Abstract
Pharmacological interventions are essential for the treatment and management of critical illness. Although women comprise a large proportion of the critically ill, sex-specific pharmacological properties are poorly described in critical care. The sex-specific effects of vitamin D3 treatment in the critically ill are not known. Therefore, we performed a metabolomics cohort study with 1215 plasma samples from 428 patients from the VITdAL-ICU trial to study sex-specific differences in the metabolic response to critical illness following high-dose oral vitamin D3 intervention. In women, despite the dose of vitamin D3 being higher, pharmacokinetics demonstrated a lower extent of vitamin D3 absorption compared to men. Metabolic response to high-dose oral vitamin D3 is sex-specific. Sex-stratified individual metabolite associations with elevations in 25(OH)D following intervention showed female-specific positive associations in long-chain acylcarnitines and male-specific positive associations in free fatty acids. In subjects who responded to vitamin D3 intervention, significant negative associations were observed in short-chain acylcarnitines and branched chain amino acid metabolites in women as compared to men. Acylcarnitines and branched chain amino acids are reflective of fatty acid B oxidation, and bioenergesis may represent notable metabolic signatures of the sex-specific response to vitamin D. Demonstrating sex-specific pharmacometabolomics differences following intervention is an important movement towards the understanding of personalized medicine.
1. Introduction
Women and men have dissimilar pharmacokinetics and pharmacodynamics in response to drug interventions [1]. In metabolism, sex-specific genetic differences are present [2,3]. Sizable studies on circulating metabolites in ambulatory patients show notable sex-specific differences [2,3,4]. Sex-specific pharmacological properties are poorly described and most drugs do not have specific dosage recommendations for women and men [5,6,7].
Critically ill patients have a profound disruption of systemic homeostasis, as reflected by circulating metabolites. Blood-based metabolomic studies early in critical illness can reflect dysregulated cellular byproducts, inform illness severity and be used to predict outcomes [8,9]. Metabolomic profiles are shown to differ in critically ill patients with and without low 25(OH)D levels [10]. Sex-specific differences are noted in 25(OH)D levels and immunomodulatory effects as well as cardiometabolic traits in ambulatory adults [11,12]. Though the pharmacokinetics is well described in outpatients, the sex-specific effects of high-dose vitamin D3 in the critically ill is not known [13,14,15,16].
Metabolomics before and after intervention can furnish mechanistic insight into drug response and severity of critical illness [17,18]. Sex-specific pharmacokinetics is likely present with oral medication use [19]. But, sex-specific pharmacometabolomics is poorly described, especially in critical care. Therefore, we studied sex-specific differences in the metabolic response to critical illness following high-dose oral vitamin D3. We hypothesize that significant sex-specific pharmacokinetic and metabolomic responses to vitamin D3 intervention exist.
We performed a post hoc metabolomics cohort study of 1215 plasma samples from 428 patients collected during the VITdAL-ICU trial [20]. The VITdAL-ICU trial was a single-center trial of 492 critically ill adults with 25-hydroxyvitamin D [25(OH)D] levels ≤ 20 ng/mL randomized to high-dose oral vitamin D3 or placebo [20]. The VITdAL-ICU trial was negative for the primary outcome of length of stay. In a secondary outcome, the VITdAL-ICU trial showed that in patients with low baseline vitamin D levels mortality was lower in patients who received high-dose oral vitamin D3 compared to those who received placebo [20]. We assessed the sex-specific changes in individual metabolites following high-dose vitamin D intervention in critical illness. Secondly, we determined if sex-specific differences in pharmacokinetics or mediation exist of the relationship between the response to intervention and individual metabolite change.
2. Results
2.1. Demographics
Baseline characteristics were similar between groups of subjects stratified by absolute increase in 25(OH)D ≥ 7.5 ng/mL between day 0 and 3 and then sex for SAPS II, C-reactive protein, baseline 25(OH)D, body mass index (BMI), diabetes and ICU type. Differences existed with respect to age, Charlson Comorbidity Index, intervention status, change in 25(OH)D and day 0 levels of glucose (Table 1). The overall 28-day mortality of the 428 subject analytic cohort was 22.2%. Twenty-eight-day mortality differed based on absolute increase in 25(OH)D levels between day 0 and day 3 (χ2(1) = 13.3; p < 0.001) but not gender (χ2(1) = 0.014; p = 0.91).

Table 1.
Characteristics of analytic cohort, according to sex and 25(OH)D response.
2.2. Pharmacokinetics
We next evaluated the sex-specific pharmacokinetics of high-dose oral vitamin D3. The amount of vitamin D3 administered was the same in men and women (oral 540,000 IU vitamin D3); the dose of vitamin D3 (IU/kg), in general, is higher in women due to their lower body weight. Despite different IU/kg doses, the pharmacokinetics of 25(OH)D in patients randomized to vitamin D3 showed similar mean serum 25(OH)D concentrations over time (Table S1, Figure S1). Compared with women, the pharmacokinetic parameters of 25(OH)D using non-compartmental analysis showed significantly higher normalized AUC0-7 in men (p < 0.05), a measure of the extent of drug absorption.
2.3. Sex-Stratified Analyses
In day 0 plasma samples of female subjects (N = 151), no significant differences were found in any individual metabolites by t-test (all q-values > 0.10) (Table S2) or in metabolomic profiles by OPLS-DA (CV-ANOVA, p = 0.99) in subjects with an increase of 25(OH)D< or ≥7.5 ng/mL from day 0 to day 3 (Table S3). In male subjects at day 0 (N = 277), no significant differences in individual metabolites were found by t-test (all q-values > 0.10) (Table S2) or in metabolomic profiles as determined by OPLS-DA (CV-ANOVA, p-value 0.99) in those with an increase in 25(OH)D ≥ 7.5 ng/mL from day 0 to 3 relative to subjects who did not (Table S3).
In sex-stratified mixed effects modeling of day 0, 3 and 7 plasma samples in 151 women (435 total samples) and 277 men (795 total samples), sex-specific differences were present, with increases in 25(OH)D between day 0 and day 3. Significant increases in long-chain acylcarnitines with elevated 25(OH)D were seen only in the female stratum (summary of data presented in Table 2, Figure 1; full data presented in Table S4). In the male but not the female stratum, significant negative associations were found with ceramides, dicarboxylate fatty acids and long chain fatty acids in the setting of increasing 25(OH)D (summarized data in Table 3, Figure 2; full data in Table S4). The common response in both the female and male strata to elevations in 25(OH)D is significantly increased branched chain amino acid (BCAA) metabolism and plasmalogens as well as decreases in pentose phosphate pathway metabolites (Table 4; full data in Table S4). In the sex-stratified mixed effects regression, additional adjustment for the Charlson Comorbidity Index did not materially alter the direction and effect size of the associations between sex and metabolite pathways (Table S5).

Table 2.
Long-chain acylcarnitines significantly associated with response to high-dose vitamin D in women but not men.
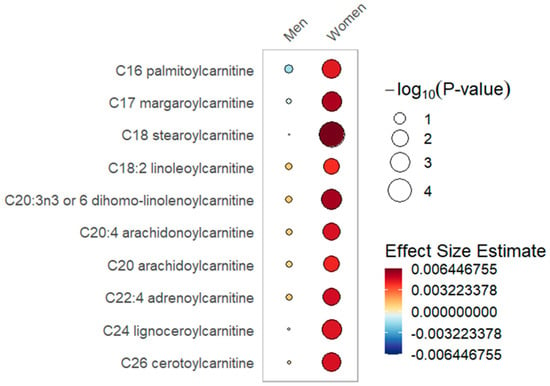
Figure 1.
Long-chain acylcarnitines rain plot. Correlations between absolute increase in 25(OH)D levels from day 0 to day 3 and individual long-chain acylcarnitine metabolite abundance at day 0, 3 or 7 in women or men determined utilizing mixed-effects linear regression models correcting for age, SAPS II, admission diagnosis and 25(OH)D at day 0. The red color fill scare indicates the magnitude of beta coefficient estimates and the size of the circle corresponds to the significance level (−log10(p-value)). Statistical significance is the multiple test-corrected threshold of −log10(p) > 1.92 which is equivalent to p < 0.0122.

Table 3.
Free fatty acid metabolites significantly associated with responses to high-dose vitamin D in men but not women.
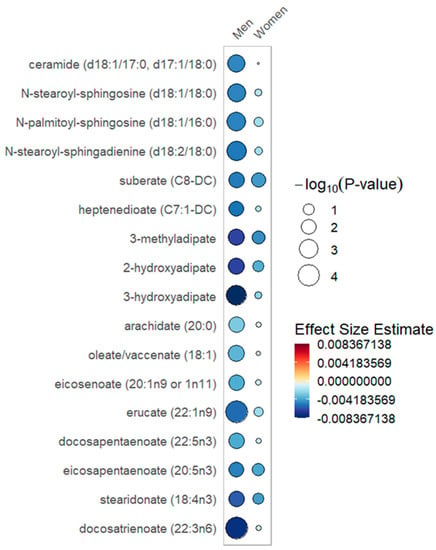
Figure 2.
Free fatty acid metabolites rain plot. Correlations between absolute increase in 25(OH)D levels from day 0 to day 3 and individual free fatty acid metabolite abundance at day 0, 3 or 7 in women or men determined utilizing mixed-effects linear regression models correcting for age, SAPS II, admission diagnosis and 25(OH)D at day 0. The blue color fill scare indicates the magnitude of beta coefficient estimates and the size of the circle corresponds to the significance level (−log10(p-value)). Statistical significance is the multiple test-corrected threshold of −log10(p) > 1.92, which is equivalent to p < 0.0122.

Table 4.
Metabolites significantly associated with response to a high dose of vitamin D in both women and men.
2.4. Responder Cohort Analysis
Restricting the mixed effects modeling of day 0, 3 and 7 plasma samples to the 153-patient responder cohort (those who received high dose vitamin D3 intervention and had an increase in 25(OH)D ≥ 7.5 ng/mL) shows sex-specific metabolite patterns over time. Compared to men, women had lower levels of BCAA metabolites and short-chain acylcarnitines and higher levels of sphingomyelin species (Table S6, Figures S2 and S3). In the mixed effects regression of the responder cohort, additional adjustment for the Charlson Comorbidity Index did not change the direction, strength or significance of the associations between sex and metabolite pathways (Table S7).
2.5. Mediation Analysis
Finally, we focused on the potential mediation by sex of the relationship between metabolite abundance and absolute increase in 25(OH)D level between day 0 and day 3. Mediation analyses in day 3 data revealed no sex-dependent influence on associations between the absolute increase in 25(OH)D and all 983 metabolites (all mediation p-values were >0.01). As both adiposity and critical illness increase free fatty acids [21,22], we evaluated BMI as a sex-specific mediator of response to vitamin D3. When individually restricted to female (N = 151) or to male subjects (N = 277), mediation analyses of the day 3 data revealed no influence of BMI on associations between the absolute increase in 25(OH)D and all 983 metabolites (all mediation p-values were >0.01).
3. Discussion
Our large post hoc metabolomics study considered temporal changes in the metabolome and 25(OH)D concentration as well as vitamin D3 dose to determine sex-specific differences in response to vitamin D3 intervention. We evaluated the response to vitamin D3 intervention in three ways: (1) a sex-specific pharmacokinetics study; (2) sex stratification to study metabolite differences relative to the change in 25(OH)D in women separately from metabolite differences in men; and (3) a responder cohort sub-analysis to directly compare metabolite differences between women and men who received vitamin D3 intervention and responded with an absolute increase in 25(OH)D ≥ 7.5 ng/mL between day 0 and 3. Our approach to evaluate sex-specific responses resulted in novel findings. First, our pharmacokinetics data identified sex-specific differences in vitamin D3 absorption. Second, we showed a sex-specific metabolomic response to vitamin D3 associated with differential metabolite patterns over time early in the course of critical illness. These analyses demonstrate evidence of sex-specific metabolism following high-dose vitamin D3.
In the sex-stratified analysis, significant associations with long-chain acylcarnitines and increased 25(OH)D over day 0 to 3 were noted within the female stratum but not in the male stratum (Table 2, Figure 1). With increased 25(OH)D, a significantly lower abundance of ceramides, dicarboxylate fatty acids and long chain fatty acids was limited to the male stratum (Table 3, Figure 2). Furthermore, the male but not the female stratum showed significant decreases in polyunsaturated fatty acids with increased 25(OH)D (Table 3, Figure 2). The common response to increased 25(OH)D in both female and male strata was a significant increase in plasmalogens and BCAA metabolites as well as a decrease in pentose phosphate pathway metabolites (Table S4). In the responder cohort data directly comparing metabolite differences in women and men who received and responded to high-dose vitamin D3 (N = 153), we note that circulating BCAA metabolites and short-chain acylcarnitines are both significantly lower in women compared to men (Table S5).
To understand the relevance of the sex-specific metabolite patterns we observed, existing studies provide context. At homeostasis, metabolism, as well as drug pharmacokinetics and pharmacodynamics, is sex-specific [19,23,24,25,26]. In healthy subjects, circulating fatty acids are incorporated into triglycerides in women but oxidized in men [27]. Compared to men, healthy women have decreased free fatty acid-induced insulin resistance, lower circulating acylcarnitines and higher levels of sphingomyelins [3]. But, studies in healthy adults have little relevance to the critically ill. The hypercatabolism of critical illness substantially alters how oxidative fuel is produced from amino acids, fatty acids and carbohydrates [28]. The physiological stress of critical illness alters such energy metabolism changes in a sex-specific fashion [29]. Drug pharmacodynamics and pharmacokinetics variability is shown in critical illness but it is not yet known to be sex-specific [30].
In the sex-stratified analysis, we found that critically ill women differed from men in the patterns of change of free fatty acids with response to vitamin D3. Specifically, with an increase in 25(OH)D, circulating free fatty acids were lower and more significant within the male stratum (Figure 2). White adipose tissue is prominent in regulating metabolic homeostasis [31]. In healthy adults, sex-specific differences exist in the adipocyte cytokines adiponectin and leptin, with higher levels in women relative to men [32,33]. Experimental models show that vitamin D has a complex active role in adipose tissue metabolism by modifying inflammation and adipocyte secretory function [34]. Small human studies note that ceramide, the highly bioactive lipid mediator, may be regulated by vitamin D [35,36]. Further study is needed to untangle the mechanism for the free fatty acid elevations noted in women responsive to vitamin D.
In the sex-stratified analysis, similar metabolomic patterns of change in the female and male strata are noted with higher levels of BCAA metabolites and plasmalogens and decreased levels of pentose phosphate pathway metabolites in response to increased 25(OH)D levels. These metabolite pathways are involved in bioenergesis, redox regulation and endothelial protection, respectively. During inflammation, amino acids are released into the circulation with protein breakdown. With inadequate mitochondrial fatty acid β-oxidation during critical illness, BCAAs are metabolized by the liver to high-energy compounds—a process that may be augmented by vitamin D signaling [37,38]. Plasmalogens are important antioxidants that act to protect endothelial cells from injury due to oxidative stress [39]. Pentose phosphate pathway metabolites increase with insufficient fatty acid β-oxidation, producing NADPH for redox regulation and ribose 5-phosphate for biosynthesis [40]. Our observation of lower levels of pentose phosphate pathway metabolites with increased 25(OH)D levels may be related to the induction of glutathione formation by vitamin D, which provides cell and mitochondrial protection via lower ROS levels and lower NADPH demand [10,41].
Fatty acid β-oxidation is the key pathway for fatty acid breakdown and energy production and is profoundly dysregulated in critical illness [42]. Fatty acid β-oxidation occurs in both mitochondria and peroxisomes. While long-, medium- and short-chain fatty acids are oxidized by mitochondria, very long-chain fatty acids, bile acids, branched-chain fatty acids and dicarboxylic fatty acids are oxidized by peroxisomes [43,44,45]. Vitamin D enhances fatty acid β-oxidation through upregulation of transcription factors, including PPAR 1α and the mitochondrial enzymes pyruvate dehydrogenase kinase 4, as well as carnitine-palmitoyl transferase 1a and 1b [46,47,48]. Further, data suggest that vitamin D is essential for mitochondrial oxidative phosphorylation capacity [49,50].
In the sex-stratified analysis, we found that critically ill women and men differ in the patterns of change in acylcarnitines. Specifically, higher and more significant increases in long-chain acylcarnitines were present in the female stratum in response to increased 25(OH)D levels. Mitochondria in women utilize fatty acids over amino acids for energy production, create less reactive oxygen species and have an increased oxygen capacity compared to men [51,52,53,54,55]. Production of long-chain acylcarnitines occurs when elevated fatty acid supply exceeds mitochondrial oxidative capacity [56]. In exercise and insulin resistance, such mitochondrial overload is observed, resulting in elevated circulating long-chain acylcarnitines [43,57]. The elevated long-chain acylcarnitines we observed in women are most likely due to increased fatty acid release that exceeds the oxidative capacity of mitochondria [56].
Important to the understanding of our observed long-chain acylcarnitine sex-specific response to vitamin D3 is the contribution of mitochondrial fatty acid uptake. The fatty acid transporter FAT/CD36 which facilitates fatty acid entry into mitochondria is shown to be more abundant in women [58,59,60]. FAT/CD36 abundance may be important for higher uptake of long-chain fatty acids by mitochondria in women [61]. Our observation of increased long-chain acylcarnitines in women relative to men following vitamin D3 may reflect an overwhelming of fatty acid β-oxidation by increased fatty acid delivery to mitochondria in women.
In the sex-stratified data, we find that BCAA catabolic metabolites increase in both men and women with increases in 25(OH)D levels. In our responder cohort analysis of the 153 patients who received and responded to high-dose vitamin D3, we observed that circulating BCAA catabolic metabolites and short-chain acylcarnitines were significantly lower in women relative to men. The relative decrease in even short-chain acylcarnitines (C4) in women is evidence of more complete fatty acid β-oxidation compared to men [43]. The C3 and C5 short-chain acylcarnitines are products of catabolism of BCAAs, threonine and methionine [62]. Our observed sex-specific differences in BCAA catabolic metabolites and short-chain acylcarnitines may reflect a greater enhancement of mitochondrial oxidative function in women with response to vitamin D3. Taken together, our results suggest that in the context of higher free fatty acids delivery, female mitochondria are more responsive to enhancement of mitochondrial oxidative function via vitamin D3, which results in a lower relative production of short-chain acylcarnitines.
Our novel study approach has several strengths. The sample size was large and the repeated measurement of subjects over time substantially increases our statistical power [37,63]. Linear mixed models are useful for determination of metabolite abundance at multiple time points [64,65]. Furthermore, we adjusted for multiple comparisons using the false discovery rate [66]. Our work has potential limitations inherent to its metabolomics cohort study design. Specifically, as an observational study it is subject to bias and may have limited causal inference. The source of samples were White patients with serum 25(OH)D ≤ 20 ng/mL, so the results are potentially not generalizable to all critically ill patients.. Incomplete adjustment for the heterogeneity of illness may exist despite adjustment for demographics and clinical data. Though expression of 25-hydroxylase is not sex-specific, our use of 25(OH)D in determining absorption does not take into account potential differences in the activation of vitamin D3 in the liver [67,68]. It is important to note that our study is post hoc and should be considered hypothesis generating.
4. Materials and Methods
Detailed methods are presented in Supplemental Methods. In the VITdAL-ICU trial, 475 critically ill adults with serum 25-hydroxyvitamin D [25(OH)D] ≤ 20 ng/mL were randomized to oral- or nasogastric tube-administered vitamin D3 540,000 IU followed by 90,000 IU monthly or placebo (oleum arachidis) [20]. Trial inclusion criteria included patients admitted to a medical or surgical ICU who were 18 years or older with an expected ICU stay of ≥48 h and a 25(OH)D level of 20 ng/mL or lower. Excluded from the trial were: patients with severely impaired gastrointestinal function; other trial participation; pregnancy; lactating women; tuberculosis; sarcoidosis; hypercalcemia; nephrolithiasis in the past year; and patients not deemed suitable for study participation (i.e., psychiatric disease, prisoner status). Blood samples were collected on days 0 (at randomization), 3 and 7. Plasma was fractionated, aliquoted and stored at −80 °C. Frozen plasma was available in 453 VITdAL-ICU trial subjects, of whom 25 were excluded due to absence of serum 25(OH)D determination at day 3.
For determination of the pharmacokinetics of oral vitamin D3, we utilized serum 25(OH)D levels, a marker of systemic vitamin D status [69]. Serum 25(OH)D levels were determined via chemiluminescence assay (IDS-iSYS, Immunodiagnostic Systems) with assay coefficients of variation for control material of 9.4% at 64 ng/mL, 10% at 31 ng/mL and 13.4% at 13 ng/mL [20]. For pharmacokinetics evaluation, the area under the plasma concentration–time curve from vitamin D3 dosing to day 7 (AUC0-7d) was calculated using the linear trapezoidal method. Patients with missing 25(OH)D levels on day 3 or 7 and those who received placebo were excluded from the pharmacokinetics evaluation. AUC normalized to vitamin D3 dose and body weight (AUCnorm) was calculated by dividing AUC0-7d by dose in IU per kg body weight. Median AUCnorm and serum 25(OH)D levels on days 0, 3 and 7 values were compared between males and females.
Metabolomics data was produced from 1215 plasma samples obtained from 428 patients: 428 samples were analyzed from day 0, 413 at day 3 and 374 at day 7. Four ultra-high-performance liquid chromatography/tandem accurate mass spectrometry (UHPLC/MS/MS) methods were utilized by Metabolon, Inc. (Morrisville, NC, USA) to identify 983 metabolites [18,29]. We cube root-transformed and Pareto-scaled the metabolomics data to generate an approximate normal distribution.
Based on our previous metabolomics analysis of the VITdAL-ICU trial [18], we considered a response to high-dose oral vitamin D3 as an absolute increase in 25(OH)D ≥ 7.5 ng/mL from day 0 to day 3. For a sex-stratified analysis of day 0 data, we utilized the Student’s t-test to determine the significance of individual metabolites between vitamin D3 response groups (25(OH)D< or ≥7.5 ng/mL from day 0 to day 3) using MetaboAnalyst in women and in men [70]. We corrected for multiple testing via the Benjamini–Hochberg procedure to adjust the false discovery rate (FDR) to 0.10, producing a q-value [66]. We analyzed sex-stratified day 0 data via orthogonal partial least squares-discriminant analysis (OPLS-DA), a statistical method for supervised classification discrimination (SIMCA 15.0 Umetrics, Umea, Sweden). We utilized the OPLS-DA approach to find the metabolomic differences at baseline (day 0) between patients who did and did not respond to high-dose vitamin D3. We analyzed male and female subjects separately to determine if such differences in metabolites at baseline were sex-specific. We used goodness of fit (R2) and predictive performance (Q2) to describe the OPLS-DA model quality. To validate the OPLS-DA model, we performed permutation testing. To determine overall OPLS-DA model significance, we used sevenfold cross-validation analysis of variance (CV-ANOVA).
For day 0, 3 and 7 repeated measures data, correlations between individual metabolites and absolute increase in 25(OH)D levels from day 0 to day 3 were determined separately in women and in men utilizing sex-stratified linear mixed effects models correcting for age, baseline 25(OH)D, SAPS II, sample day, admission diagnosis, and a subject-specific random intercept. A false discovery rate adjusted p-value (q-value) threshold of 0.10 was utilized to identify all significant differences [66]. Additionally, we performed a sub-analysis in a responder cohort of patients who received high-dose vitamin D3 intervention and had an increase in 25(OH)D ≥ 7.5 ng/mL from day 0 to day 3. For day 0, 3 and 7 repeated measures data, we determined correlations between individual metabolites over time relative to sex (as the exposure) using linear mixed effects models adjusted for age, baseline 25(OH)D, SAPS II, sample day, admission diagnosis and a subject-specific random intercept. All mixed effects models were analyzed using STATA 16.1 (College Station, TX, USA). For data visualization purposes, rain plots were produced in R-3.6.2 [71].
Lastly, we evaluated a potential sex-specific mediating effect on the association between the absolute rise in 25(OH)D levels from day 0 to day 3 and individual metabolite abundance, adjusting for baseline 25(OH)D, age, SAPS II and diagnosis at admission. Each of the 983 metabolites at day 3 were analyzed using mediation, an R package for causal mediation analysis [72], to determine bootstrap p-values (N = 2000) for the sex-specific mediating effect. Mediation was significantly present if ≥10% of the association was mediated through sex and the p-value was <0.01 [73].
5. Conclusions
In a large metabolomics study, we demonstrated substantial differences between women and men in the response to high-dose vitamin D3 early in critical illness. Specifically, robust sex-specific differences in pharmacokinetics and metabolomics were found in the response over time to high-dose vitamin D3. Demonstrating sex-specific differences in the metabolic response to pharmacologic intervention is a crucial first step in the understanding of personalized medicine.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/metabo12030207/s1, Figure S1: Day 0, 3 and 7 serum 25(OH)D levels in women and men. Whisker and box plot overlaid with a dot plot showing median and quartile values of the 25(OH)D at day 0, 3 and 7 in women (N = 78) and men (N = 134), randomized to vitamin D3, Figure S2: Responder cohort short-chain acylcarnitines. Day 0, 3 and 7 short-chain acylcarnitines in the 153-patient responder cohort (those who received high-dose vitamin D3 intervention and had an increase in 25(OH)D ≥ 7.5 ng/mL), Figure S3: Responder cohort BCAA metabolites. Day 0, 3 and 7 BCAA metabolites in the 153-patient responder cohort (those who received high-dose vitamin D3 intervention and had an increase in 25(OH)D ≥ 7.5 ng/mL), Table S1: Pharmacokinetics of high-dose vitamin D3, Table S2: Day 0 t-test in female and male subjects. Significance of individual metabolites in subjects with an absolute increase of 25(OH)D< or ≥7.5 ng/mL from day 0 to day 3 as determined by t-test adjusted for false discovery rate, Table S3: OPLS-DA model goodness of fit and predictive ability, Table S4: Sex-stratified mixed effects regression, Table S5: Sex-stratified mixed effects regression model with additional adjustment for the Charlson Comorbidity Index, Table S6: Responder cohort mixed effects regression, Table S7: Responder cohort mixed effects regression model with additional adjustment for the Charlson Comorbidity Index, Methods S1: Details regarding the VITdAL-ICU clinical trial, sample preparation, quality assurance and quality control, ultrahigh performance liquid chromatography-tandem mass spectroscopy, data extraction and compound identification, curation, metabolite quantification and data normalization, pharmacokinetics and statistical analyses, Data S1: Details of the 983 metabolites analyzed, including biochemical name, retention time, mass-to-charge ratio, metabolic pathway, analysis platform, Chemical Abstracts Service number, PUBCHEM identifier, KEGG pathway and HMDB ID.
Author Contributions
Conceptualization, S.C., K.A., J.A.L.-S. and K.B.C.; methodology, S.C., S.H.M., J.A.L.-S. and K.B.C.; software, K.B.C.; validation, S.C., K.A., and K.B.C.; formal analysis, S.C. and K.B.C.; investigation, S.C., K.A., S.H.M. and K.B.C.; resources, K.B.C.; data curation, K.A. and K.B.C.; writing—original draft preparation, S.C., K.A., S.H.M., J.L-S. and K.B.C.; writing—review and editing, S.C., K.A. and K.B.C.; visualization, S.C. and K.B.C.; supervision, K.B.C.; project administration, K.A. and K.B.C.; funding acquisition, K.A. and K.B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health grant number R01 GM115774. The VITdAL-ICU trial was supported by the European Society for Clinical Nutrition and Metabolism (ESPEN), a research grant including provision of study medication from Fresenius Kabi (Germany) and the Austrian National Bank (Jubiläumsfonds, Project Nr. 14143). The APC was funded by Medizinischen Universität Graz.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by Mass General Brigham Human Research Committee (Protocol 2015P002766 in February 2016) and the Ethikkommission der Medizinischen Universität Graz, Austria (Ethikkommission number 21-214 in September 2010).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. At VITdAL-ICU trial enrollment, written informed consent was obtained, if possible, directly from the patient or from a legal surrogate. Consent included permission for plasma specimens to be saved for future research studies.
Data Availability Statement
The data presented in this study are available in Tables S1–S7.
Acknowledgments
This article is dedicated to the memory of our dear friend and colleague Nathan Edward Hellman.
Conflicts of Interest
S. Chary was a master’s student at Harvard Medical School during the implementation of the study. During her master’s, S. Chary was employed at Takeda Pharmaceutical Company and is currently employed by Biogen, Inc. S. Chary reports receiving salary and stock options from Takeda and from Biogen. Neither Takeda nor Biogen had any involvement in the VITdAL-ICU trial, the identification of metabolites, the post hoc study design, the analysis of metabolomics, interpretation of the data, access to the data or the writing of the manuscript. K. Amrein reports receiving lecture fees from Fresenius Kabi. No other financial or other relationships exist that might lead to a conflict of interest.
References
- Gandhi, M.; Aweeka, F.; Greenblatt, R.M.; Blaschke, T.F. Sex differences in pharmacokinetics and pharmacodynamics. Annu. Rev. Pharm. Toxicol. 2004, 44, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Kolz, M.; Johnson, T.; Sanna, S.; Teumer, A.; Vitart, V.; Perola, M.; Mangino, M.; Albrecht, E.; Wallace, C.; Farrall, M.; et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 2009, 5, e1000504. [Google Scholar] [CrossRef] [PubMed]
- Mittelstrass, K.; Ried, J.S.; Yu, Z.; Krumsiek, J.; Gieger, C.; Prehn, C.; Roemisch-Margl, W.; Polonikov, A.; Peters, A.; Theis, F.J.; et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet 2011, 7, e1002215. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Lin, W.; Broadhurst, D.; Begley, P.; Brown, M.; Zelena, E.; Vaughan, A.A.; Halsall, A.; Harding, N.; Knowles, J.D.; et al. Molecular phenotyping of a UK population: Defining the human serum metabolome. Metabolomics 2015, 11, 9–26. [Google Scholar] [CrossRef]
- Franconi, F.; Campesi, I. Sex Impact on Biomarkers, Pharmacokinetics and Pharmacodynamics. Curr. Med. Chem. 2017, 24, 2561–2575. [Google Scholar] [CrossRef]
- Kantae, V.; Krekels, E.H.J.; Esdonk, M.J.V.; Lindenburg, P.; Harms, A.C.; Knibbe, C.A.J.; Van der Graaf, P.H.; Hankemeier, T. Integration of pharmacometabolomics with pharmacokinetics and pharmacodynamics: Towards personalized drug therapy. Metabolomics 2017, 13, 9. [Google Scholar] [CrossRef]
- Karlsson Lind, L.; von Euler, M.; Korkmaz, S.; Schenck-Gustafsson, K. Sex differences in drugs: The development of a comprehensive knowledge base to improve gender awareness prescribing. Biol. Sex Differ. 2017, 8, 32. [Google Scholar] [CrossRef]
- Kiehntopf, M.; Nin, N.; Bauer, M. Metabolism, metabolome, and metabolomics in intensive care: Is it time to move beyond monitoring of glucose and lactate? Am. J. Respir. Crit. Care Med. 2013, 187, 906–907. [Google Scholar] [CrossRef]
- Johansson, P.I.; Nakahira, K.; Rogers, A.J.; McGeachie, M.J.; Baron, R.M.; Fredenburgh, L.E.; Harrington, J.; Choi, A.M.K.; Christopher, K.B. Plasma mitochondrial DNA and metabolomic alterations in severe critical illness. Crit. Care 2018, 22, 360. [Google Scholar] [CrossRef]
- Lasky-Su, J.; Dahlin, A.; Litonjua, A.A.; Rogers, A.J.; McGeachie, M.J.; Baron, R.M.; Gazourian, L.; Barragan-Bradford, D.; Fredenburgh, L.E.; Choi, A.M.K.; et al. Metabolome alterations in severe critical illness and vitamin D status. Crit. Care 2017, 21, 193. [Google Scholar] [CrossRef]
- Dupuis, M.L.; Pagano, M.T.; Pierdominici, M.; Ortona, E. The role of vitamin D in autoimmune diseases: Could sex make the difference? Biol. Sex Differ. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Sanghera, D.K.; Sapkota, B.R.; Aston, C.E.; Blackett, P.R. Vitamin D Status, Gender Differences, and Cardiometabolic Health Disparities. Ann. Nutr. Metab. 2017, 70, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, M.; Armas, L.A.; Heaney, R.P. Pharmacokinetics of a single, large dose of cholecalciferol. Am. J. Clin. Nutr. 2008, 87, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, E.; Mascia, M.L.; Cipriani, C.; Fassino, V.; Mazzei, F.; D’Erasmo, E.; Carnevale, V.; Scillitani, A.; Minisola, S. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J. Clin. Endocrinol. Metab. 2008, 93, 3015–3020. [Google Scholar] [CrossRef]
- Cipriani, C.; Romagnoli, E.; Scillitani, A.; Chiodini, I.; Clerico, R.; Carnevale, V.; Mascia, M.L.; Battista, C.; Viti, R.; Pileri, M.; et al. Effect of a single oral dose of 600,000 IU of cholecalciferol on serum calciotropic hormones in young subjects with vitamin D deficiency: A prospective intervention study. J. Clin. Endocrinol. Metab. 2010, 95, 4771–4777. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.J.; Simpson, J.A.; Kotowicz, M.A.; Young, D.; Nicholson, G.C. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; Kristal, B.S.; Weinshilboum, R.M. Metabolomics: A global biochemical approach to drug response and disease. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 653–683. [Google Scholar] [CrossRef]
- Amrein, K.; Lasky-Su, J.A.; Dobnig, H.; Christopher, K.B. Metabolomic basis for response to high dose vitamin D in critical illness. Clin. Nutr. 2021, 40, 2053–2060. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharm. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.B.; Pachler, C.; Urbanic Purkart, T.; Waltensdorfer, A.; Munch, A.; Warnkross, H.; et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA 2014, 312, 1520–1530. [Google Scholar] [CrossRef]
- Fong, Y.M.; Marano, M.A.; Moldawer, L.L.; Wei, H.; Calvano, S.E.; Kenney, J.S.; Allison, A.C.; Cerami, A.; Shires, G.T.; Lowry, S.F. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J. Clin. Investg. 1990, 85, 1896–1904. [Google Scholar] [CrossRef]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef]
- Murphy, M.O.; Loria, A.S. Sex-specific effects of stress on metabolic and cardiovascular disease: Are women at higher risk? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 2015, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Roeters van Lennep, J.E.; Westerveld, H.T.; Erkelens, D.W.; van der Wall, E.E. Risk factors for coronary heart disease: Implications of gender. Cardiovasc. Res. 2002, 53, 538–549. [Google Scholar] [CrossRef]
- Sugiyama, M.G.; Agellon, L.B. Sex differences in lipid metabolism and metabolic disease risk. Biochem. Cell Biol. 2012, 90, 124–141. [Google Scholar] [CrossRef]
- Uranga, A.P.; Levine, J.; Jensen, M. Isotope tracer measures of meal fatty acid metabolism: Reproducibility and effects of the menstrual cycle. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E547–E555. [Google Scholar] [CrossRef]
- Preiser, J.C.; Ichai, C.; Orban, J.C.; Groeneveld, A.B. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Chary, S.; Amrein, K.; Lasky-Su, J.; Dobnig, H.; Christopher, K.B. The Sex-specific Metabolic Response to Critical Illness: A post-hoc metabolomics study of the VITdAL-ICU trial. Sci. Rep. 2021, 11, 3951. [Google Scholar] [CrossRef]
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient--concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Barrett-Connor, E.; May, S. Sex-specific determinants of serum adiponectin in older adults: The role of endogenous sex hormones. Int. J. Obes. 2007, 31, 457–465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kennedy, A.; Gettys, T.W.; Watson, P.; Wallace, P.; Ganaway, E.; Pan, Q.; Garvey, W.T. The metabolic significance of leptin in humans: Gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J. Clin. Endocrinol. Metab. 1997, 82, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Mutt, S.J.; Hypponen, E.; Saarnio, J.; Jarvelin, M.R.; Herzig, K.H. Vitamin D and adipose tissue-more than storage. Front. Physiol. 2014, 5, 228. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dong, Y.; Bhagatwala, J.; Raed, A.; Huang, Y.; Zhu, H. Vitamin D3 Supplementation Increases Long-Chain Ceramide Levels in Overweight/Obese African Americans: A Post-Hoc Analysis of a Randomized Controlled Trial. Nutrients 2020, 12, 981. [Google Scholar] [CrossRef]
- Koch, A.; Grammatikos, G.; Trautmann, S.; Schreiber, Y.; Thomas, D.; Bruns, F.; Pfeilschifter, J.; Badenhoop, K.; Penna-Martinez, M. Vitamin D Supplementation Enhances C18(dihydro)ceramide Levels in Type 2 Diabetes Patients. Int. J. Mol. Sci. 2017, 18, 1532. [Google Scholar] [CrossRef]
- Kobayashi, H.; Amrein, K.; Lasky-Su, J.; Christopher, K.B. Procalcitonin Metabolomics in the Critically Ill reveal relationships between inflammation intensity and energy utilization pathways. Sci. Rep. 2021, 11, 23194. [Google Scholar] [CrossRef]
- Dimitrov, V.; Barbier, C.; Ismailova, A.; Wang, Y.; Dmowski, K.; Salehi-Tabar, R.; Memari, B.; Groulx-Boivin, E.; White, J.H. Vitamin D-regulated Gene Expression Profiles: Species-specificity and Cell-specific Effects on Metabolism and Immunity. Endocrinology 2021, 162. [Google Scholar] [CrossRef]
- Wallner, S.; Schmitz, G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids 2011, 164, 573–589. [Google Scholar] [CrossRef]
- Nalos, M.; Parnell, G.; Robergs, R.; Booth, D.; McLean, A.S.; Tang, B.M. Transcriptional reprogramming of metabolic pathways in critically ill patients. Intensive Care Med. Exp. 2016, 4, 21. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.J.; Tsalik, E.L.; van Velkinburgh, J.C.; Glickman, S.W.; Rice, B.J.; Wang, C.; Chen, B.; Carin, L.; Suarez, A.; Mohney, R.P.; et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci. Transl. Med. 2013, 5, 195ra195. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Makrecka-Kuka, M.; Sevostjanovs, E.; Vilks, K.; Volska, K.; Antone, U.; Kuka, J.; Makarova, E.; Pugovics, O.; Dambrova, M.; Liepinsh, E. Plasma acylcarnitine concentrations reflect the acylcarnitine profile in cardiac tissues. Sci. Rep. 2017, 7, 17528. [Google Scholar] [CrossRef]
- Violante, S.; Ijlst, L.; Te Brinke, H.; Koster, J.; Tavares de Almeida, I.; Wanders, R.J.; Ventura, F.V.; Houten, S.M. Peroxisomes contribute to the acylcarnitine production when the carnitine shuttle is deficient. Biochim. Biophys. Acta 2013, 1831, 1467–1474. [Google Scholar] [CrossRef]
- Yin, Y.; Yu, Z.; Xia, M.; Luo, X.; Lu, X.; Ling, W. Vitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur. J. Clin. Investig. 2012, 42, 1189–1196. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.J.; Bendahan, D.; Bernard, M.; Martin, J.C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Song, Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol. Nutr. Food Res. 2014, 58, 1342–1348. [Google Scholar] [CrossRef]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 660498. [Google Scholar] [CrossRef]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Malorni, W.; Campesi, I.; Straface, E.; Vella, S.; Franconi, F. Redox features of the cell: A gender perspective. Antioxid. Redox Signal. 2007, 9, 1779–1801. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Borras, C. Women live longer than men: Understanding molecular mechanisms offers opportunities to intervene by using estrogenic compounds. Antioxid. Redox Signal. 2010, 13, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Demarest, T.G.; McCarthy, M.M. Sex differences in mitochondrial (dys)function: Implications for neuroprotection. J. Bioenerg. Biomembr. 2015, 47, 173–188. [Google Scholar] [CrossRef]
- Austad, S.N.; Fischer, K.E. Sex Differences in Lifespan. Cell Metab. 2016, 23, 1022–1033. [Google Scholar] [CrossRef]
- Bruls, Y.M.; de Ligt, M.; Lindeboom, L.; Phielix, E.; Havekes, B.; Schaart, G.; Kornips, E.; Wildberger, J.E.; Hesselink, M.K.; Muoio, D.; et al. Carnitine supplementation improves metabolic flexibility and skeletal muscle acetylcarnitine formation in volunteers with impaired glucose tolerance: A randomised controlled trial. EBioMedicine 2019, 49, 318–330. [Google Scholar] [CrossRef]
- Zhang, J.; Light, A.R.; Hoppel, C.L.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Hughen, R.W.; Keim, N.L.; et al. Acylcarnitines as markers of exercise-associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp. Physiol. 2017, 102, 48–69. [Google Scholar] [CrossRef]
- Holloway, G.P.; Bezaire, V.; Heigenhauser, G.J.; Tandon, N.N.; Glatz, J.F.; Luiken, J.J.; Bonen, A.; Spriet, L.L. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J. Physiol. 2006, 571, 201–210. [Google Scholar] [CrossRef]
- Bezaire, V.; Bruce, C.R.; Heigenhauser, G.J.; Tandon, N.N.; Glatz, J.F.; Luiken, J.J.; Bonen, A.; Spriet, L.L. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: Essential role in fatty acid oxidation. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E509–E515. [Google Scholar] [CrossRef]
- Kiens, B.; Roepstorff, C.; Glatz, J.F.; Bonen, A.; Schjerling, P.; Knudsen, J.; Nielsen, J.N. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: Influence of physical activity and gender. J. Appl. Physiol. 2004, 97, 1209–1218. [Google Scholar] [CrossRef]
- Maher, A.C.; Akhtar, M.; Vockley, J.; Tarnopolsky, M.A. Women have higher protein content of beta-oxidation enzymes in skeletal muscle than men. PLoS ONE 2010, 5, e12025. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J. How many repeated measures in repeated measures designs? Statistical issues for comparative trials. BMC Med. Res. Methodol. 2003, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Kim, B.S.; Tsui, K. Linear mixed effects models for feature selection in high dimensional NMR spectra. Exprt Syst. Applic. 2009, 36, 4703–4708. [Google Scholar] [CrossRef]
- Ernest, B.; Gooding, J.R.; Campagna, S.R.; Saxton, A.M.; Voy, B.H. MetabR: An R script for linear model analysis of quantitative metabolomic data. BMC Res. Notes 2012, 5, 596. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Cheng, J.B.; Motola, D.L.; Mangelsdorf, D.J.; Russell, D.W. De-orphanization of cytochrome P450 2R1: A microsomal vitamin D 25-hydroxilase. J. Biol. Chem. 2003, 278, 38084–38093. [Google Scholar] [CrossRef]
- Yamasaki, T.; Izumi, S.; Ide, H.; Ohyama, Y. Identification of a novel rat microsomal vitamin D3 25-hydroxylase. J. Biol. Chem. 2004, 279, 22848–22856. [Google Scholar] [CrossRef]
- Seamans, K.M.; Cashman, K.D. Existing and potentially novel functional markers of vitamin D status: A systematic review. Am. J. Clin. Nutr. 2009, 89, 1997S–2008S. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. Using MetaboAnalyst 4.0 for Metabolomics Data Analysis, Interpretation, and Integration with Other Omics Data. Methods Mol. Biol. 2020, 2104, 337–360. [Google Scholar] [CrossRef]
- Henglin, M.; Niiranen, T.; Watrous, J.D.; Lagerborg, K.A.; Antonelli, J.; Claggett, B.L.; Demosthenes, E.J.; von Jeinsen, B.; Demler, O.; Vasan, R.S.; et al. A Single Visualization Technique for Displaying Multiple Metabolite-Phenotype Associations. Metabolites 2019, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Dustin, T.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. mediation: R package for causal mediation analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar]
- Pietzner, M.; Budde, K.; Homuth, G.; Kastenmuller, G.; Henning, A.K.; Artati, A.; Krumsiek, J.; Volzke, H.; Adamski, J.; Lerch, M.M.; et al. Hepatic Steatosis Is Associated with Adverse Molecular Signatures in Subjects Without Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3856–3868. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).