Metabolomic Analysis Reveals the Effect of Insecticide Chlorpyrifos on Rice Plant Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Preparation and Treatment
2.3. Physiological Activities
2.4. Metabolomic Analysis
3. Results
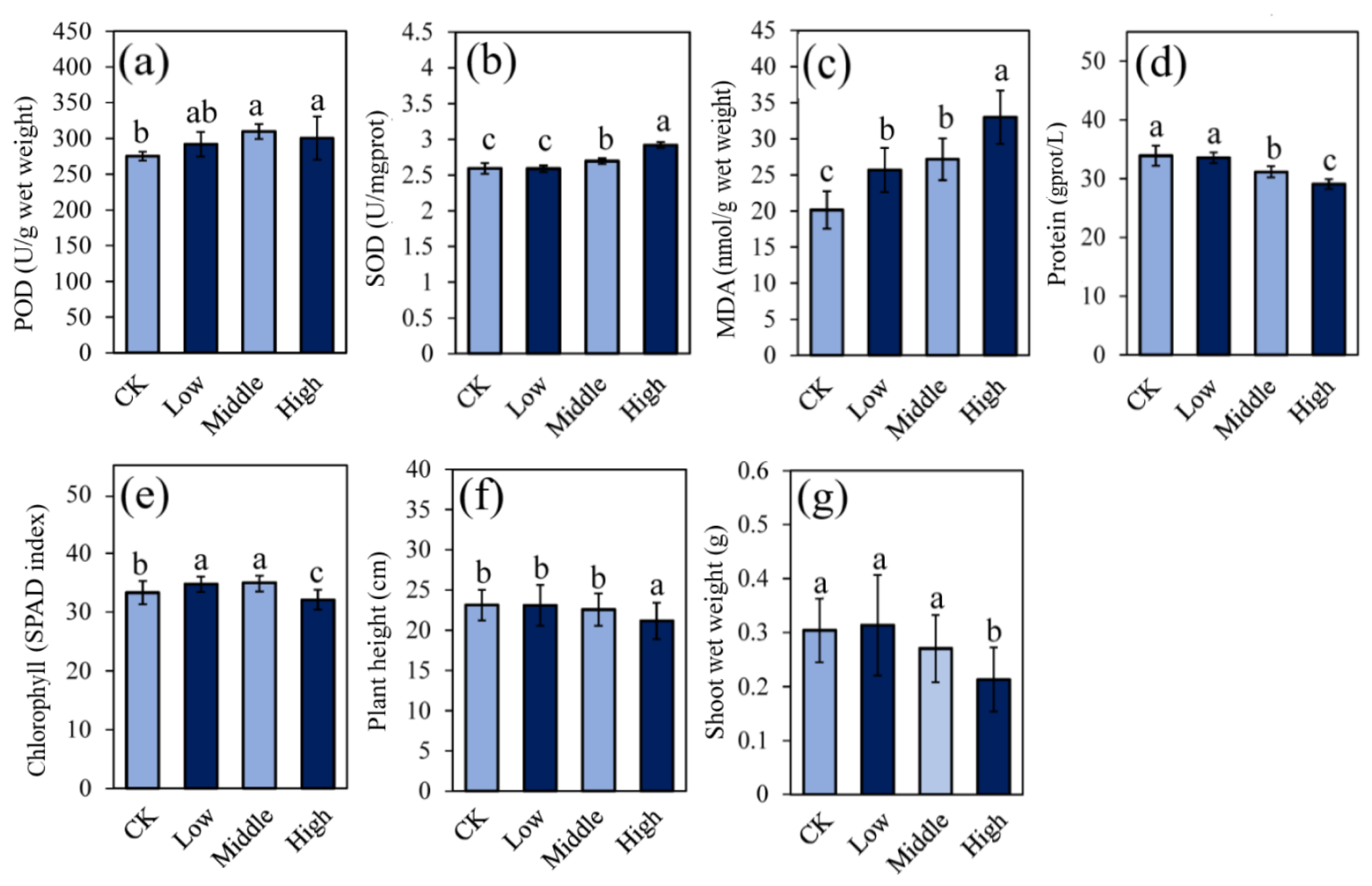
3.1. Plant Growth and Physiological Activities
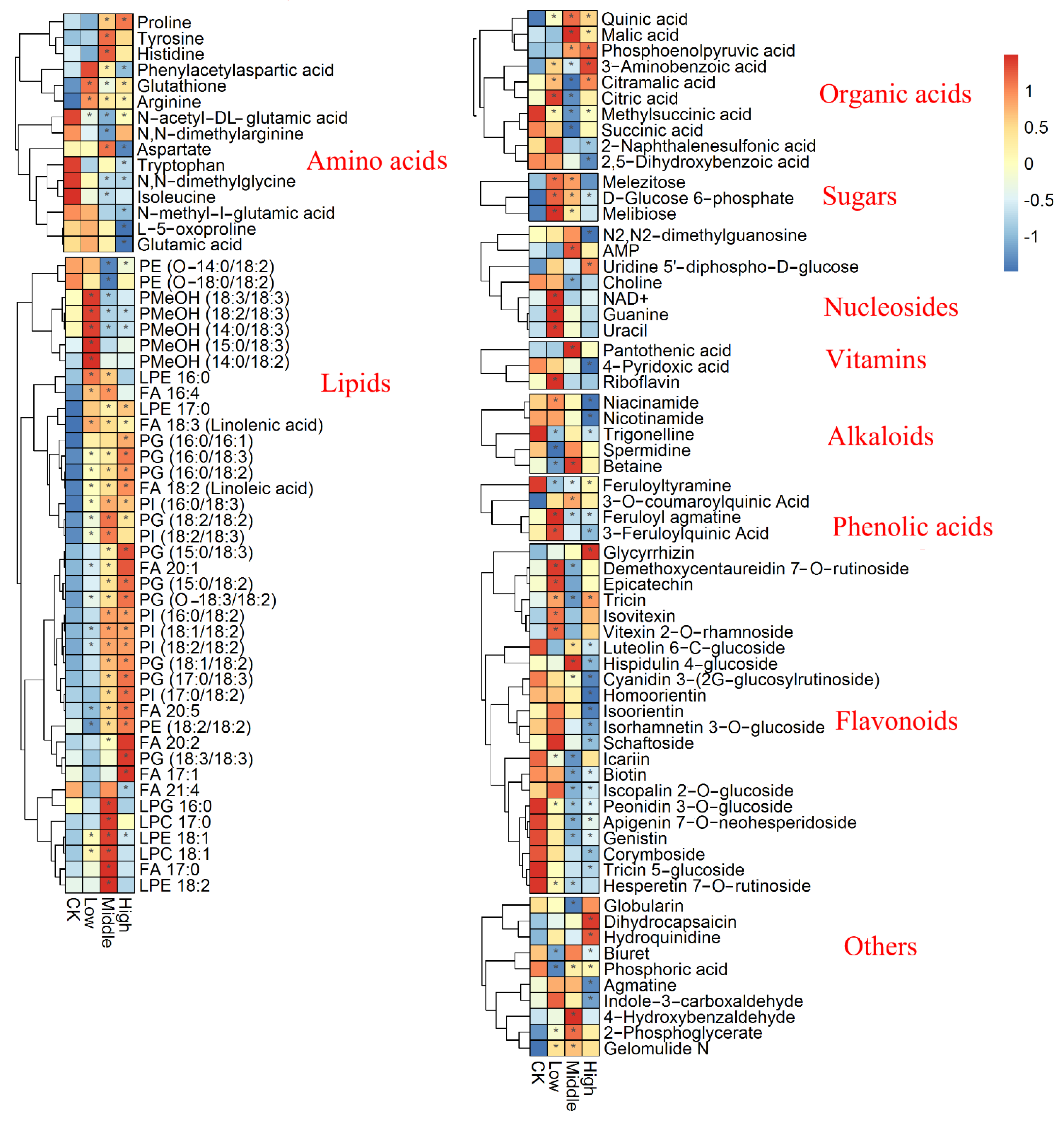
3.2. Metabolic Profiling Analysis
3.3. Metabolic Pathways Analysis
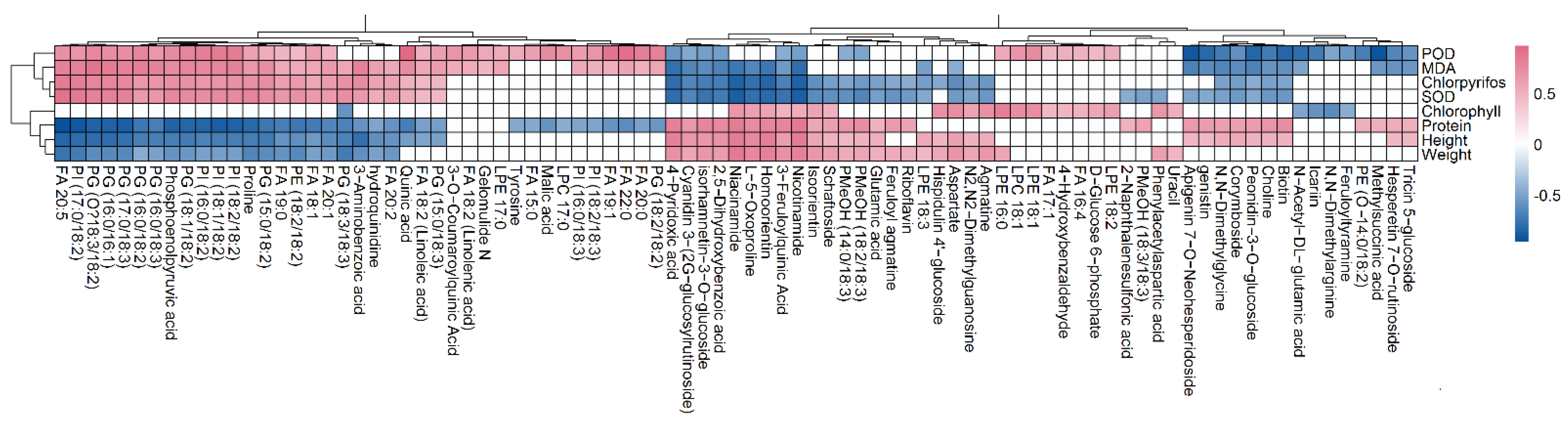
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, H.-Q.; Zhou, H.; Miao, S.; Guo, D.-A.; Zhang, X.-L.; Hu, Q.; Mao, X.-H.; Ji, S. Plant metabolomics for studying the effect of two insecticides on comprehensive constituents of Lonicerae Japonicae Flos. Chin. J. Nat. Med. 2021, 19, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Hageman, K.J.; Taylor, M.; Michelsen-Heath, S.; Stewart, I. Fate of the organophosphate insecticide, chlorpyrifos, in leaves, soil, and air following application. Chemosphere 2020, 243, 125194. [Google Scholar] [CrossRef]
- Agarwal, T.; Khillare, P.S.; Shridhar, V.; Ray, S. Pattern, sources and toxic potential of PAHs in the agricultural soils of Delhi, India. J. Hazard. Mater. 2009, 163, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kumar, P.S.; Vo, D.N. A review on the microbial degradation of chlorpyrifos and its metabolite TCP. Chemosphere 2021, 283, 131447. [Google Scholar] [CrossRef] [PubMed]
- Ahir, U.N.; Vyas, T.K.; Gandhi, K.D.; Faldu, P.R.; Patel, K.G. In Vitro Efficacy for Chlorpyrifos Degradation by Novel Isolate Tistrella sp. AUC10 Isolated from Chlorpyrifos Contaminated Field. Curr. Microbiol. 2020, 77, 2226–2232. [Google Scholar] [CrossRef]
- Pope, C.; Karanth, S.; Liu, J. Pharmacology and toxicology of cholinesterase inhibitors: Uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 2005, 19, 433–446. [Google Scholar] [CrossRef]
- John, E.M.; Shaike, J.M. Chlorpyrifos: Pollution and remediation. Environ. Chem. Lett. 2015, 13, 269–291. [Google Scholar] [CrossRef]
- Das, S.; Adhya, T.K. Degradation of chlorpyrifos in tropical rice soils. J. Environ. Manag. 2015, 152, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.K.; Shao, J.; Zhu, B.; Chen, M.; Xia, Y.; Kaciroti, N.; Lozoff, B.; Meeker, J.D. Prenatal naled and chlorpyrifos exposure is associated with deficits in infant motor function in a cohort of Chinese infants. Environ. Int. 2017, 106, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yang, Z.; Liu, J.; Liao, Q.; Ling, W.; Waigi, M.G.; Odinga, E.S. Chlorpyrifos inhibits nitrogen fixation in rice-vegetated soil containing Pseudomonas stutzeri A1501. Chemosphere 2020, 256, 127098. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, R.; Sharma, S. Effects of chemical and biological pesticides on plant growth parameters and rhizospheric bacterial community structure in Vigna radiata. J. Hazard. Mater. 2015, 291, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, S.; Sun, L.-N.; Wang, H.; Bao, T.; Adeel, M. Identification of root exudates from the Pb-accumulator Sedum alfredii under Pb stresses and assessment of their roles. J. Plant Interact. 2017, 12, 272–278. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Q.; Miao, X.; Fan, T.; Meng, Z.; Chen, X.; Zhu, W. Study on toxicity effects of environmental pollutants based on metabolomics: A review. Chemosphere 2022, 286, 131815. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Adeleye, A.S.; Zhao, L.; Minakova, A.S.; Anumol, T.; Keller, A.A. Antioxidant response of cucumber (Cucumis sativus) exposed to nano copper pesticide: Quantitative determination via LC-MS/MS. Food Chem. 2019, 270, 47–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, L.; Liu, L.; Cao, X.; Sun, C.; Lin, X. Metabolic disturbance in lettuce (Lactuca sativa) plants triggered by imidacloprid and fenvalerate. Sci. Total. Environ. 2022, 802, 149764. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, L. Metabolomic and Transcriptomic Investigation of Metabolic Perturbations in Oryza sativa L. Triggered by Three Pesticides. Environ. Sci. Technol. 2020, 54, 6115–6124. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Li, Y.; Yu, X.; Nie, J. Effect of neonicotinoid dinotefuran on root exudates of Brassica rapa var. chinensis. Chemosphere 2021, 266, 129020. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, H. Metabolism and detoxification of pesticides in plants. Sci. Total. Environ. 2021, 790, 148034. [Google Scholar] [CrossRef]
- Shakir, S.K.; Irfan, S.; Akhtar, B.; Rehman, S.U.; Daud, M.K.; Taimur, N.; Azizullah, A. Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings. Ecotoxicology 2018, 27, 919–935. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, G. Signal Function Studies of ROS, Especially RBOH-Dependent ROS, in Plant Growth, Development and Environmental Stress. J. Plant Growth Regul. 2019, 39, 157–171. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, N.; Lu, H.; Zhu, L. Disturbed phospholipid metabolism by three polycyclic aromatic hydrocarbons in Oryza sativa. Environ. Pollut. 2021, 283, 117073. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Qi, Y.; Song, W.; Xu, H. Effects of di-n-butyl phthalate and di (2-ethylhexyl) phthalate on the growth, photosynthesis, and chlorophyll fluorescence of wheat seedlings. Chemosphere 2016, 151, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.Z.; Zhang, F.; Wu, Z.L.; Yu, Z.Y.; Wu, G. Chlorpyrifos-induced hormesis in insecticide-resistant and -susceptible Plutella xylostella under normal and high temperatures. Bull. Entomol. Res. 2016, 106, 378–386. [Google Scholar] [CrossRef]
- Sáenz, M.E.; Marzio, W.D.D.; Alberdi, J.L. Assessment of Cyfluthrin commercial formulation on growth, photosynthesis and catalase activity of green algae. Pestic. Biochem. Physiol. 2012, 104, 50–57. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Zhao, X.; Zhao, S.; Gu, X.; Du, W.; Wei, H.; Ji, R.; Zhao, L. Metabolomics Reveals the “Invisible” Responses of Spinach Plants Exposed to CeO2 Nanoparticles. Environ. Sci. Technol. 2019, 53, 6007–6017. [Google Scholar] [CrossRef]
- Less, H.; Galili, G. Principal transcriptional programs regulating plant amino acid metabolism in response to abiotic stresses. Plant Physiol. 2008, 147, 316–330. [Google Scholar] [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione Metabolism in Plants under Stress: Beyond Reactive Oxygen Species Detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Xie, J.; Gan, Y.; Coulter, J.A.; Yu, J.; Li, J.; Wang, J.; Zhang, X. Nitrogen Source Affects the Composition of Metabolites in Pepper (Capsicum annuum L.) and Regulates the Synthesis of Capsaicinoids through the GOGAT-GS Pathway. Foods 2020, 9, 150. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, P.; Zhao, X.; Ji, R.; Zhao, L. Physiological and metabolic responses of maize (Zea mays) plants to Fe3O4 nanoparticles. Sci. Total Environ. 2020, 718, 137400. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Editorial: Amino Acids in Plants: Regulation and Functions in Development and Stress Defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef] [PubMed]
- Ragaey, M.M.; Sadak, M.S.; Dawood, M.F.A.; Mousa, N.H.S.; Hanafy, R.S.; Latef, A. Role of Signaling Molecules Sodium Nitroprusside and Arginine in Alleviating Salt-Induced Oxidative Stress in Wheat. Plants 2022, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Kallscheuer, N.; Vogt, M.; Marienhagen, J. A Novel Synthetic Pathway Enables Microbial Production of Polyphenols Independent from the Endogenous Aromatic Amino Acid Metabolism. ACS Synth. Biol. 2017, 6, 410–415. [Google Scholar] [CrossRef]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef]
- Li, P.; Li, J. Perfluorooctanoic acid (PFOA) caused oxidative stress and metabolic disorders in lettuce (Lactuca sativa) root. Sci. Total Environ. 2021, 770, 144726. [Google Scholar] [CrossRef]
- Zhang, Y.; Swart, C.; Alseekh, S.; Scossa, F.; Jiang, L.; Obata, T.; Graf, A.; Fernie, A.R. The Extra-Pathway Interactome of the TCA Cycle: Expected and Unexpected Metabolic Interactions. Plant Physiol. 2018, 177, 966–979. [Google Scholar] [CrossRef]
- Huang, S.; Lee, C.P.; Millar, A.H. Activity assay for plant mitochondrial enzymes. Methods Mol. Biol. 2015, 1305, 139–149. [Google Scholar] [CrossRef]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol. 2020, 225, 1166–1180. [Google Scholar] [CrossRef]
- Wang, M.; Gu, Z.; Wang, R.; Guo, J.; Ling, N.; Firbank, L.G.; Guo, S. Plant Primary Metabolism Regulated by Nitrogen Contributes to Plant-Pathogen Interactions. Plant Cell Physiol. 2019, 60, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Sumalan, R.L.; Croitor, L.; Petric, M.; Radulov, I.; Bourosh, P.; Sumalan, R.M.; Crisan, M. p-Aminobenzoate Organic Salts as Potential Plant Growth Regulators for Tomatoes. Molecules 2020, 25, 1635. [Google Scholar] [CrossRef] [PubMed]
- Marbois, B.; Xie, L.X.; Choi, S.; Hirano, K.; Hyman, K.; Clarke, C.F. para-Aminobenzoic acid is a precursor in coenzyme Q6 biosynthesis in Saccharomyces cerevisiae. J. Biol. Chem. 2010, 285, 27827–27838. [Google Scholar] [CrossRef] [PubMed]
- Naranjo Pinta, M.; Montoliu, I.; Aura, A.M.; Seppanen-Laakso, T.; Barron, D.; Moco, S. In Vitro Gut Metabolism of [U-(13) C]-Quinic Acid, The Other Hydrolysis Product of Chlorogenic Acid. Mol. Nutr. Food Res. 2018, 62, e1800396. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ishitsuka, Y.; Kadowaki, D.; Kuroda, M.; Tanaka, Y.; Nagatome, M.; Irikura, M.; Hirata, S.; Sato, K.; Maruyama, T.; et al. Phosphoenolpyruvic acid, an intermediary metabolite of glycolysis, as a potential cytoprotectant and anti-oxidant in HeLa cells. Biol. Pharm. Bull. 2012, 35, 606–611. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Chang, Y.; Lu, X.; Zhu, Z.; Xu, G. Alteration of leaf metabolism in Bt-transgenic rice (Oryza sativa L.) and its wild type under insecticide stress. J. Proteome. Res. 2012, 11, 4351–4360. [Google Scholar] [CrossRef]
- Macabuhay, A.; Arsova, B.; Walker, R.; Johnson, A.; Watt, M.; Roessner, U. Modulators or facilitators? Roles of lipids in plant root-microbe interactions. Trends Plant Sci. 2022, 27, 180–190. [Google Scholar] [CrossRef]
- Li, N.; Xu, C.; Li-Beisson, Y.; Philippar, K. Fatty Acid and Lipid Transport in Plant Cells. Trends Plant Sci. 2016, 21, 145–158. [Google Scholar] [CrossRef]
- Zhang, M.; Barg, R.; Yin, M.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef]
- Reszczynska, E.; Hanaka, A. Lipids Composition in Plant Membranes. Cell Biochem. Biophys 2020, 78, 401–414. [Google Scholar] [CrossRef]
- Tsukada, K.; Takahashi, K.; Nabeta, K. Biosynthesis of jasmonic acid in a plant pathogenic fungus, Lasiodiplodia theobromae. Phytochemistry 2010, 71, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Tan, X.; Liu, C.; Zeng, Y.; Li, Y. Effects of lead stress on rice (Oryza sativa L.) growth and metabolism in the rhizosphere microenvironment: The role of eicosanoid compounds. Plant Growth Regul. 2022, 96, 483–495. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Kofeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid. Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Murakami, M. Lipoquality control by phospholipase A2 enzymes. Proc. Jpn. Acad. Ser. B 2017, 93, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Filippou, P.; Antoniou, C.; Obata, T.; Van Der Kelen, K.; Harokopos, V.; Kanetis, L.; Aidinis, V.; Van Breusegem, F.; Fernie, A.R.; Fotopoulos, V. Kresoxim-methyl primes Medicago truncatula plants against abiotic stress factors via altered reactive oxygen and nitrogen species signalling leading to downstream transcriptional and metabolic readjustment. J. Exp. Bot. 2016, 67, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Li, Y.; Long, L.; Ge, J.; Li, H.; Zhang, M.; Wan, Q.; Yu, X. Effect of Imidacloprid Uptake from Contaminated Soils on Vegetable Growth. J. Agric. Food Chem. 2019, 67, 7232–7242. [Google Scholar] [CrossRef]
- Pereira, S.I.; Figueiredo, P.I.; Barros, A.S.; Dias, M.C.; Santos, C.; Duarte, I.F.; Gil, A.M. Changes in the metabolome of lettuce leaves due to exposure to mancozeb pesticide. Food Chem. 2014, 154, 291–298. [Google Scholar] [CrossRef]
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Q.; Zhang, M.; Li, Y.; Feng, F.; Yu, X.; Nie, J. Metabolomic Analysis Reveals the Effect of Insecticide Chlorpyrifos on Rice Plant Metabolism. Metabolites 2022, 12, 1289. https://doi.org/10.3390/metabo12121289
Mu Q, Zhang M, Li Y, Feng F, Yu X, Nie J. Metabolomic Analysis Reveals the Effect of Insecticide Chlorpyrifos on Rice Plant Metabolism. Metabolites. 2022; 12(12):1289. https://doi.org/10.3390/metabo12121289
Chicago/Turabian StyleMu, Qi’er, Mingxia Zhang, Yong Li, Fayun Feng, Xiangyang Yu, and Jinfang Nie. 2022. "Metabolomic Analysis Reveals the Effect of Insecticide Chlorpyrifos on Rice Plant Metabolism" Metabolites 12, no. 12: 1289. https://doi.org/10.3390/metabo12121289
APA StyleMu, Q., Zhang, M., Li, Y., Feng, F., Yu, X., & Nie, J. (2022). Metabolomic Analysis Reveals the Effect of Insecticide Chlorpyrifos on Rice Plant Metabolism. Metabolites, 12(12), 1289. https://doi.org/10.3390/metabo12121289






