Bone Fragility in Chronic Kidney Disease Stage 3 to 5: The Use of Vitamin D Supplementation
Abstract
1. Introduction
2. Epidemiology of Bone Fractures in CKD
3. Vitamin D and Its Metabolism in CKD
4. Circulating Vitamin D Levels and Fractures in CKD
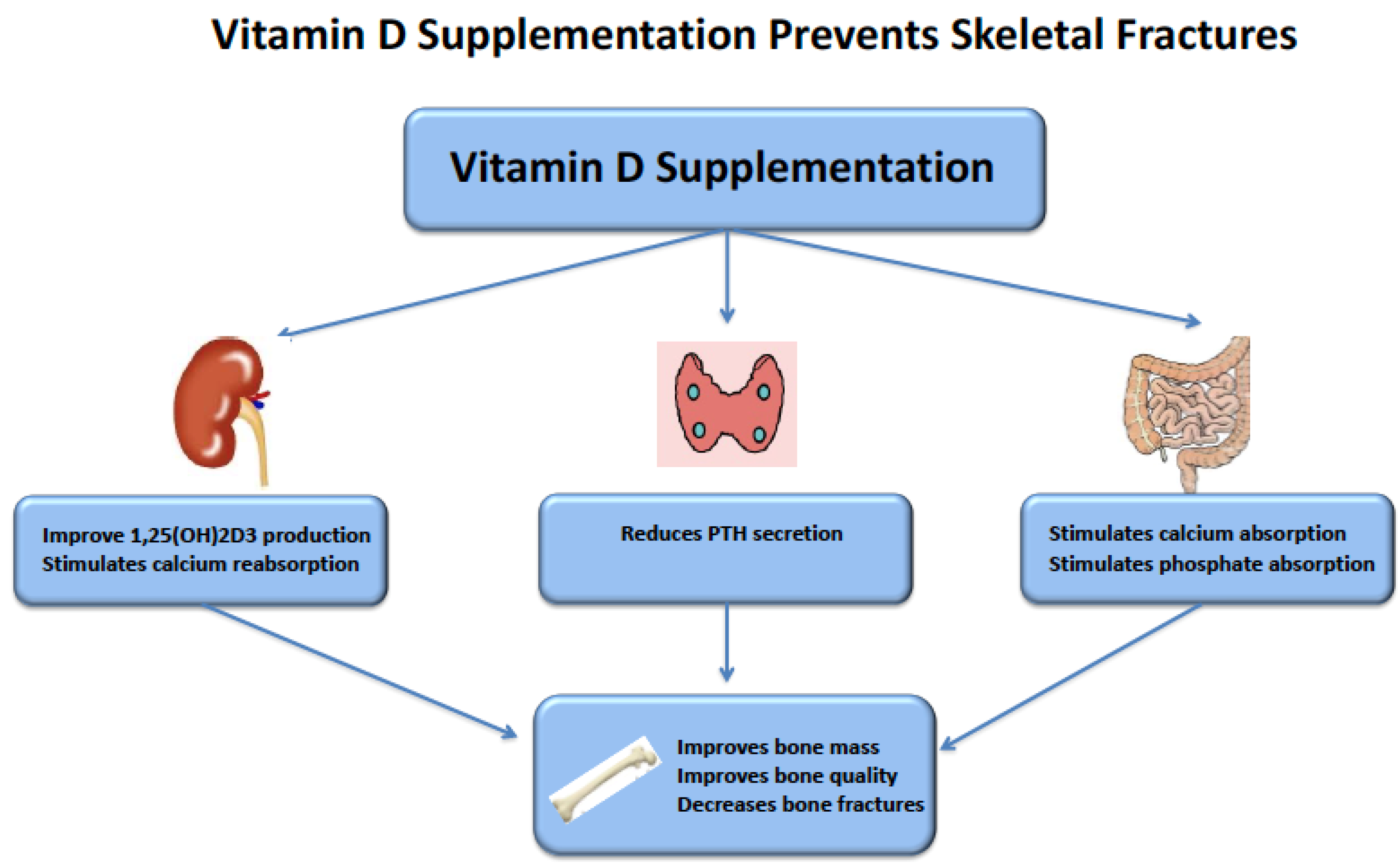
5. Vitamin D Supplementation and Fractures in CKD
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moe, S.M.; Nickolas, T.L. Fractures in Patients with CKD: Time for Action. Clin. J. Am. Soc. Nephrol. 2016, 11, 1929–1931. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.L.; McArthur, E.; Leslie, W.D.; Fraser, L.A.; Jamal, S.A.; Cadarette, S.M.; Pouget, J.G.; Lok, C.E.; Hodsman, A.B.; Adachi, J.D.; et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014, 86, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.; Houillier, P.; Gauci, C.; Haymann, J.P.; Flamant, M.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Froissart, M.; Stengel, B.; et al. Relation between circulating levels of 25(OH) vitamin D and parathyroid hormone in chronic kidney disease: Quest for a threshold. J. Clin. Endocrinol. Metab. 2013, 98, 2922–2928. [Google Scholar] [CrossRef] [PubMed]
- Moranne, O.; Froissart, M.; Rossert, J.; Gauci, C.; Boffa, J.J.; Haymann, J.P.; M’Rad, M.B.; Jacquot, C.; Houillier, P.; Stengel, B.; et al. Timing of onset of CKD-related metabolic complications. J. Am. Soc. Nephrol. 2009, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Malberti, F.; Surian, M.; Cosci, P. Effect of chronic intravenous calcitriol on parathyroid function and set point of calcium in dialysis patients with refractory secondary hyperparathyroidism. Nephrol. Dial. Transplant. 1992, 7, 822–828. [Google Scholar] [PubMed]
- Gogusev, J.; Duchambon, P.; Hory, B.; Giovannini, M.; Goureau, Y.; Sarfati, E.; Drueke, T.B. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997, 51, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Slatopolsky, E.; Bricker, N.S. The role of phosphorus restriction in the prevention of secondary hyperparathyroidism in chronic renal disease. Kidney Int. 1973, 4, 141–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naveh-Many, T. Minireview: The play of proteins on the parathyroid hormone messenger ribonucleic Acid regulates its expression. Endocrinology 2010, 151, 1398–1402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar] [CrossRef]
- Cozzolino, M.; Urena-Torres, P.; Vervloet, M.G.; Brandenburg, V.; Bover, J.; Goldsmith, D.; Larsson, T.E.; Massy, Z.A.; Mazzaferro, S.; on behalf of the CKD-MBD Working Group of ERA-EDTA. Is chronic kidney disease-mineral bone disorder (CKD-MBD) really a syndrome? Nephrol. Dial. Transplant. 2014, 29, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- KDIGO. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 2009, 76, S1–S130. [Google Scholar] [CrossRef]
- Covic, A.; Vervloet, M.; Massy, Z.A.; Torres, P.U.; Goldsmith, D.; Brandenburg, V.; Mazzaferro, S.; Evenepoel, P.; Bover, J.; Apetrii, M.; et al. Bone and mineral disorders in chronic kidney disease: Implications for cardiovascular health and ageing in the general population. Lancet Diabetes Endocrinol. 2017, 6, 319–331. [Google Scholar] [CrossRef]
- Maravic, M.; Ostertag, A.; Torres, P.U.; Cohen-Solal, M. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos. Int. 2014, 25, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Maravic, M.; Ostertag, A.; Urena, P.; Cohen-Solal, M. Dementia is a major risk factor for hip fractures in patients with chronic kidney disease. Osteoporos. Int. 2016, 27, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Urena-Torres, P.; Zillikens, M.C.; Bover, J.; Cohen-Solal, M. Fractures in patients with CKD-diagnosis, treatment, and prevention: A review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int. 2017, 92, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Souberbielle, J.C.; Body, J.J.; Lappe, J.M.; Plebani, M.; Shoenfeld, Y.; Wang, T.J.; Bischoff-Ferrari, H.A.; Cavalier, E.; Ebeling, P.R.; Fardellone, P.; et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev. 2010, 9, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Strugnell, S.A.; Sprague, S.M.; Ashfaq, A.; Petkovich, M.; Bishop, C.W. Rationale for Raising Current Clinical Practice Guideline Target for Serum 25-Hydroxyvitamin D in Chronic Kidney Disease. Am. J. Nephrol. 2019, 49, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Cailleaux, P.E.; Ostertag, A.; Metzger, M.; Stengel, B.; Boucquemont, J.; Houillier, P.; Flamant, M.; Urena-Torres, P.; Cohen-Solal, M.; on the behalf of the NephroTest Study Group. Longitudinal Bone Loss Occurs at the Radius in CKD. Kidney Int. Rep. 2021, 6, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Nickolas, T.L.; McMahon, D.J.; Shane, E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J. Am. Soc. Nephrol. 2006, 17, 3223–3232. [Google Scholar] [CrossRef] [PubMed]
- Urena, P.; Bernard-Poenaru, O.; Ostertag, A.; Baudoin, C.; Cohen-Solal, M.; Cantor, T.; de Vernejoul, M.C. Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients. Nephrol. Dial. Transplant. 2003, 18, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Goto, R.; Toyama, S.; Sawada, K.; Takamuku, K.; Kubo, T.; Takahashi, T. The Usefulness of Basic Movement Scale in Hip Fracture Patients: Construct Validity from a Cross-Sectional Study. Am. J. Phys. Med. Rehabil. 2019, 98, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Iseri, K.; Carrero, J.J.; Evans, M.; Fellander-Tsai, L.; Berg, H.E.; Runesson, B.; Stenvinkel, P.; Lindholm, B.; Qureshi, A.R. Incidence of Fractures Before and After Dialysis Initiation. J. Bone Miner. Res. 2020, 35, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.L.; Garg, A.X.; Zou, G.; Langsetmo, L.; Leslie, W.D.; Fraser, L.A.; Adachi, J.D.; Morin, S.; Goltzman, D.; Lentle, B.; et al. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin. J. Am. Soc. Nephrol. 2015, 10, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.H.; Nuttleman, P.R.; Ketcham, C.M.; Roberts, R.M. Purification and characterization of human bone tartrate-resistant acid phosphatase. J. Bone Miner. Res. 1989, 4, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Schuit, S.C.; van der Klift, M.; Weel, A.E.; de Laet, C.E.; Burger, H.; Seeman, E.; Hofman, A.; Uitterlinden, A.G.; van Leeuwen, J.P.; Pols, H.A. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam Study. Bone 2004, 34, 195–202. [Google Scholar] [CrossRef]
- Stein, M.S.; Packham, D.K.; Ebeling, P.R.; Wark, J.D.; Becker, G.J. Prevalence and risk factors for osteopenia in dialysis patients. Am. J. Kidney Dis. 1996, 28, 515–522. [Google Scholar] [CrossRef]
- Kim, S.M.; Long, J.; Montez-Rath, M.E.; Leonard, M.B.; Norton, J.A.; Chertow, G.M. Rates and Outcomes of Parathyroidectomy for Secondary Hyperparathyroidism in the United States. Clin. J. Am. Soc. Nephrol. 2016, 11, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Tentori, F.; McCullough, K.; Kilpatrick, R.D.; Bradbury, B.D.; Robinson, B.M.; Kerr, P.G.; Pisoni, R.L. Response to High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2013, 85, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, D.; Mylne, A.; Roderick, P.J.; Smeeth, L.; Hubbard, R.; Fletcher, A. Chronic kidney disease and hip fracture-related mortality in older people in the UK. Nephrol. Dial. Transplant. 2009, 24, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Mitani, A.A.; Goldstein, B.A.; Chertow, G.M.; Lowenberg, D.W.; Winkelmayer, W.C. Temporal trends in the incidence, treatment, and outcomes of hip fracture in older patients initiating dialysis in the United States. Clin. J. Am. Soc. Nephrol. 2013, 8, 1336–1342. [Google Scholar] [CrossRef][Green Version]
- Bhan, I.; Powe, C.E.; Berg, A.H.; Ankers, E.; Wenger, J.B.; Karumanchi, S.A.; Thadhani, R.I. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012, 82, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Mendel, C.M. The free hormone hypothesis: A physiologically based mathematical model. Endocr. Rev. 1989, 10, 232–274. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- van Driel, M.; Koedam, M.; Buurman, C.J.; Hewison, M.; Chiba, H.; Uitterlinden, A.G.; Pols, H.A.; van Leeuwen, J.P. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006, 20, 2417–2419. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.H.; Atkins, G.J.; Turner, A.G.; Kogawa, M.; Findlay, D.M.; Morris, H.A. Vitamin D metabolism within bone cells: Effects on bone structure and strength. Mol. Cell. Endocrinol. 2011, 347, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Eisman, J.A.; Bouillon, R. Vitamin D: Direct effects of vitamin D metabolites on bone: Lessons from genetically modified mice. BoneKEy Rep. 2014, 3, 499. [Google Scholar] [CrossRef] [PubMed]
- Martineau, C.; Naja, R.P.; Husseini, A.; Hamade, B.; Kaufmann, M.; Akhouayri, O.; Arabian, A.; Jones, G.; St-Arnaud, R. Optimal bone fracture repair requires 24R,25-dihydroxyvitamin D3 and its effector molecule FAM57B2. J. Clin. Investig. 2018, 128, 3546–3557. [Google Scholar] [CrossRef] [PubMed]
- Bienaime, F.; Prie, D.; Friedlander, G.; Souberbielle, J.C. Vitamin D metabolism and activity in the parathyroid gland. Mol. Cell. Endocrinol. 2011, 347, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Rickers, H.; Christiansen, C.; Christensen, P.; Christensen, M.; Rodbro, P. Serum concentrations of vitamin D metabolites in different degrees of impaired renal function. Estimation of renal and extrarenal secretion rate of 24,25-dihydroxyvitamin D. Nephron 1985, 39, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Urena-Torres, P.; Metzger, M.; Haymann, J.P.; Karras, A.; Boffa, J.J.; Flamant, M.; Vrtovsnik, F.; Gauci, C.; Froissart, M.; Houillier, P.; et al. Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am. J. Kidney Dis. 2011, 58, 544–553. [Google Scholar] [CrossRef]
- Abdelbaqi-Salhab, M.; Shalhub, S.; Morgan, M.B. A current review of the cutaneous manifestations of renal disease. J. Cutan. Pathol. 2003, 30, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.; Negri, A.L.; Aguirre, C.; Fradinger, E.; Zanchetta, J.R. Prevalence of 25(OH) vitamin D insufficiency and deficiency in chronic kidney disease stage 5 patients on hemodialysis. Hemodial. Int. 2007, 11, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.A.; Sachdeva, A.; Oliver, D.A.; Martin, K.J. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am. J. Nephrol. 2004, 24, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.I.; Sallman, A.; Santiz, Z.; Hollis, B.W. Defective photoproduction of cholecalciferol in normal and uremic humans. J. Nutr. 1984, 114, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Mawer, E.B.; Taylor, C.M.; Backhouse, J.; Lumb, G.A.; Stanbury, S.W. Failure of formation of 1,25-dihydroxycholecalciferol in chronic renal insufficiency. Lancet 1973, 1, 626–628. [Google Scholar] [CrossRef]
- Sato, K.A.; Gray, R.W.; Lemann, J., Jr. Urinary excretion of 25-hydroxyvitamin D in health and the nephrotic syndrome. J. Lab. Clin. Med. 1982, 99, 325–330. [Google Scholar] [PubMed]
- Vaziri, N.D.; Hollander, D.; Hung, E.K.; Vo, M.; Dadufalza, L. Impaired intestinal absorption of vitamin D3 in azotemic rats. Am. J. Clin. Nutr. 1983, 37, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999, 96, 507–515. [Google Scholar] [CrossRef]
- Takemoto, F.; Shinki, T.; Yokoyama, K.; Inokami, T.; Hara, S.; Yamada, A.; Kurokawa, K.; Uchida, S. Gene expression of vitamin D hydroxylase and megalin in the remnant kidney of nephrectomized rats. Kidney Int. 2003, 64, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Tanaka, H.; Tominaga, Y.; Fukagawa, M.; Kurokawa, K.; Seino, Y. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J. Clin. Investig. 1993, 92, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Patel, S.R. Altered vitamin D metabolism and receptor interaction with the target genes in renal failure: Calcitriol receptor interaction with its target gene in renal failure. Curr. Opin. Nephrol. Hypertens. 1995, 4, 302–306. [Google Scholar] [CrossRef]
- Patel, S.R.; Ke, H.Q.; Vanholder, R.; Koenig, R.J.; Hsu, C.H. Inhibition of calcitriol receptor binding to vitamin D response elements by uremic toxins. J. Clin. Investig. 1995, 96, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.A.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Melamed, M.L.; Chonchol, M.; Gutierrez, O.M.; Kalantar-Zadeh, K.; Kendrick, J.; Norris, K.; Scialla, J.J.; Thadhani, R. The Role of Vitamin D in CKD Stages 3 to 4: Report of a Scientific Workshop Sponsored by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 72, 834–845. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Ravani, P.; Malberti, F.; Tripepi, G.; Pecchini, P.; Cutrupi, S.; Pizzini, P.; Mallamaci, F.; Zoccali, C. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009, 75, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mucsi, I.; Almasi, C.; Deak, G.; Marton, A.; Ambrus, C.; Berta, K.; Lakatos, P.; Szabo, A.; Horvath, C. Serum 25(OH)-vitamin D levels and bone metabolism in patients on maintenance hemodialysis. Clin. Nephrol. 2005, 64, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Bordier, P.; Gueris, J.; Sebert, J.L.; Marie, P.; Ferriere, C.; Bedrossian, J.; DeLuca, H.F. Comparison of 1α-hydroxycholecalciferol and 25-hydroxycholecalciferol in the treatment of renal osteodystrophy: Greater effect of 25-hydroxycholecalciferol on bone mineralization. Kidney Int. 1979, 15, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Stanbury, S.W. Azotaemic renal osteodystrophy. Br. Med. Bull. 1957, 13, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, C.; Almasi, C.; Berta, K.; Deak, G.; Marton, A.; Molnar, M.Z.; Nemeth, Z.; Horvath, C.; Lakatos, P.; Szathmari, M.; et al. Vitamin D insufficiency and bone fractures in patients on maintenance hemodialysis. Int. Urol. Nephrol. 2011, 43, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Coen, G.; Mantella, D.; Manni, M.; Balducci, A.; Nofroni, I.; Sardella, D.; Ballanti, P.; Bonucci, E. 25-hydroxyvitamin D levels and bone histomorphometry in hemodialysis renal osteodystrophy. Kidney Int. 2005, 68, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Liu, S.; Looker, A.C.; Wallace, T.C.; Wang, D.D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016, 27, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.; Avenell, A. Effects of vitamin D supplementation on musculoskeletal health: A systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018, 6, 847–858. [Google Scholar] [CrossRef]
- Mazess, R.B.; Bischoff-Ferrari, H.A.; Dawson-Hughes, B. Vitamin D: Bolus Is Bogus-A Narrative Review. JBMR Plus 2021, 5, e10567. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Orav, E.J.; Abderhalden, L.; Dawson-Hughes, B.; Willett, W.C. Vitamin D supplementation and musculoskeletal health. Lancet Diabetes Endocrinol. 2019, 7, 85. [Google Scholar] [CrossRef]
- Carmel, A.S.; Shieh, A.; Bang, H.; Bockman, R.S. The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥33 ng/mL. Osteoporos. Int. 2012, 23, 2479–2487. [Google Scholar] [CrossRef]
- Bover, J.; Bailone, L.; Lopez-Baez, V.; Benito, S.; Ciceri, P.; Galassi, A.; Cozzolino, M. Osteoporosis, bone mineral density and CKD-MBD: Treatment considerations. J. Nephrol. 2017, 30, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, C.; de Boer, I.H.; Targher, G.; Kendrick, J.; Smits, G.; Chonchol, M. The effect of combined calcium and cholecalciferol supplementation on bone mineral density in elderly women with moderate chronic kidney disease. Clin. Nephrol. 2012, 77, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, M.; Doi, Y.; Obi, Y.; Hamano, T.; Tomosugi, T.; Futamura, K.; Okada, M.; Hiramitsu, T.; Goto, N.; Isaka, Y.; et al. Cholecalciferol Supplementation Attenuates Bone Loss in Incident Kidney Transplant Recipients: A Prespecified Secondary Endpoint Analysis of a Randomized Controlled Trial. J. Bone Miner. Res. 2021, 37, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.; Erkoc, R.; Sayarlioglu, H.; Soyoral, Y.; Dulger, H. Effect of depot oral cholecalciferol treatment on secondary hyperparathyroidism in stage 3 and stage 4 chronic kidney diseases patients. Ren. Fail. 2008, 30, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Binongo, J.N.; Ziegler, T.R.; Schlanger, L.E.; Wang, W.; Someren, J.T.; Tangpricha, V. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: A randomized controlled pilot study. Endocr. Pract. 2008, 14, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.R.; Jackson, S.T.; Hoffmann, M.R.; Jindal, K.; Senior, P.A. Vitamin D3 supplementation, bone health and quality of life in adults with diabetes and chronic kidney disease: Results of an open label randomized clinical trial. Clin. Nutr. 2017, 36, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Kumar, V.; Kumar, V.; Banerjee, D.; Gupta, K.L.; Jha, V. The Effect of Vitamin D Supplementation on Bone Metabolic Markers in Chronic Kidney Disease. J. Bone Miner. Res. 2018, 33, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Kumar, V.; Banerjee, D.; Gupta, K.L.; Jha, V. Effect of vitamin D supplementation on serum sclerostin levels in chronic kidney disease. J. Steroid Biochem. Mol. Biol. 2018, 180, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.; Aspray, T.J.; Schoenmakers, I. Vitamin D Supplementation for Patients with Chronic Kidney Disease: A Systematic Review and Meta-analyses of Trials Investigating the Response to Supplementation and an Overview of Guidelines. Calcif. Tissue Int. 2021, 109, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Kandula, P.; Dobre, M.; Schold, J.D.; Schreiber, M.J., Jr.; Mehrotra, R.; Navaneethan, S.D. Vitamin D supplementation in chronic kidney disease: A systematic review and meta-analysis of observational studies and randomized controlled trials. Clin. J. Am. Soc. Nephrol. 2011, 6, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Bover, J.; Gunnarsson, J.; Csomor, P.; Kaiser, E.; Cianciolo, G.; Lauppe, R. Impact of nutritional vitamin D supplementation on parathyroid hormone and 25-hydroxyvitamin D levels in non-dialysis chronic kidney disease: A meta-analysis. Clin. Kidney J. 2021, 14, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Tang, M.; Perry, T.; Zalunardo, N.; Beaulieu, M.; Dubland, J.A.; Zerr, K.; Djurdjev, O. Randomized Controlled Trial for the Effect of Vitamin D Supplementation on Vascular Stiffness in CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Sprague, S.M.; Silva, A.L.; Al-Saghir, F.; Damle, R.; Tabash, S.P.; Petkovich, M.; Messner, E.J.; White, J.A.; Melnick, J.Z.; Bishop, C.W. Modified-release calcifediol effectively controls secondary hyperparathyroidism associated with vitamin D insufficiency in chronic kidney disease. Am. J. Nephrol. 2014, 40, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Sprague, S.M.; Crawford, P.W.; Melnick, J.Z.; Strugnell, S.A.; Ali, S.; Mangoo-Karim, R.; Lee, S.; Petkovich, P.M.; Bishop, C.W. Use of Extended-Release Calcifediol to Treat Secondary Hyperparathyroidism in Stages 3 and 4 Chronic Kidney Disease. Am. J. Nephrol. 2016, 44, 316–325. [Google Scholar] [CrossRef]
- Jackson, R.D.; LaCroix, A.Z.; Gass, M.; Wallace, R.B.; Robbins, J.; Lewis, C.E.; Bassford, T.; Beresford, S.A.; Black, H.R.; Blanchette, P.; et al. Calcium plus vitamin D supplementation and the risk of fractures. N. Engl. J. Med. 2006, 354, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Malihi, Z.; Lawes, C.M.M.; Wu, Z.; Huang, Y.; Waayer, D.; Toop, L.; Khaw, K.T.; Camargo, C.A.; Scragg, R. Monthly high-dose vitamin D supplementation does not increase kidney stone risk or serum calcium: Results from a randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, M.; Ferraro, P.M.; Vittori, M.; Lombardi, G.; Gambaro, G.; Somani, B. Calcium and Vitamin D Supplementation and Their Association with Kidney Stone Disease: A Narrative Review. Nutrients 2021, 13, 4363. [Google Scholar] [CrossRef] [PubMed]
- Larsson, T.; Nisbeth, U.; Ljunggren, O.; Juppner, H.; Jonsson, K.B. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003, 64, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Xie, H.; Yang, W.; Xie, D.; Anderson, A.H.; Scialla, J.; Wahl, P.; Gutierrez, O.M.; Steigerwalt, S.; He, J.; et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011, 305, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Bitarafan, S.; Mousavi, S.M.; Zargarzadeh, N.; Mokhtari, P.; Hawkins, J.; Meysamie, A.; Koohdani, F. The effect of vitamin D supplementation on fibroblast growth factor-23 in patients with chronic kidney disease: A systematic review and meta-analysis. Phytother. Res. 2021, 35, 5339–5351. [Google Scholar] [CrossRef] [PubMed]

| Number | Duration | Number of Patients | CKD Stage | Treatment | Main Results | Authors |
|---|---|---|---|---|---|---|
| Bone Mineral Density | ||||||
| 1 | 2 years | 610 | 3 and 4 | 1200 mg of calcium + 800 IU of vitamin D3 versus placebo | Loss of BMD at the distal radius was reduced | Bosworth C. 2012 DECALYOS Study [69] |
| 2 | 11 months | 193 | Kidney transplanted patients | 4000 IU/day of vitamin D Versus placebo | Bone loss at the LS in treated subjects was attenuated (−0.2 versus −1.9%) | Tsujita M. 2021 [70] |
| Circulating Biomarkers of Bone Metabolism | ||||||
| 3 | 1 month | 40 | 3 to 4 | 300,000 IU/month of vitamin D3 versus placebo | Decrease of PTH roughly from 368 to 279 pg/mL in the treated group | Dogan E. 2008 [71] |
| 4 | 3 months | 34 | 3 to 4 | 50,000 IU/week of vitamin D3 versus placebo | PTH showed a trend of reduction in the treated group | Chandra P. 2008 [72] |
| 5 | 6 months | - | CKD diabetic patients | 2000 IU/day versus 40,000 IU/day | Significant reduction in BSAP | Mager D.R. 2017 [73] |
| 6 | 4 months | 120 | 3 to 4 | 300,000 IU vitamin D3 every 2 months versus placebo | No significant change in serum sclerostin levels | Yadav A. 2018 [74] |
| 7 | 4 months | 120 | 3 to 4 | 300,000 IU vitamin D3 every 2 months versus placebo | BSAP decreased by 29 ng/mL CTX-1 decreased by 18 ng/mL No change in FGF23 | Yadav A. 2017 [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ureña Torres, P.A.; Souberbielle, J.C.; Solal, M.C. Bone Fragility in Chronic Kidney Disease Stage 3 to 5: The Use of Vitamin D Supplementation. Metabolites 2022, 12, 266. https://doi.org/10.3390/metabo12030266
Ureña Torres PA, Souberbielle JC, Solal MC. Bone Fragility in Chronic Kidney Disease Stage 3 to 5: The Use of Vitamin D Supplementation. Metabolites. 2022; 12(3):266. https://doi.org/10.3390/metabo12030266
Chicago/Turabian StyleUreña Torres, Pablo Antonio, Jean Claude Souberbielle, and Martine Cohen Solal. 2022. "Bone Fragility in Chronic Kidney Disease Stage 3 to 5: The Use of Vitamin D Supplementation" Metabolites 12, no. 3: 266. https://doi.org/10.3390/metabo12030266
APA StyleUreña Torres, P. A., Souberbielle, J. C., & Solal, M. C. (2022). Bone Fragility in Chronic Kidney Disease Stage 3 to 5: The Use of Vitamin D Supplementation. Metabolites, 12(3), 266. https://doi.org/10.3390/metabo12030266






