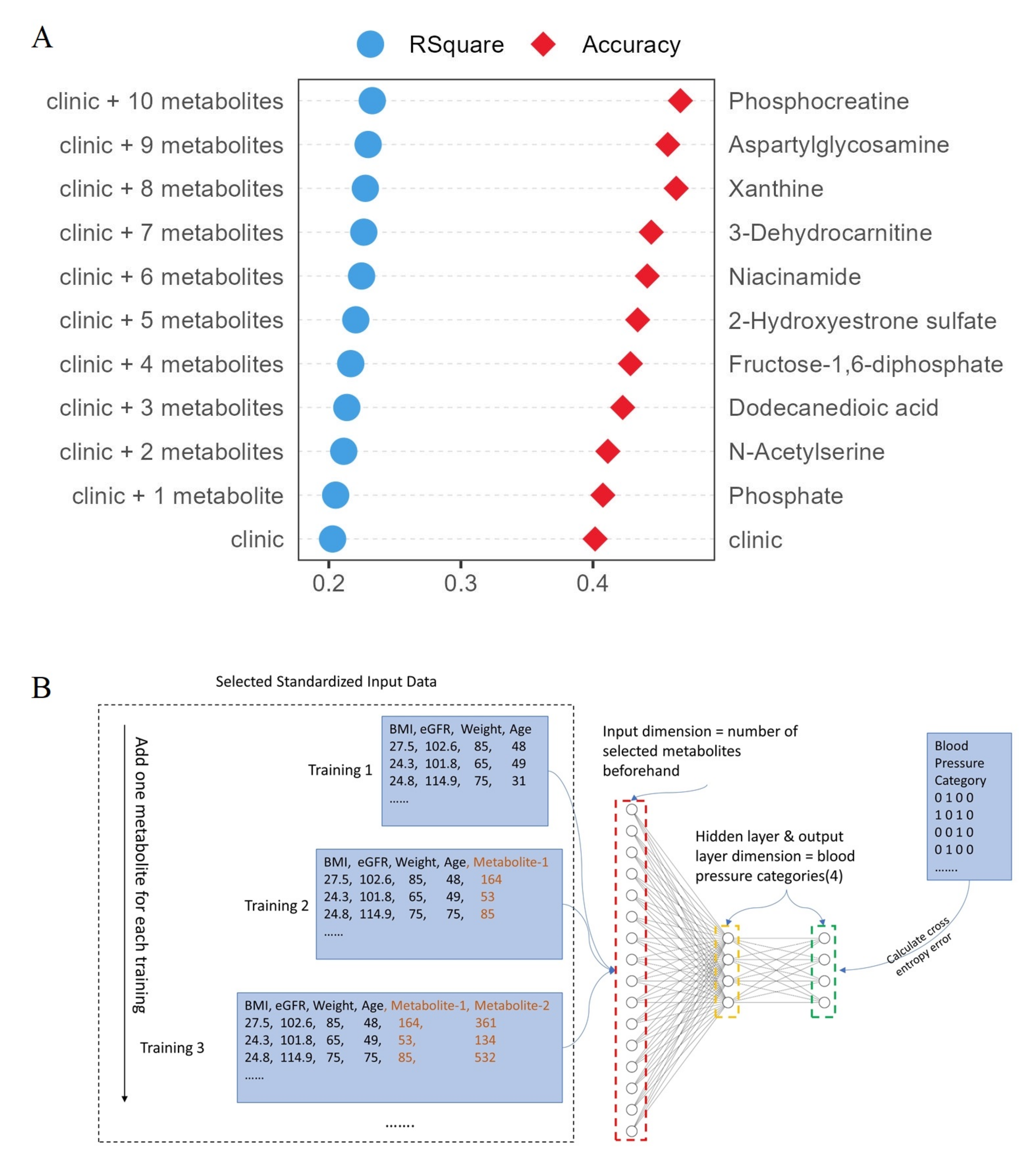
| Dataset | Clinical Characteristics | Normal | CKD1 | CKD2 | CKD3 | CKD4 | CKD5 |
|---|---|---|---|---|---|---|---|
| Discovery | Sample Size | 144 | 125 | 133 | 131 | 150 | 141 |
| Men (%) | 62.50% | 45.60% | 57.10% | 58.80% | 54.70% | 48.90% | |
| Age (years) | 57.28 ± 17.66 | 54.65 ± 8.54 | 56.41 ± 10.2 | 55.36 ± 15.44 | 59.51 ± 14.27 | 59.86 ± 16.41 | |
| eGFR | 107.03 ± 15.73 | 109.75 ± 16.48 | 78.95 ± 12.32 | 44.53 ± 11.75 | 21.7 ± 4.95 | 8.18 ± 3.07 | |
| Weight | 70.36 ± 11.9 | 69.18 ± 12.83 | 73.13 ± 11.13 | 74.08 ± 12.08 | 73.07 ± 13.29 | 72.41 ± 13.1 | |
| BMI | 24.34 ± 3.36 | 24.39 ± 3.49 | 23.78 ± 3.08 | 24.09 ± 3.39 | 24.68 ± 3.11 | 25.58 ± 3.28 | |
| Systolic pressure | 124.93 ± 17.65 | 127.19 ± 19.86 | 127.99 ± 15.44 | 146.52 ± 25.25 | 142.75 ± 20.42 | 146.48 ± 20.77 | |
| Diastolic pressure | 77.6 ± 11.67 | 79.18 ± 12.64 | 80.63 ± 11.87 | 89 ± 16.9 | 77.97 ± 13.78 | 81.56 ± 15.62 | |
| Validation | Sample Size | 96 | 97 | 76 | 94 | 93 | 96 |
| Men (%) | 61.50% | 52.60% | 56.60% | 61.70% | 54.80% | 52.10% | |
| Age (years) | 57.74 ± 15.69 | 55.94 ± 7.79 | 52.78 ± 9.18 | 57.62 ± 14.64 | 59.05 ± 14.45 | 58.56 ± 14.5 | |
| eGFR | 106.06 ± 11.64 | 106.39 ± 11.53 | 78.54 ± 10.72 | 44.48 ± 13.26 | 21.55 ± 4.54 | 8.77 ± 3 | |
| Weight | 69.79 ± 11.41 | 71.21 ± 13.14 | 73.53 ± 11.95 | 71.9 ± 12.76 | 72.48 ± 11.56 | 72.54 ± 12.61 | |
| BMI | 24.18 ± 3.19 | 24.68 ± 3.25 | 23.6 ± 3.11 | 24.8 ± 3.5 | 25.27 ± 3.63 | 25.76 ± 3.47 | |
| Systolic pressure | 125.12 ± 19.72 | 127.1 ± 17.99 | 127.76 ± 16.73 | 145.18 ± 27.47 | 140.94 ± 21.44 | 149.59 ± 20.78 | |
| Diastolic pressure | 78.26 ± 13.11 | 78.33 ± 12.16 | 80.51 ± 12.83 | 85.4 ± 15.49 | 76.44 ± 11.37 | 83.64 ± 14.71 |
| Cohort | Clinical Characteristics | Estimate 1 | Stderr 2 | p3 |
|---|---|---|---|---|
| Discovery (n = 824) | (Intercept) | 1.5874 | 0.3458 | 5.11 × 10−6 |
| CKD | 0.0819 | 0.0443 | 6.49 × 10−2 | |
| eGFR | −0.0042 | 0.0019 | 2.56 × 10−2 | |
| Sex | 0.0667 | 0.0530 | 2.09 × 10−1 | |
| Age | 0.0037 | 0.0019 | 4.88 × 10−2 | |
| Weight | 0.0095 | 0.0022 | 1.32 × 10−5 | |
| BMI | 0.0337 | 0.0082 | 4.42 × 10−5 | |
| Validation (n = 552) | (Intercept) | 1.8901 | 0.4203 | 8.44 × 10−6 |
| CKD | 0.0284 | 0.0601 | 6.37 × 10−1 | |
| eGFR | −0.0062 | 0.0026 | 1.62 × 10−2 | |
| Sex | 0.0624 | 0.0657 | 3.43 × 10−1 | |
| Age | 0.0061 | 0.0025 | 1.44 × 10−2 | |
| Weight | 0.0082 | 0.0028 | 3.17 × 10−3 | |
| BMI | 0.0300 | 0.0100 | 2.81 × 10−3 | |
| Combined (n = 1376) | (Intercept) | 1.7042 | 0.2657 | 1.94 × 10−10 |
| CKD | 0.0631 | 0.0355 | 7.55 × 10−2 | |
| eGFR | −0.0049 | 0.0015 | 1.27 × 10−3 | |
| Sex | 0.0660 | 0.0411 | 1.09 × 10−1 | |
| Age | 0.0045 | 0.0015 | 2.44 × 10−3 | |
| Weight | 0.0090 | 0.0017 | 1.36 × 10−7 | |
| BMI | 0.0320 | 0.0063 | 4.59 × 10−7 |
| Discovery | Validation | Combined | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Identification Confidence a | MS | Retention Time | Estimate b | pc | Estimate b | pc | Estimate b | pc | Adjusted p d |
| Aspartylglycosamine | EC, MS, MSE, database | 412.0567 | 4.53 | 0.0015 | 1.44 × 10−2 | 0.002 | 5.63 × 10−3 | 0.0007 | 4.58 × 10−5 | 1.42 × 10−3 |
| Fructose-1,6-diphosphate | EC, MS, MSE, database | 447.9958 | 2.89 | 0.0008 | 3.61 × 10−2 | 0.001 | 4.48 × 10−2 | 0.0004 | 1.19 × 10−4 | 1.84 × 10−3 |
| L-Glutamic acid | Reference standard | 226.074 | 0.98 | 0.0004 | 1.36 × 10−3 | 0.0003 | 4.27 × 10−2 | 0.0001 | 3.27 × 10−4 | 3.38 × 10−3 |
| Niacinamide | EC, MS, MSE, database | 283.0602 | 3.44 | 0.0028 | 1.32 × 10−2 | 0.0077 | 1.09 × 10−4 | 0.0017 | 5.30 × 10−4 | 4.11 × 10−3 |
| 3-Dehydrocarnitine | EC, MS, MSE, database | 236.0078 | 3.57 | 0.0011 | 2.17 × 10−2 | 0.0014 | 1.84 × 10−2 | 0.0004 | 1.27 × 10−3 | 7.87 × 10−3 |
| Phosphocreatine | Reference standard | 294.0924 | 5.14 | −0.0012 | 4.44 × 10−2 | −0.0015 | 1.24 × 10−2 | −0.0006 | 6.39 × 10−3 | 3.30 × 10−2 |
| Dodecanedioic acid | Reference standard | 269.1144 | 3.47 | −0.0035 | 1.79 × 10−2 | −0.0046 | 2.64 × 10−2 | −0.0011 | 1.38 × 10−2 | 6.11 × 10−2 |
| 2-Hydroxyestrone sulfate | EC, MS, MSE, database | 408.1494 | 3.01 | −0.0006 | 4.72 × 10−2 | −0.001 | 2.20 × 10−2 | −0.0003 | 2.07 × 10−2 | 8.02 × 10−2 |
| Xanthine | Reference standard | 368.0554 | 3.86 | 0.0013 | 2.51 × 10−2 | 0.0016 | 3.19 × 10−2 | 0.0003 | 4.01 × 10−2 | 1.38 × 10−1 |
| Phosphate | EC, MS, MSE, database | 181.0374 | 0.87 | −0.0003 | 1.03 × 10−2 | −0.0003 | 4.94 × 10−2 | −0.0001 | 4.56 × 10−2 | 1.41 × 10−1 |
| NADP+ | EC, MS, MSE, database | 391.0605 | 4.09 | 0.0004 | 5.68 × 10−3 | 0.0003 | 2.39 × 10−2 | 0 | 8.34 × 10−2 | 2.24 × 10−1 |
| Coenzyme A | Reference standard | 393.0802 | 4.33 | 0.0002 | 2.99 × 10−2 | 0.0002 | 7.57 × 10−3 | 0 | 8.69 × 10−2 | 2.24 × 10−1 |
| Nicotine glucuronide | EC, MS, MSE, database | 415.0683 | 0.87 | 0.0005 | 1.40 × 10−2 | −0.0005 | 2.10 × 10−2 | −0.0001 | 1.12 × 10−1 | 2.55 × 10−1 |
| Dihydroasparagusic acid | EC, MS, MSE, database | 305.0033 | 1.34 | 0.0029 | 2.08 × 10−4 | 0.0036 | 1.67 × 10−4 | 0.0003 | 1.15 × 10−1 | 2.55 × 10−1 |
| N2-Methylguanine | EC, MS, MSE, database | 210.0359 | 5.48 | 0.0061 | 1.78 × 10−4 | 0.0048 | 1.29 × 10−2 | 0.0003 | 1.25 × 10−1 | 2.58 × 10−1 |
| Butyl acetate | Reference standard | 81.0702 | 4.96 | 0.0131 | 3.78 × 10−2 | −0.0088 | 4.61 × 10−2 | −0.0026 | 1.51 × 10−1 | 2.93 × 10−1 |
| Kynuramine | Reference standard | 392.2132 | 3.8 | −0.0004 | 3.72 × 10−2 | −0.0005 | 3.93 × 10−2 | −0.0001 | 1.75 × 10−1 | 3.19 × 10 −1 |
| N-Myristoyl Alanine | EC, MS, MSE, database | 306.2628 | 4.67 | 0.007 | 4.45 × 10−2 | 0.0091 | 2.23 × 10−2 | −0.0002 | 2.09 × 10−1 | 3.60 × 10−1 |
| N-Acetylputrescine | Reference standard | 207.0297 | 0.85 | 0.0047 | 2.64 × 10−3 | 0.0052 | 1.26 × 10−2 | 0.0003 | 2.65 × 10−1 | 4.32 × 10−1 |
| Undecanedioic acid | EC, MS, MSE, database | 199.136 | 4.75 | 0.0028 | 8.12 × 10−3 | 0.0034 | 2.63 × 10−2 | 0.0004 | 3.28 × 10−1 | 5.00 × 10−1 |
| dUDP | EC, MS, MSE, database | 206.0009 | 4.65 | 0.0029 | 3.64 × 10−2 | −0.0045 | 1.98 × 10−2 | 0.0005 | 3.39 × 10−1 | 5.00 × 10−1 |
| 5-Hydroxytryptamine | Reference standard | 177.1022 | 3.18 | 0 | 1.63 × 10−2 | 0 | 1.76 × 10−2 | 0 | 4.39 × 10−1 | 6.00 × 10−1 |
| Methionine sulfoxide | EC, MS, MSE, database | 244.065 | 2.42 | 0.0103 | 2.80 × 10−3 | 0.0131 | 1.04 × 10−3 | 0 | 4.45 × 10−1 | 6.00 × 10−1 |
| Selenocysteine | EC, MS, MSE, database | 355.9162 | 2.93 | −0.0018 | 2.56 × 10−2 | −0.0028 | 1.38 × 10−2 | −0.0002 | 5.33 × 10−1 | 6.88 × 10−1 |
| N-Acetylneuraminic acid | Reference standard | 332.0959 | 0.98 | −0.0285 | 4.95 × 10−2 | −0.0483 | 4.48 × 10−2 | 0 | 5.90 × 10−1 | 7.00 × 10−1 |
| N-Acetylgalactosamine 6-sulfate | EC, MS, MSE, database | 365.0669 | 2.73 | 0.0085 | 1.47 × 10−2 | 0.0088 | 3.97 × 10−2 | 0.0001 | 6.04 × 10−1 | 7.00 × 10−1 |
| 3-Methyladenine | Reference standard | 172.0588 | 2.89 | 0.0016 | 9.56 × 10−3 | 0.0018 | 2.67 × 10−2 | 0 | 6.10 × 10−1 | 7.00 × 10−1 |
| N-Acetylaspartylglutamic acid | EC, MS, MSE, database | 322.1209 | 4.21 | −0.0004 | 1.90x10−2 | 0.0005 | 4.01 × 10−2 | 0 | 7.17 × 10−1 | 7.71 × 10−1 |
| Sphingosine-1-phosphate | EC, MS, MSE, database | 356.1989 | 5.44 | −0.0015 | 3.84 × 10−2 | 0.0019 | 2.80 × 10−2 | 0.0001 | 7.21 × 10−1 | 7.71 × 10−1 |
| Oxodecanoylcarnitine | EC, MS, MSE, database | 271.1503 | 4.52 | 0.0004 | 3.69 × 10−2 | −0.0006 | 4.41 × 10−2 | 0 | 8.79 × 10−1 | 8.88 × 10 −1 |
| 2-Methylguanosine | EC, MS, MSE, database | 342.0801 | 3.26 | −0.0004 | 2.41 × 10−2 | 0.0002 | 4.79 × 10−2 | 0 | 8.88 × 10−1 | 8.88 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, F.; Chen, D.-Q.; Tang, J.; Zhao, Y.-Y.; Guo, Y. Serum Metabolites Associated with Blood Pressure in Chronic Kidney Disease Patients. Metabolites 2022, 12, 281. https://doi.org/10.3390/metabo12040281
Yan F, Chen D-Q, Tang J, Zhao Y-Y, Guo Y. Serum Metabolites Associated with Blood Pressure in Chronic Kidney Disease Patients. Metabolites. 2022; 12(4):281. https://doi.org/10.3390/metabo12040281
Chicago/Turabian StyleYan, Fengyao, Dan-Qian Chen, Jijun Tang, Ying-Yong Zhao, and Yan Guo. 2022. "Serum Metabolites Associated with Blood Pressure in Chronic Kidney Disease Patients" Metabolites 12, no. 4: 281. https://doi.org/10.3390/metabo12040281
APA StyleYan, F., Chen, D.-Q., Tang, J., Zhao, Y.-Y., & Guo, Y. (2022). Serum Metabolites Associated with Blood Pressure in Chronic Kidney Disease Patients. Metabolites, 12(4), 281. https://doi.org/10.3390/metabo12040281










