Lipidomic Profiling Identifies Serum Lipids Associated with Persistent Multisite Musculoskeletal Pain
Abstract
1. Introduction
2. Results
2.1. Lipid Markers and MSMP
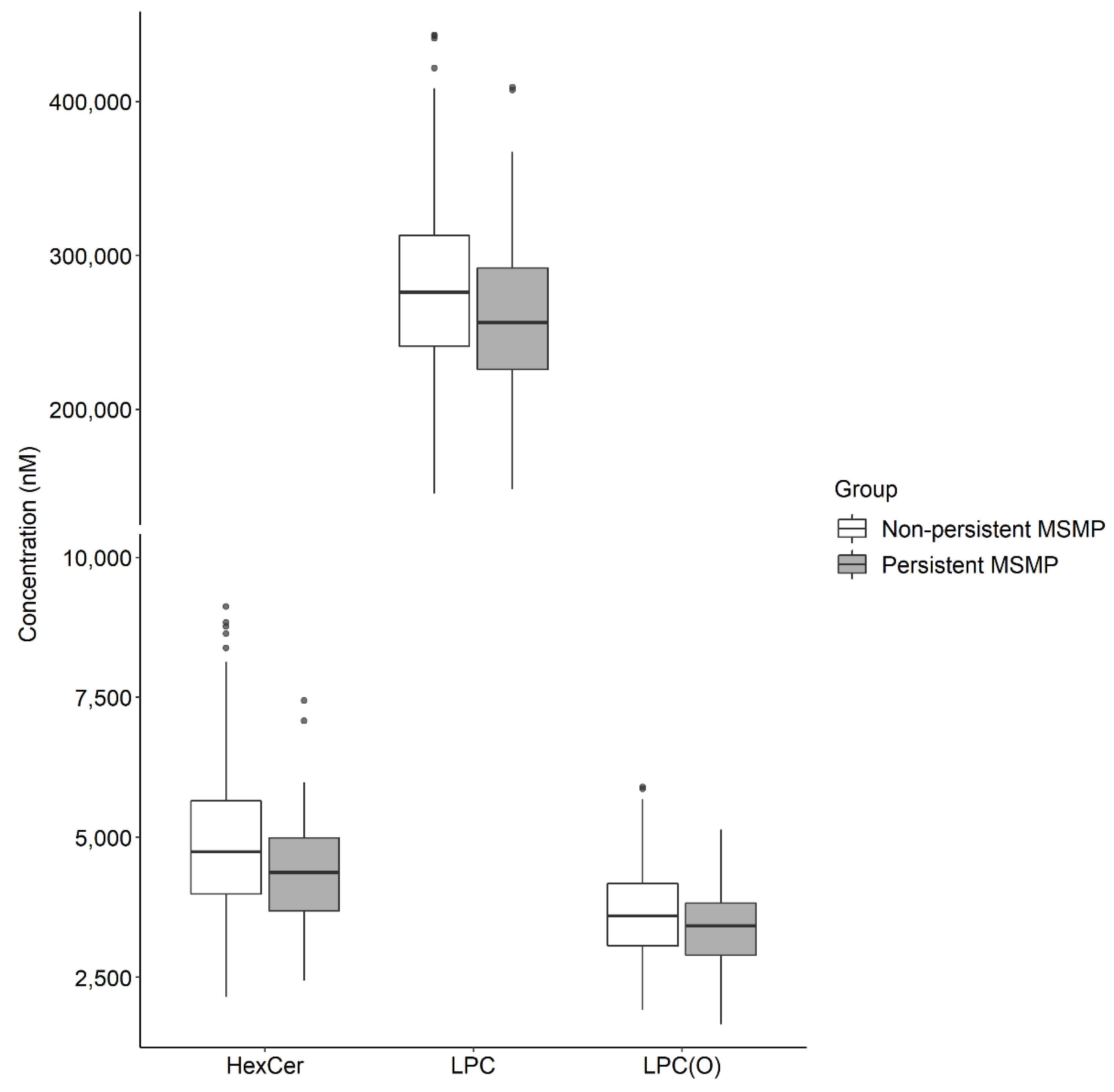
2.2. Lipid Markers for Persistent MSMP
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Demographic and Medical Information Collection
4.3. MSMP Assessment
4.4. Lipidomic Profiling
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blyth, F.M.; Noguchi, N. Chronic musculoskeletal pain and its impact on older people. Best Pract. Res. Clin. Rheumatol. 2017, 31, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Blyth, F.M.; Briggs, A.M.; Schneider, C.H.; Hoy, D.G.; March, L.M. The Global Burden of Musculoskeletal Pain-Where to From Here? Am. J. Public Health 2019, 109, 35–40. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Dargan, P.; Lanas, A.; Wiffen, P. The burden of musculoskeletal pain and the role of topical non-steroidal anti-inflammatory drugs (NSAIDs) in its treatment. Ten underpinning statements from a global pain faculty. Curr. Med Res. Opin. 2021, 37, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Carnes, D.; Parsons, S.; Ashby, D.; Breen, A.; Foster, N.E.; Pincus, T.; Vogel, S.; Underwood, M. Chronic musculoskeletal pain rarely presents in a single body site: Results from a UK population study. Rheumatology 2007, 46, 1168–1170. [Google Scholar] [CrossRef]
- Lacey, R.J.; Belcher, J.; Rathod, T.; Wilkie, R.; Thomas, E.; McBeth, J. Pain at multiple body sites and health-related quality of life in older adults: Results from the North Staffordshire Osteoarthritis Project. Rheumatology 2014, 53, 2071–2079. [Google Scholar] [CrossRef]
- Pan, F.; Tian, J.; Cicuttini, F.; Jones, G. Sleep Disturbance and Its Association with Pain Severity and Multisite Pain: A Prospective 10.7-Year Study. Pain Ther. 2020, 9, 751–763. [Google Scholar] [CrossRef]
- Pan, F.; Tian, J.; Aitken, D.; Cicuttini, F.; Jones, G. Pain at multiple sites is associated with prevalent and incident fractures in older adults. J. Bone Miner. Res. 2019, 34, 2012–2018. [Google Scholar] [CrossRef]
- Welsh, V.K.; Clarson, L.E.; Mallen, C.D.; McBeth, J. Multisite pain and self-reported falls in older people: Systematic review and meta-analysis. Arthritis Res. Ther. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Generaal, E.; Vogelzangs, N.; Penninx, B.W.J.H.; Dekker, J. Insomnia, Sleep Duration, Depressive Symptoms, and the Onset of Chronic Multisite Musculoskeletal Pain. Sleep 2016, 40, 1–10. [Google Scholar]
- Aroke, E.N.; Powell-Roach, K.L. The Metabolomics of Chronic Pain Conditions: A Systematic Review. Biol. Res. Nurs. 2020, 22, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, K.V.; Aykas, D.P.; Sigurdson, G.T.; Plans, M.; Madiai, F.; Yu, L.; Buffington, C.A.T.; Giusti, M.M.; Rodriguez-Saona, L. Metabolic fingerprinting for diagnosis of fibromyalgia and other rheumatologic disorders. J. Biol. Chem. 2019, 294, 2555–2568. [Google Scholar] [CrossRef] [PubMed]
- Stockstill, K.; Doyle, T.M.; Yan, X.; Chen, Z.; Janes, K.; Little, J.W.; Braden, K.; Lauro, F.; Giancotti, L.A.; Harada, C.M.; et al. Dysregulation of sphingolipid metabolism contributes to bortezomib-induced neuropathic pain. J. Exp. Med. 2018, 215, 1301–1313. [Google Scholar] [CrossRef]
- Kaluarachchi, M.; Lewis, M.R.; Lindon, J.C. Standardized Protocols for MS-Based Metabolic Phenotyping. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Oxford, UK, 2017; pp. 224–231. [Google Scholar]
- Teckchandani, S.; Nagana Gowda, G.A.; Raftery, D.; Curatolo, M. Metabolomics in chronic pain research. Eur. J. Pain 2021, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D.; Sasso, O. Peripheral gating of pain signals by endogenous lipid mediators. Nat. Neurosci. 2014, 17, 164–174. [Google Scholar] [CrossRef]
- Ueda, H. Pathogenic mechanisms of lipid mediator lysophosphatidic acid in chronic pain. Prog. Lipid Res. 2021, 81, 101079. [Google Scholar] [CrossRef] [PubMed]
- Osthues, T.; Sisignano, M. Oxidized Lipids in Persistent Pain States. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Piomelli, D.; Hohmann, A.G.; Seybold, V.; Hammock, B.D. A Lipid Gate for the Peripheral Control of Pain. J. Neurosci. 2014, 34, 15184–15191. [Google Scholar] [CrossRef]
- Pousinis, P.; Gowler, P.R.W.; Burston, J.J.; Ortori, C.A.; Chapman, V.; Barrett, D.A. Lipidomic identification of plasma lipids associated with pain behaviour and pathology in a mouse model of osteoarthritis. Metabolomics 2020, 16, 32. [Google Scholar] [CrossRef]
- Pan, F.; Liu, M.; Randell, E.W.; Rahman, P.; Jones, G.; Zhai, G. Sphingomyelin is involved in multisite musculoskeletal pain: Evidence from metabolomic analysis in 2 independent cohorts. Pain 2021, 162, 1876–1881. [Google Scholar] [CrossRef]
- Liu, M.; Xie, Z.; Costello, C.A.; Zhang, W.; Chen, L.; Qi, D.; Furey, A.; Randell, E.W.; Rahman, P.; Zhai, G. Metabolomic analysis coupled with extreme phenotype sampling identified that lysophosphatidylcholines are associated with multisite musculoskeletal pain. Pain 2021, 162, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Macgregor, A.J.; Gieger, C.; Malkin, I.; Moayyeri, A.; Grallert, H.; Emeny, R.T.; Spector, T.; Kastenmüller, G.; Williams, F.M.K. An omics investigation into chronic widespread musculoskeletal pain reveals epiandrosterone sulfate as a potential biomarker. Pain 2015, 156, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Liori, B.; Kumar, A.; Santoru, M.L.; Asthana, S.; Pieroni, E.; Fais, A.; Era, B.; Cacace, E.; Ruggiero, V.; et al. Metabolomics Analysis and Modeling Suggest a Lysophosphocholines-PAF Receptor Interaction in Fibromyalgia. PLoS ONE 2014, 9, e107626. [Google Scholar] [CrossRef] [PubMed]
- Grenald, S.A.; Doyle, T.M.; Zhang, H.; Slosky, L.M.; Chen, Z.; Largent-Milnes, T.M.; Spiegel, S.; Vanderah, T.W.; Salvemini, D. Targeting the S1P/S1PR1 axis mitigates cancer-induced bone pain and neuroinflammation. Pain 2017, 158, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Watanabe, S.; Oyama, M.; Iwai, T.; Tanabe, M. Glycosphingolipid Biosynthesis Pathway in the Spinal Cord and Dorsal Root Ganglia During Inflammatory Pain: Early and Late Changes in Expression Patterns of Glycosyltransferase Genes. Neuroscience 2020, 428, 217–227. [Google Scholar] [CrossRef]
- Farwanah, H.; Kolter, T. Lipidomics of Glycosphingolipids. Metabolites 2012, 2, 134–164. [Google Scholar] [CrossRef]
- Jatooratthawichot, P.; Talabnin, C.; Ngiwsara, L.; Rustam, Y.H.; Svasti, J.; Reid, G.E.; Ketudat Cairns, J.R. Effect of Expression of Human Glucosylceramidase 2 Isoforms on Lipid Profiles in COS-7 Cells. Metabolites 2020, 10, 488. [Google Scholar] [CrossRef]
- Sántha, P.; Dobos, I.; Kis, G.; Jancsó, G. Role of Gangliosides in Peripheral Pain Mechanisms. Int. J. Mol. Sci. 2020, 21, 1005. [Google Scholar] [CrossRef]
- Salvemini, D.; Doyle, T.; Kress, M.; Nicol, G. Therapeutic targeting of the ceramide-to-sphingosine 1-phosphate pathway in pain. Trends Pharmacol. Sci. 2013, 34, 110–118. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids1 J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Squillace, S.; Spiegel, S.; Salvemini, D. Targeting the sphingosine-1-phosphate axis for developing non-narcotic pain therapeutics. Trends Pharmacol. Sci. 2020, 41, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-H.; Lee, C.-H.; Tsai, M.-H.; Chen, C.-H.; Lin, H.-F.; Hsu, C.-Y.; Lai, C.-L.; Chen, C.-C. Activation of acid-sensing ion channel 3 by lysophosphatidylcholine 16:0 mediates psychological stress-induced fibromyalgia-like pain. Ann. Rheum. Dis. 2020, 79, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Rimola, V.; Hahnefeld, L.; Zhao, J.; Jiang, C.; Angioni, C.; Schreiber, Y.; Osthues, T.; Pierre, S.; Geisslinger, G.; Ji, R.R. Lysophospholipids Contribute to Oxaliplatin-Induced Acute Peripheral Pain. J. Neurosci. 2020, 40, 9519–9532. [Google Scholar] [CrossRef] [PubMed]
- Tigyi, G. Lipids: LPA activates TRPV1--and it hurts. Nat. Chem. Biol. 2011, 8, 22–23. [Google Scholar] [CrossRef][Green Version]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Tsai, Y.-J.; Chen, S.-H.; Lin, C.-T.; Lue, J.-H. Lysophosphatidylcholine causes neuropathic pain via the increase of neuronal nitric oxide synthase in the dorsal root ganglion and cuneate nucleus. Pharmacol. Biochem. Behav. 2013, 106, 47–56. [Google Scholar] [CrossRef]
- Kuwajima, K.; Sumitani, M.; Kurano, M.; Kano, K.; Nishikawa, M.; Uranbileg, B.; Tsuchida, R.; Ogata, T.; Aoki, J.; Yatomi, Y. Lysophosphatidic acid is associated with neuropathic pain intensity in humans: An exploratory study. PLoS ONE 2018, 13, e0207310. [Google Scholar] [CrossRef]
- Langeslag, M.; Kress, M. The ceramide-S1P pathway as a druggable target to alleviate peripheral neuropathic pain. Expert Opin. Ther. Targets 2020, 24, 869–884. [Google Scholar] [CrossRef]
- Oude Elferink, R.P.J.; Bolier, R.; Beuers, U.H. Lysophosphatidic acid and signaling in sensory neurons. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2015, 1851, 61–65. [Google Scholar] [CrossRef]
- O’Brien, M.S.; Philpott, H.T.A.; McDougall, J.J. Targeting the Nav1.8 ion channel engenders sex-specific responses in lysophosphatidic acid–induced joint neuropathy. Pain 2019, 160, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, H.; von Hegedus, J.; Toes, R.; Kloppenburg, M.; Ioan-Facsinay, A. Lipid mediators of inflammation in rheumatoid arthritis and osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 741–755. [Google Scholar] [CrossRef]
- Tootsi, K.; Vilba, K.; Märtson, A.; Kals, J.; Paapstel, K.; Zilmer, M. Metabolomic Signature of Amino Acids, Biogenic Amines and Lipids in Blood Serum of Patients with Severe Osteoarthritis. Metabolites 2020, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, G.; Aitken, D.; Likhodii, S.; Liu, M.; Martin, G.; Furey, A.; Randell, E.; Rahman, P.; Jones, G.; et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology 2016, 55, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Hauser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Gureje, O.; Von Korff, M.; Simon, G.E.; Gater, R. Persistent pain and well-being: A World Health Organization Study in Primary Care. Jama 1998, 280, 147–151. [Google Scholar] [CrossRef]
- Beyene, H.B.; Olshansky, G.; Smith, A.A.T.; Giles, C.; Huynh, K.; Cinel, M.; Mellett, N.A.; Cadby, G.; Hung, J.; Hui, J.; et al. High-coverage plasma lipidomics reveals novel sex-specific lipidomic fingerprints of age and BMI: Evidence from two large population cohort studies. PLoS Biol. 2020, 18, e3000870. [Google Scholar]
- Pan, F.; Byrne, K.S.; Ramakrishnan, R.; Ferreira, M.; Dwyer, T.; Jones, G. Association between musculoskeletal pain at multiple sites and objectively measured physical activity and work capacity: Results from UK Biobank study. J. Sci. Med. Sport 2019, 22, 444–449. [Google Scholar] [CrossRef]
- Pan, F.; Laslett, L.; Blizzard, L.; Cicuttini, F.; Winzenberg, T.; Ding, C.; Jones, G. Associations Between Fat Mass and Multisite Pain: A Five-Year Longitudinal Study. Arthritis Care Res. 2017, 69, 509–516. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef]
- Zhai, G. Alteration of Metabolic Pathways in Osteoarthritis. Metabolites 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Miltenberger-Miltenyi, G.; Cruz-Machado, A.R.; Saville, J.; Conceição, V.A.; Calado, Â.; Lopes, I.; Fuller, M.; Fonseca, J.E. Increased monohexosylceramide levels in the serum of established rheumatoid arthritis patients. Rheumatology 2019, 59, 2085–2089. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Han, F.; Gong, L.; Lv, Y.; Wan, Z.; Liu, H.; Zhang, D.; Jia, Y.; Yang, S.; Ren, L.; et al. Association between chronic obstructive pulmonary disease and serum lipid levels: A meta-analysis. Lipids Health Dis. 2018, 17, 263. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.K.; Toth, P.P. Trends in Lipids, Obesity, Metabolic Syndrome, and Diabetes Mellitus in the United States: An NHANES Analysis (2003–2004 to 2013–2014). Obesity 2019, 27, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]

| Total (n = 530) | Persistent MSMP (n = 112) | Non-Persistent MSMP (n = 418) | p Value | |
|---|---|---|---|---|
| Age (years) | 61.54 ± 6.57 | 61.71 ± 6.65 | 61.49 ± 6.56 | 0.80 |
| BMI (kg/m2) | 27.73 ± 4.54 | 28.13 ± 4.73 | 27.62 ± 4.48 | 0.31 |
| Females (%) | 50 | 67 | 46 | <0.001 |
| Physical activity (steps per day) | 7865.21 ± 3253.78 | 7234.72 ± 3009.63 | 8034.60 ± 3299.35 | 0.04 |
| Comorbidities (%) | 40 | 70 | 31 | <0.001 |
| OA (%) | 37 | 65 | 30 | <0.001 |
| RA (%) | 3 | 5 | 3 | 0.23 |
| Emphysema (%) | 3 | 7 | 2 | 0.01 |
| Diabetes (%) | 2 | 4 | 1 | 0.23 |
| Univariable | Multivariable * | |||||||
|---|---|---|---|---|---|---|---|---|
| p Value | OR | 2.5% CI | 97.5% CI | p Value | OR | 2.5% CI | 97.5% CI | |
| Lipid species | ||||||||
| SM(38:3) (a) | 3.30 × 10−4 | 4.45 | 1.99 | 10.19 | 6.12 × 10−2 | 2.61 | 0.96 | 7.23 |
| HexCer(d18:1/24:0) | 3.60 × 10−3 | 0.38 | 0.20 | 0.73 | 7.50 × 10−2 | 0.51 | 0.25 | 1.07 |
| SM(40:4) | 3.91 × 10−3 | 4.79 | 1.67 | 14.04 | 1.59 × 10−1 | 2.55 | 0.70 | 9.44 |
| AC(26:0) | 5.27 × 10−3 | 0.42 | 0.23 | 0.77 | 6.84 × 10−1 | 0.86 | 0.41 | 1.80 |
| Lipid class | ||||||||
| HexCer | 2.21 × 10−2 | 0.44 | 0.21 | 0.88 | 1.21 × 10−1 | 0.53 | 0.24 | 1.18 |
| Univariable | Multivariable * | |||||||
|---|---|---|---|---|---|---|---|---|
| p Value | OR | 2.5% CI | 97.5% CI | p Value | OR | 2.5% CI | 97.5% CI | |
| HexCer(d18:1/22:0) | 3.46 × 10−4 | 0.26 | 0.12 | 0.54 | 7.71 × 10−3 | 0.33 | 0.14 | 0.74 |
| HexCer(d18:1/24:0) | 4.55 × 10−5 | 0.20 | 0.09 | 0.43 | 2.15 × 10−3 | 0.25 | 0.10 | 0.60 |
| LPC(18:1) [sn1] | 6.60 × 10−4 | 0.23 | 0.09 | 0.53 | 1.46 × 10−2 | 0.31 | 0.12 | 0.78 |
| LPC(15-MHDA) [sn1] [104_sn1] | 6.48 × 10−4 | 0.32 | 0.16 | 0.61 | 7.95 × 10−3 | 0.36 | 0.17 | 0.76 |
| LPC(18:2) [sn1] | 6.53 × 10−4 | 0.28 | 0.13 | 0.58 | 4.70 × 10−2 | 0.41 | 0.17 | 0.98 |
| LPC(18:2) [sn2] | 4.06 × 10−4 | 0.21 | 0.09 | 0.50 | 1.91 × 10−2 | 0.30 | 0.11 | 0.82 |
| AC(26:0) | 2.19 × 10−4 | 0.25 | 0.12 | 0.52 | 5.01 × 10−2 | 0.41 | 0.17 | 0.99 |
| Univariable | Multivariable * | |||||||
|---|---|---|---|---|---|---|---|---|
| p Value | OR | 2.5% CI | 97.5% CI | p Value | OR | 2.5% CI | 97.5% CI | |
| HexCer | 2.23 × 10−4 | 0.20 | 0.08 | 0.46 | 2.02 × 10−3 | 0.22 | 0.08 | 0.57 |
| LPC | 1.02 × 10−3 | 0.18 | 0.06 | 0.49 | 2.50 × 10−2 | 0.27 | 0.08 | 0.84 |
| LPC(O) | 2.35 × 10−3 | 0.22 | 0.08 | 0.58 | 6.88 × 10−2 | 0.36 | 0.12 | 1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Liu, M.; Tian, J.; Zhai, G.; Cicuttini, F.; Schooneveldt, Y.L.; Meikle, P.J.; Jones, G.; Pan, F. Lipidomic Profiling Identifies Serum Lipids Associated with Persistent Multisite Musculoskeletal Pain. Metabolites 2022, 12, 206. https://doi.org/10.3390/metabo12030206
Ma C, Liu M, Tian J, Zhai G, Cicuttini F, Schooneveldt YL, Meikle PJ, Jones G, Pan F. Lipidomic Profiling Identifies Serum Lipids Associated with Persistent Multisite Musculoskeletal Pain. Metabolites. 2022; 12(3):206. https://doi.org/10.3390/metabo12030206
Chicago/Turabian StyleMa, Canchen, Ming Liu, Jing Tian, Guangju Zhai, Flavia Cicuttini, Yvette L. Schooneveldt, Peter J. Meikle, Graeme Jones, and Feng Pan. 2022. "Lipidomic Profiling Identifies Serum Lipids Associated with Persistent Multisite Musculoskeletal Pain" Metabolites 12, no. 3: 206. https://doi.org/10.3390/metabo12030206
APA StyleMa, C., Liu, M., Tian, J., Zhai, G., Cicuttini, F., Schooneveldt, Y. L., Meikle, P. J., Jones, G., & Pan, F. (2022). Lipidomic Profiling Identifies Serum Lipids Associated with Persistent Multisite Musculoskeletal Pain. Metabolites, 12(3), 206. https://doi.org/10.3390/metabo12030206







