Diet-Related and Gut-Derived Metabolites and Health Outcomes: A Scoping Review
Abstract
:1. Introduction
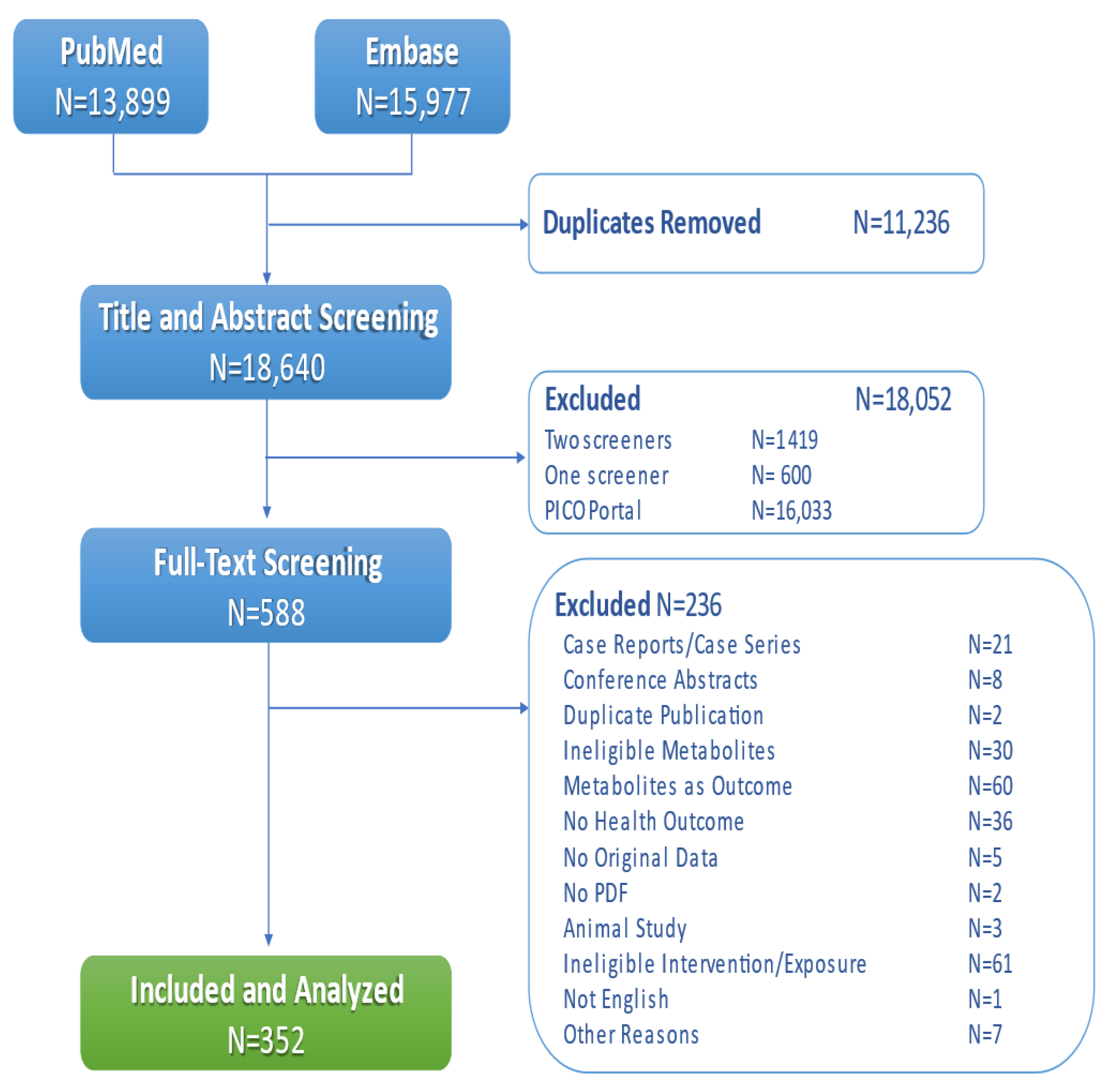
2. Materials and Methods
2.1. Scope of the Review
2.2. Literature Search
2.3. Data Abstraction and Analysis
3. Results
3.1. Study Characteristics
3.2. Metabolites
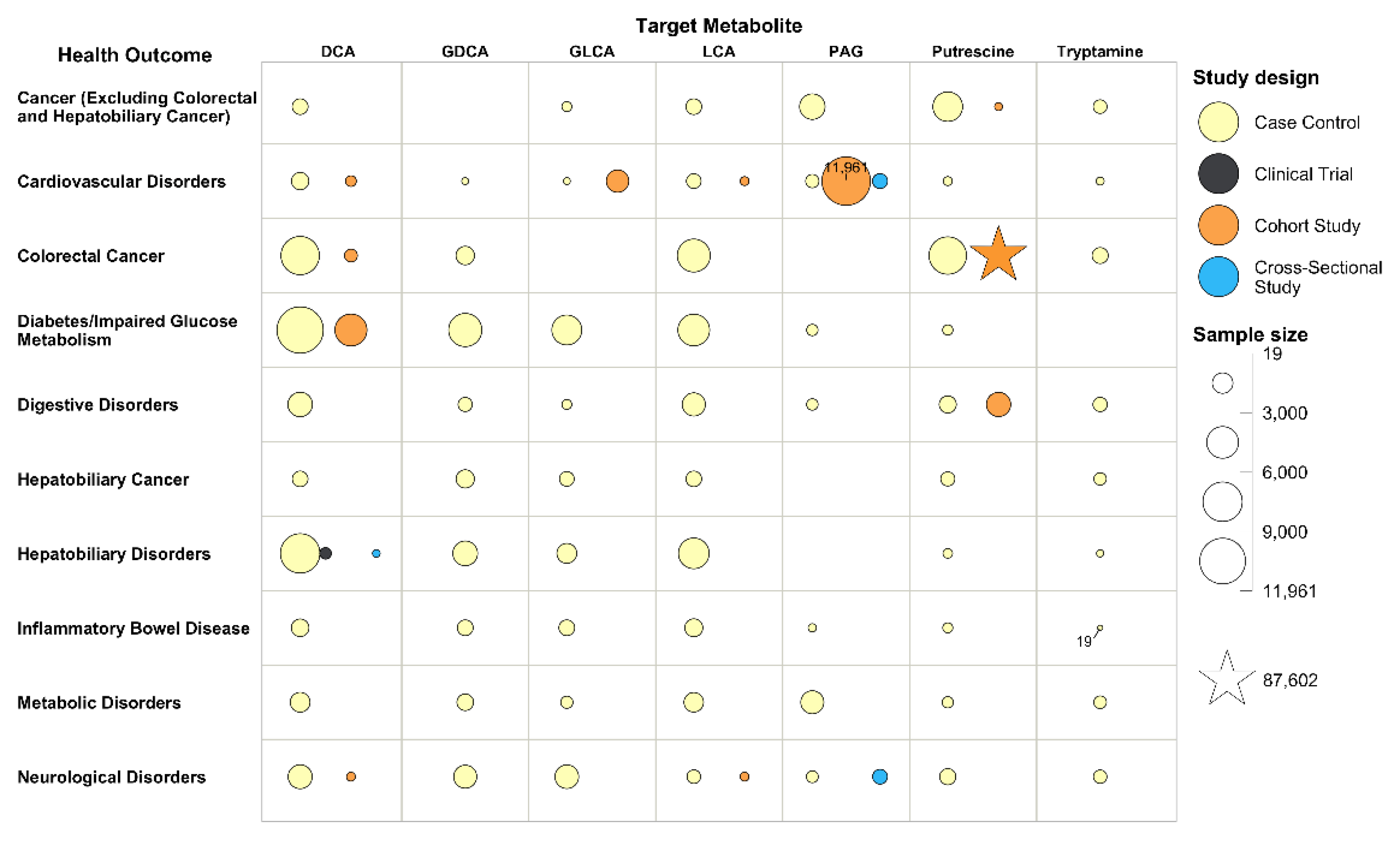
3.3. Health Outcomes
3.4. Participants
3.5. Evidence Maps and Research Gaps
4. Discussion
4.1. Suggestions for Systematic Reviews
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holmes, E.; Li, J.V.; Athanasiou, T.; Ashrafian, H.; Nicholson, J.K. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol. 2011, 19, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Ding, Y.; Saeidi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2019, 28, 3285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, D.; Zimetti, F.; Caffarra, P.; Tassotti, M.; Bernini, F.; Brighenti, F.; Zini, A.; Zanotti, I. The Gut Microbial Metabolite Trimethylamine-N-Oxide Is Present in Human Cerebrospinal Fluid. Nutrients 2017, 9, 1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sittipo, P.; Shim, J.W.; Lee, Y.K. Microbial Metabolites Determine Host Health and the Status of Some Diseases. Int. J. Mol. Sci. 2019, 20, 5296. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.; Wang, X.; Zhao, W.; Su, Z.; Chen, Q. Gut microbe–generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.-E.; Liao, P.-D.; Zhao, X.-J.; Wang, L. Trimethylamine-N-oxide has prognostic value in coronary heart disease: A meta-analysis and dose-response analysis. BMC Cardiovasc. Disord. 2020, 20, 7. [Google Scholar] [CrossRef]
- Li, W.; Huang, A.; Zhu, H.; Liu, X.; Huang, X.; Huang, Y.; Cai, X.; Lu, J.; Huang, Y. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. Med. J. Aust. 2020, 213, 374–379. [Google Scholar] [CrossRef]
- Glisic, M.; Kastrati, N.; Gonzalez-Jaramillo, V.; Bramer, W.M.; Ahmadizar, F.; Chowdhury, R.; Danser, A.H.J.; Roks, A.J.M.; Voortman, T.; Franco, O.H.; et al. Associations between phytoestrogens, glucose homeostasis, and risk of diabetes in women: A systematic review and meta-analysis. Adv. Nutr. 2018, 9, 726–740. [Google Scholar] [CrossRef] [Green Version]
- Crider, K.S.; Cordero, A.M.; Qi, Y.P.; Mulinare, J.; Dowling, N.F.; Berry, R.J. Prenatal folic acid and risk of asthma in children: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1272–1281. [Google Scholar] [CrossRef] [Green Version]
- Posadzki, P.; Lee, M.S.; Onakpoya, I.; Lee, H.W.; Ko, B.S.; Ernst, E. Dietary supplements and prostate cancer: A systematic review of double-blind, placebo-controlled randomised clinical trials. Maturitas 2013, 75, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Althuis, M.D.; Weed, D.L. Evidence mapping: Methodologic foundations and application to intervention and observational research on sugar-sweetened beverages and health outcomes. Am. J. Clin. Nutr. 2013, 98, 755–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, G.; Haslam, D.; Avendano, E.; Johnson, E.J. Lutein/zeaxanthin intake and visual outcomes in adults with healthy eyes: Qualitative gap analysis. Cogent Med. 2019, 6, 1683939. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef] [Green Version]
- McGowan, J.; Straus, S.; Moher, D.; Langlois, E.V.; O’Brien, K.K.; Horsley, T.; Aldcroft, A.; Zarin, W.; Garitty, C.M.; Hempel, S.; et al. Reporting scoping reviews—PRISMA ScR extension. J. Clin. Epidemiol. 2020, 123, 177–179. [Google Scholar] [CrossRef]
- Peters, M.D.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Implement. 2021, 19, 3–10. [Google Scholar] [CrossRef]
- Metabolon, I. Reference Library. 2022. Available online: https://www.metabolon.com/technology-knowledgebase/ (accessed on 28 January 2022).
- Mandal, R.; Cano, R.; Davis, C.D.; Hayashi, D.; Jackson, S.A.; Jones, C.M.; Lampe, J.W.; Latulippe, M.E.; Lin, N.J.; Lippa, K.A.; et al. Workshop report: Toward the development of a human whole stool reference material for metabolomic and metagenomic gut microbiome measurements. Metabolomics 2020, 16, 119. [Google Scholar] [CrossRef]
- Minion, J.; Egunsola, O.; Mastikhina, L.; Mastikhina, L.; Farkas, B.; Hofmeister, M.; Flanagan, J.; Salmon, C.; Clement, F. PICO Portal (product review). J. Can. Health Libr. Assoc. /J. L’association Bibliothèques St. Can. 2021, 42, 3. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases 11th Revision. 2022. Available online: https://icd.who.int/en (accessed on 28 January 2022).
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. BMJ Evid.-Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Leuven, J.A.G.; Havekes, L.; Kempen, H.J. Effect of deoxycholic acid on lipoprotein and apolipoprotein levels in patients with familial hypercholesterolemia. Atherosclerosis 1986, 62, 21–25. [Google Scholar] [CrossRef]
- Carulli, N.; De Leon, M.P.; Zironi, F.; Iori, R.; Loria, P. Bile acid feeding and hepatic sterol metabolism: Effect of deoxycholic acid. Gastroenterology 1980, 79, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Carulli, N.; De Leon, M.P.; Zironi, F.; Iori, R.; Loria, P. Deoxycholic acid treatment in patients with cholesterol gallstones: Failure to detect a suppression of cholesterol 7α-hydroxylase activity. J. Intern. Med. 1999, 246, 399–407. [Google Scholar]
- Cahill, C.; Pain, J.; Bailey, M. Bile salts, endotoxin and renal function in obstructive jaundice. Surg. Gynecol. Obstet. 1987, 165, 519–522. [Google Scholar] [PubMed]
- Cahill, C.J. Prevention of postoperative renal failure in patients with obstructive jaundice—The role of bile salts. J. Br. Surg. 1983, 70, 590–595. [Google Scholar] [CrossRef]
- Pain, J.; Cahill, C.; Gilbert, J.; Johnson, C.; Trapnell, J.; Bailey, M. Prevention of postoperative renal dysfunction in patients with obstructive jaundice: A multicentre study of bile salts and lactulose. J. Br. Surg. 1991, 78, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, N.; Malicdan, M.C.; Leoyklang, P.; Shrader, J.A.; Joe, G.; Slota, C.; Perreault, J.; Heiss, J.D.; Class, B.; Liu, C.-Y.; et al. Safety and efficacy of N-acetylmannosamine (ManNAc) in patients with GNE myopathy: An open-label phase 2 study. Genet. Med. 2021, 23, 2067–2075. [Google Scholar] [CrossRef]
- Xu, X.; Wang, A.Q.; Latham, L.L.; Celeste, F.; Ciccone, C.; Malicdan, M.C.; Goldspiel, B.; Terse, P.; Cradock, J.; Yang, N.; et al. Safety, pharmacokinetics and sialic acid production after oral administration of N-acetylmannosamine (ManNAc) to subjects with GNE myopathy. Mol. Genet. Metab. 2017, 122, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Bogiatzi, C.; Gloor, G.; Allen-Vercoe, E.; Reid, G.; Wong, R.G.; Urquhart, B.L.; Dinculescu, V.; Ruetz, K.N.; Velenosi, T.J.; Pignanelli, M.; et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis 2018, 273, 91–97. [Google Scholar] [CrossRef]
- Vargas, A.J.; Wertheim, B.C.; Gerner, E.W.; Thomson, C.A.; Rock, C.L.; Thompson, P.A. Dietary polyamine intake and risk of colorectal adenomatous polyps. Am. J. Clin. Nutr. 2012, 96, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-Y.; Fang, Y.-J.; Abulimiti, A.; Yang, X.; Li, L.; Liu, K.-Y.; Zhang, X.; Feng, X.-L.; Chen, Y.-M.; Zhang, C.-X. Dietary Polyamines Intake and Risk of Colorectal Cancer: A Case-Control Study. Nutrients 2020, 12, 3575. [Google Scholar] [CrossRef]
- Vargas, A.J.; Ashbeck, E.L.; Wertheim, B.C.; Wallace, R.B.; Neuhouser, M.L.; Thomson, A.C.; Thompson, A.P. Dietary polyamine intake and colorectal cancer risk in postmenopausal women. Am. J. Clin. Nutr. 2015, 102, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneghan, C.; Goldacre, B.; Mahtani, K.R. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017, 18, 122. [Google Scholar] [CrossRef] [PubMed]
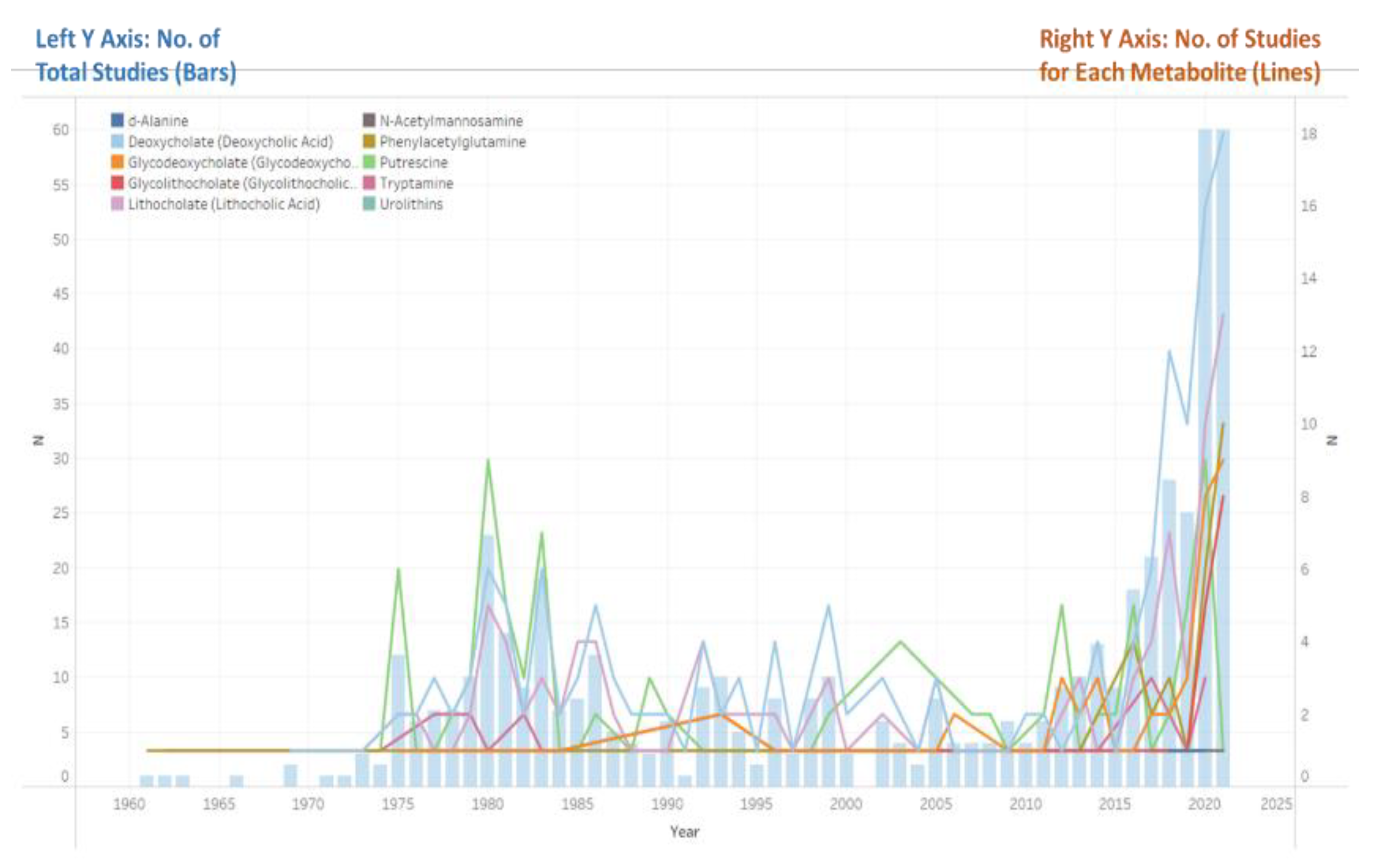

| Metabolite | No. of Studies (%) | Sample Size (%) |
|---|---|---|
| DCA | 166 (47.2) | 33,231 (19.8) |
| LCA | 111 (31.5) | 18,591 (11.1) |
| Putrescine | 100 (28.4) | 103,272 (61.4) |
| GDCA | 48 (13.6) | 12,340 (7.3) |
| PAG | 36 (10.2) | 23,931 (14.2) |
| GLCA | 30 (8.5) | 10,377 (6.2) |
| Tryptamine | 26 (7.4) | 2422 (1.4) |
| ManNAc | 3 (0.9) | 140 (0.1) |
| d-Alanine | 3 (0.9) | 281 (0.2) |
| Urolithins | 2 (0.6) | 857 (0.5) |
| Total | 352 (100.0) | 168,076 (100.0) |
| Health Outcomes | No. of Studies (%) | Sample Size (%) |
|---|---|---|
| Hepatobiliary Disorders | 80 (22.7) | 9786 (5.8) |
| Cancer (Excluding Colorectal and Hepatobiliary Cancer) | 61 (17.3) | 7801 (4.6) |
| Digestive Disorders | 41 (11.6) | 5891 (3.5) |
| Other | 35 (9.9) | 2743 (1.6) |
| Colorectal Cancer | 35 (9.9) | 100,977 (60.1) |
| Neurological Disorders | 27 (7.7) | 5317 (3.2) |
| Cardiovascular Disorders | 24 (6.8) | 16,579 (9.9) |
| Renal Disorders | 23 (6.5) | 7804 (4.6) |
| Diabetes/Impaired Glucose Metabolism | 21 (6.0) | 11,348 (6.8) |
| Metabolic Disorders | 20 (5.7) | 4900 (2.9) |
| Inflammatory Bowel Disease | 15 (4.3) | 1379 (0.8) |
| Hepatobiliary Cancer | 14 (4.0) | 1906 (1.1) |
| Mental Health | 7 (2.0) | 598 (0.4) |
| Dermatological Disorders | 7 (2.0) | 410 (0.2) |
| Respiratory Disorders | 6 (1.7) | 645 (0.4) |
| Total | 352 (100.0) | 168,076 (100.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Yang, X.; Wilson, L.M.; Mueller, N.T.; Sears, C.L.; Treisman, G.J.; Robinson, K.A. Diet-Related and Gut-Derived Metabolites and Health Outcomes: A Scoping Review. Metabolites 2022, 12, 1261. https://doi.org/10.3390/metabo12121261
Jia Y, Yang X, Wilson LM, Mueller NT, Sears CL, Treisman GJ, Robinson KA. Diet-Related and Gut-Derived Metabolites and Health Outcomes: A Scoping Review. Metabolites. 2022; 12(12):1261. https://doi.org/10.3390/metabo12121261
Chicago/Turabian StyleJia, Yuanxi, Xuhao Yang, Lisa M. Wilson, Noel T. Mueller, Cynthia L. Sears, Glenn J. Treisman, and Karen A. Robinson. 2022. "Diet-Related and Gut-Derived Metabolites and Health Outcomes: A Scoping Review" Metabolites 12, no. 12: 1261. https://doi.org/10.3390/metabo12121261
APA StyleJia, Y., Yang, X., Wilson, L. M., Mueller, N. T., Sears, C. L., Treisman, G. J., & Robinson, K. A. (2022). Diet-Related and Gut-Derived Metabolites and Health Outcomes: A Scoping Review. Metabolites, 12(12), 1261. https://doi.org/10.3390/metabo12121261






