Antibacterial and Antibiofilm Activity of Carvacrol against Oral Pathogenic Bacteria
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Carvacrol Samples
2.2. Microorganisms and Culture Conditions
2.3. Antibacterial Activity
2.4. Growth Kinetics and Effect of Subinhibitory Concentrations on Biofilm Formation
2.5. Lethality Kinetics
2.6. Activity on Mature Biofilms
2.7. Scanning Electron Microscopy of Biofilm
2.8. Influence on Erythrocytes
2.9. Statistical Analysis
3. Results and Discussion
3.1. Determination of MICs and MBCs
3.2. Effect of Sub-Inhibitory Concentrations
3.2.1. Growth Curves
3.2.2. Biofilm Formation
3.3. Bacterial Lethality Curves
3.4. CAR Activity in Mature Biofilms
3.5. Ultramicroscopic Study of Biofilm
3.6. Influence of CAR on Erythrocytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bacali, C.; Vulturar, R.; Buduru, S.; Cozma, A.; Fodor, A.; Chis, A.; Lucaciu, O.; Damian, L.; Moldovan, M.L. Oral Microbiome: Getting to Know and Befriend Neighbors, a Biological Approach. Biomedicines 2022, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Valm, A.M. Microbial Interactions in Oral Communities Mediate Emergent Biofilm Properties. J. Dent. Res. 2020, 99, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.L.M.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791–E800. [Google Scholar] [CrossRef]
- Scharnow, A.M.; Solinski, A.E.; Wuest, W.M. Targeting S. mutans biofilms: A perspective on preventing dental caries. Medchemcomm 2019, 10, 1057–1067. [Google Scholar] [CrossRef]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus mutans: Dental Caries Onset Linked to Multiple Species by 16S rRNA Community Analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef]
- Redanz, S.; Cheng, X.; Giacaman, R.A. Live and let die: Hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol. Oral Microbiol. 2018, 33, 337–352. [Google Scholar] [CrossRef]
- Kreth, J.; Giacaman, R.A.; Raghavan, R.; Merritt, J. The road less traveled—Defining molecular commensalism with Streptococcus sanguinis. Mol. Oral Microbiol. 2017, 32, 181–196. [Google Scholar] [CrossRef]
- Zhu, B.; Macleod, L.C.; Kitten, T.; Xu, P. Streptococcus sanguinis biofilm formation & interaction with oral pathogens. Future Microbiol. 2018, 13, 915–932. [Google Scholar] [CrossRef]
- Kreth, J.; Merritt, J.; Shi, W.Y.; Qi, F.X. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 2005, 187, 7193–7203. [Google Scholar] [CrossRef]
- Costerton, J.W. Introduction to biofilm. Int. J. Antimicrob. Agents 1999, 11, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Martinez, F.J.; Barrajon-Catalan, E.; Micol, V. Tackling Antibiotic Resistance with Compounds of Natural Origin: A Comprehensive Review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Kazek-Kęsik, A. Recent Advances in the Control of Clinically Important Biofilms. Mol. Sci. 2022, 23, 9526. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tian, J.; Yang, C.; Chen, J.; Wu, L.; Fan, M.; Cai, X. L-Arg-Rich Amphiphilic Dendritic Peptide as a Versatile NO Donor for NO/Photodynamic Synergistic Treatment of Bacterial Infections and Promoting Wound Healing. Small 2021, 17, 2101495. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tian, J.; Zhu, J.; Chen, J.; Li, L.; Yang, C.; Chen, J.; Chen, D. Photodynamic and photothermal co-driven CO-enhanced multi-mode synergistic antibacterial nanoplatform to effectively fight against biofilm infections. Chem. Eng. J. 2021, 426, 131919. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sanchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Nasrollahzadeh, M.S.; Tajani, A.S.; Soheili, V. Bacterial biofilms and their resistance mechanisms: A brief look at treatment with natural agents. Folia Microbiol. 2022, 67, 535–554. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Altern. Med. eCAM 2014, 2014, 651593. [Google Scholar] [CrossRef]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevao-Silva, C.F.; Carvalho, M.D.D.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of Thymol and Carvacrol, Constituents of Thymus vulgaris L. Essential Oil, on the Inflammatory Response. Evid. Based Complement. Altern. Med. 2012, 2012, 65702. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.O.; Silva, E.R.; Gomes, I.A.; Santana, H.S.; Santos, D.D.; Souza, G.P.D.; Silva, D.D.; Monteiro, J.C.M.; de Albuquerque, R.L.C.; Araujo, A.A.D.; et al. Anti-inflammatory and antioxidant activity of carvacrol in the respiratory system: A systematic review and meta-analysis. Phytother. Res. 2020, 34, 2214–2229. [Google Scholar] [CrossRef]
- Zeytinoglu, H.; Incesu, Z.; Baser, K.H.C. Inhibition of DNA synthesis by carvacrol in mouse myoblast cells bearing a human N-RAS oncogene. Phytomedicine 2003, 10, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, S.; Moran, A.; Martinez-Blanco, H.; Ferrero, M.A.; Rodriguez-Aparicio, L.B. The Usefulness of Non-Toxic Plant Metabolites in the Control of Bacterial Proliferation. Probiotics Antimicrob. Proteins 2017, 9, 323–333. [Google Scholar] [CrossRef]
- Rao, S.Q.; Xu, G.W.; Lu, X.N.; Zhang, R.Y.; Gao, L.; Wang, Q.Y.; Yang, Z.Q.; Jiao, X.A. Characterization of ovalbumin-carvacrol inclusion complexes as delivery systems with antibacterial application. Food Hydrocoll. 2020, 105, 105753. [Google Scholar] [CrossRef]
- M07-A10; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015.
- Pérez-Giraldo, C.; Rodríguez-Benito, A.; Morán, F.J.; Hurtado, C.; Blanco, M.T.; Gómez-García, A.C. Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J. Antimicrob. Chemother. 1997, 39, 643–646. [Google Scholar] [CrossRef]
- Navarro-Perez, M.L.; Vadillo-Rodriguez, V.; Fernandez-Babiano, I.; Perez-Giraldo, C.; Fernandez-Calderon, M.C. Antimicrobial activity of a novel Spanish propolis against planktonic and sessile oral Streptococcus spp. Sci. Rep. 2021, 11, 23860. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef]
- Stone, T.A.; Cole, G.B.; Ravamehr-Lake, D.; Nguyen, H.Q.; Khan, F.; Sharpe, S.; Deber, C.M. Positive Charge Patterning and Hydrophobicity of Membrane-Active Antimicrobial Peptides as Determinants of Activity, Toxicity, and Pharmacokinetic Stability. J. Med. Chem. 2019, 62, 6276–6286. [Google Scholar] [CrossRef]
- Burt, S.A.; van der Zee, R.; Koets, A.P.; de Graaff, A.M.; van Knapen, F.; Gaastra, W.; Haagsman, H.P.; Veldhuizen, E.J. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 4484–4490. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.A.S.; Santiago, M.B. Antibacterial Activity of Essential Oils against Oral Pathogens. Chem. Biodivers. 2022, 19, e202200097. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the Medicinal Use of Eleven Lamiaceae Species in Lebanon and Rationalization of Their Antimicrobial Potential by Examination of the Chemical Composition and Antimicrobial Activity of Their Essential Oils. Evid. Based Complement. Altern. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Lee, S.F.; Cheng, Z.Y.; Davidson, R.; Rupasinghe, H.P.V. Carvacrol exhibits rapid bactericidal activity against Streptococcus pyogenes through cell membrane damage. Sci. Rep. 2021, 11, 1487. [Google Scholar] [CrossRef]
- D’Amato, S.; Serio, A.; Lopez, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Faleiro, M.L.; Miguel, M.G.; Ladeiro, F.; Venancio, F.; Tavares, R.; Brito, J.C.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antimicrobial activity of essential oils isolated from Portuguese endemic species of Thymus. Lett. Appl. Microbiol. 2003, 36, 35–40. [Google Scholar] [CrossRef]
- Nostro, A.; Papalia, T. Antimicrobial activity of carvacrol: Current progress and future prospectives. Recent Pat. Anti Infect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef]
- Ciandrini, E.; Campana, R.; Federici, S.; Manti, A.; Battistelli, M.; Falcieri, E.; Papa, S.; Baffone, W. In vitro activity of Carvacrol against titanium-adherent oral biofilms and planktonic cultures. Clin. Oral Investig. 2014, 18, 2001–2013. [Google Scholar] [CrossRef]
- Wang, T.H.; Hsia, S.M.; Wu, C.H.; Ko, S.Y.; Chen, M.Y.; Shih, Y.H.; Shieh, T.M.; Chuang, L.C.; Wu, C.Y. Evaluation of the Antibacterial Potential of Liquid and Vapor Phase Phenolic Essential Oil Compounds against Oral Microorganisms. PLoS ONE 2016, 11, e0163147. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, R.; Abd-Elkader, O.H.; Musarrat, J.; Alkhathlan, H.Z.; Al-Kedhairy, A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express 2017, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca Genus as Antimicrobial Agents: From Farm to Pharmacy. Phytother. Res. 2017, 31, 1475–1494. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.A.; Nogueira, N.A.; Bastos, G.M.; Fonseca, S.G.; Lemos, T.L.; Matos, F.J.; Montenegro, D.; Heukelbach, J.; Rao, V.S.; Brito, G.A. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz. J. Med. Biol. Res. 2007, 40, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999, 65, 4606–4610. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, F.; Ji, B.P.; Pei, R.S.; Xu, N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett. Appl. Microbiol. 2008, 47, 174–179. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; Tjeerdsma-Van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural requirements for the antimicrobial activity of carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Lee, S.F.; Rupasinghe, H.P.V. Carvacrol inhibits Streptococcus pyogenes biofilms by suppressing the expression of genes associated with quorum-sensing and reducing cell surface hydrophobicity. Microb. Pathog. 2022, 169, 105684. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Rezvi, F.B.; Roy, A. Carvacrol: A well-known phytochemical for modern dentistry. Drug Invent. Today 2019, 11, 1460–1463. [Google Scholar]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.S.; Roder, H.L.; Russel, J.; Sorensen, H.; Burmolle, M.; Sorensen, S.J. Coexistence facilitates interspecific biofilm formation in complex microbial communities. Environ. Microbiol. 2016, 18, 2565–2574. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The mechanisms of action of carvacrol and its synergism with nisin against Listeria monocytogenes on sliced bologna sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- De Souza, E.L.; de Barros, J.C.; de Oliveira, C.E.V.; da Conceicao, M.L. Influence of Origanum vulgare L. essential oil on enterotoxin production, membrane permeability and surface characteristics of Staphylococcus aureus. Int. J. Food Microbiol. 2010, 137, 308–311. [Google Scholar] [CrossRef]
- La Storia, A.; Ercolini, D.; Marinello, F.; Di Pasqua, R.; Villani, F.; Mauriello, G. Atomic force microscopy analysis shows surface structure changes in carvacrol-treated bacterial cells. Res. Microbiol. 2011, 162, 164–172. [Google Scholar] [CrossRef]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Krzysciak, W.; Jurczak, A.; Koscielniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, V.; Alavinezhad, A.; Rajabi, O.; Mohammadpour, A.H.; Boskabady, M.H. Safety and tolerability of carvacrol in healthy subjects: A phase I clinical study. Drug Chem. Toxicol. 2021, 44, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. Biomed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Fan, M.; Si, J.; Xu, X.; Chen, L.; Chen, J.; Yang, C.; Zhu, J.; Wu, L.; Tian, J.; Chen, X.; et al. A versatile chitosan nanogel capable of generating AgNPs in-situ and long-acting slow-release of Ag+ for highly efficient antibacterial. Carbohydr. Polym. 2021, 257, 117636. [Google Scholar] [CrossRef]
- Ma, W.; Chen, X.; Fu, L.; Zhu, J.; Fan, M.; Chen, J.; Yang, C.; Yang, G.; Wu, L.; Mao, G.; et al. Ultra-efficient Antibacterial System Based on Photodynamic Therapy and CO Gas Therapy for Synergistic Antibacterial and Ablation Biofilms. ACS Appl. Mater. Interfaces 2020, 12, 22479–22491. [Google Scholar] [CrossRef]

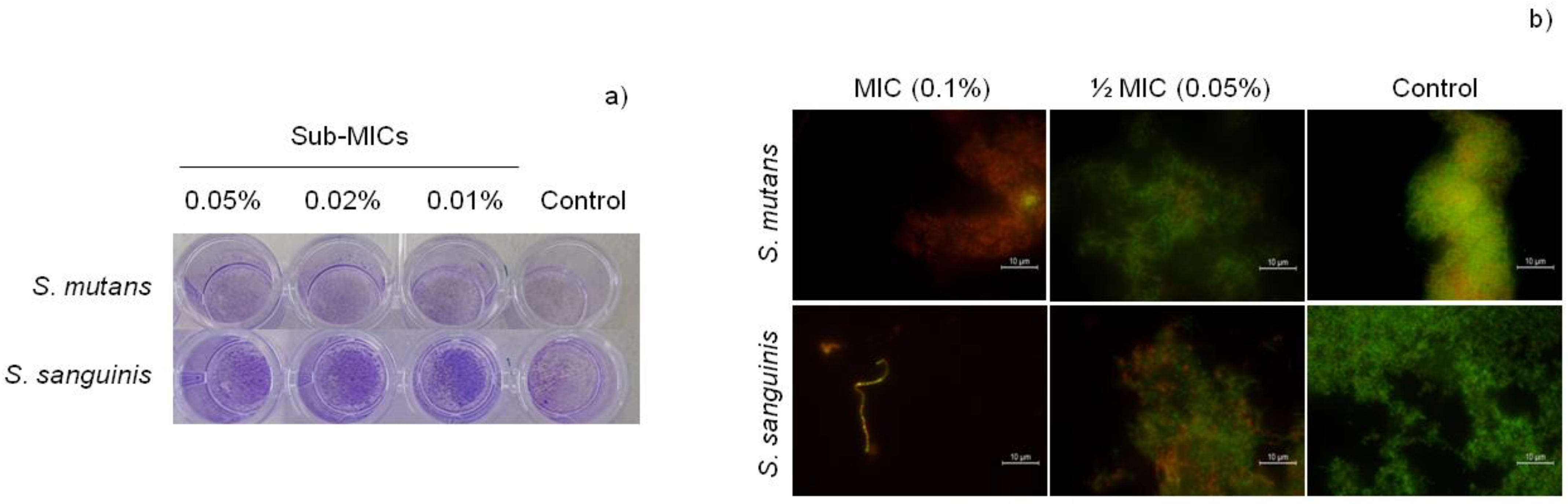

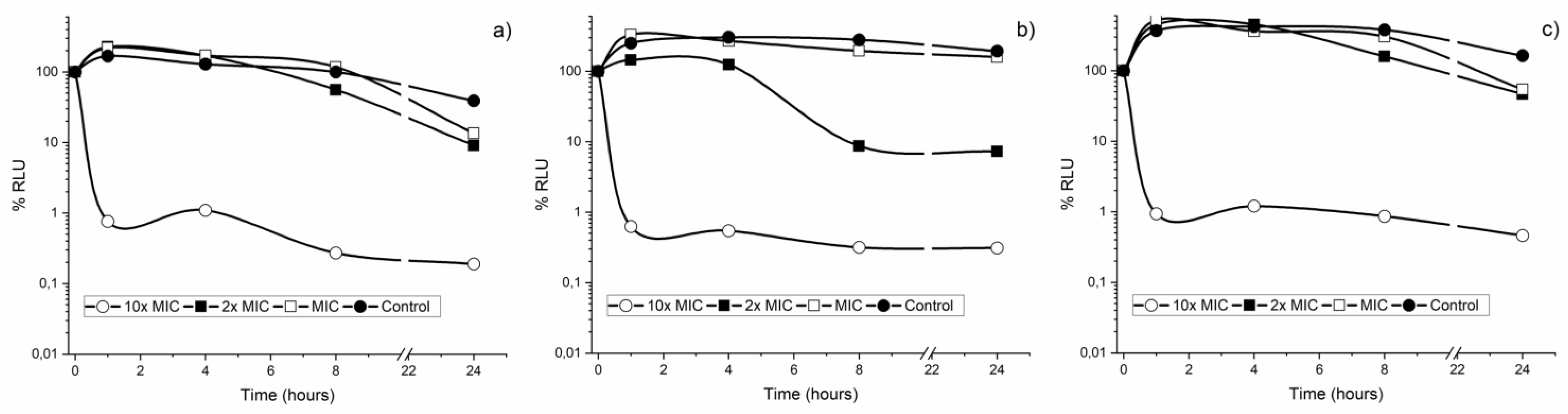
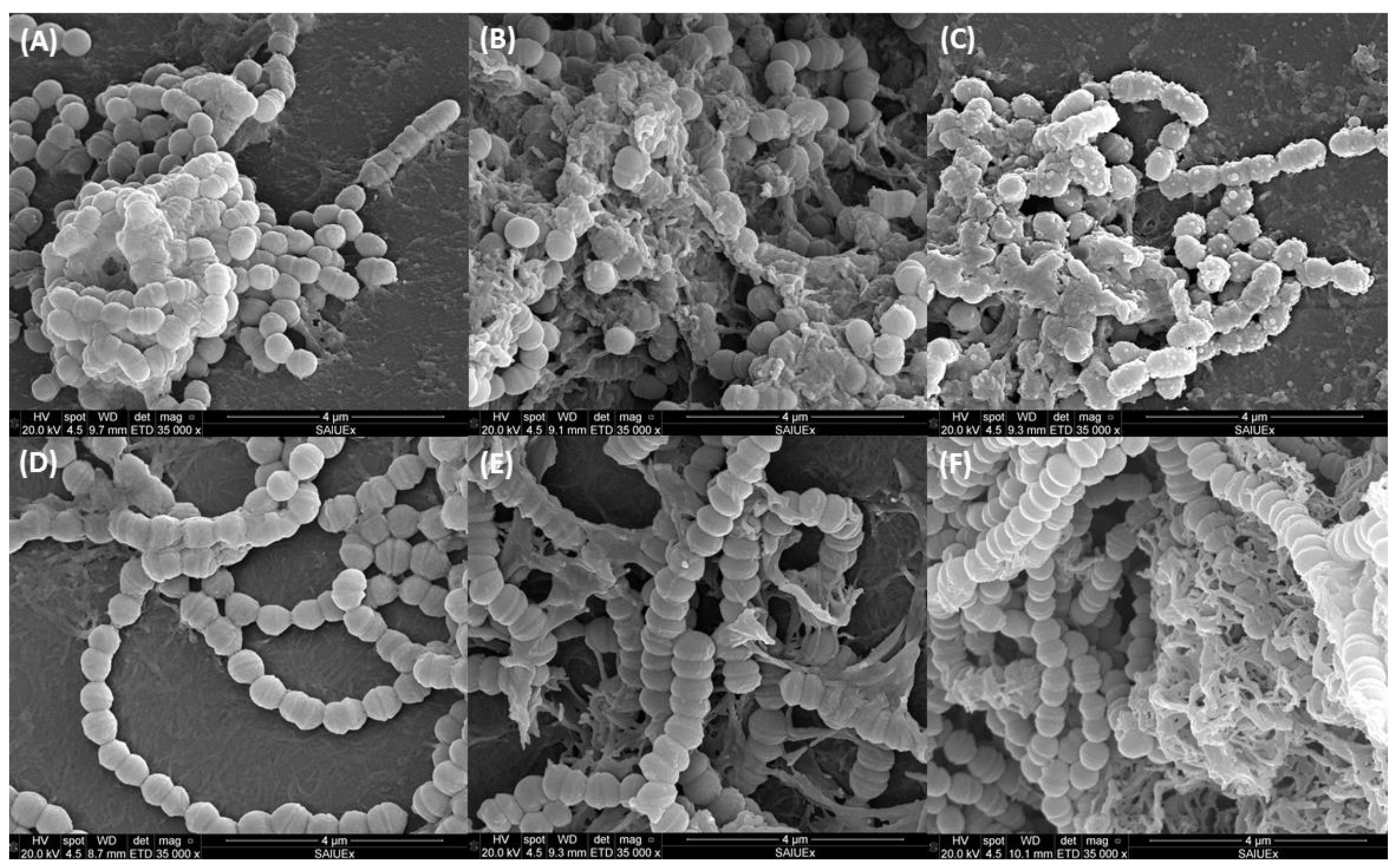
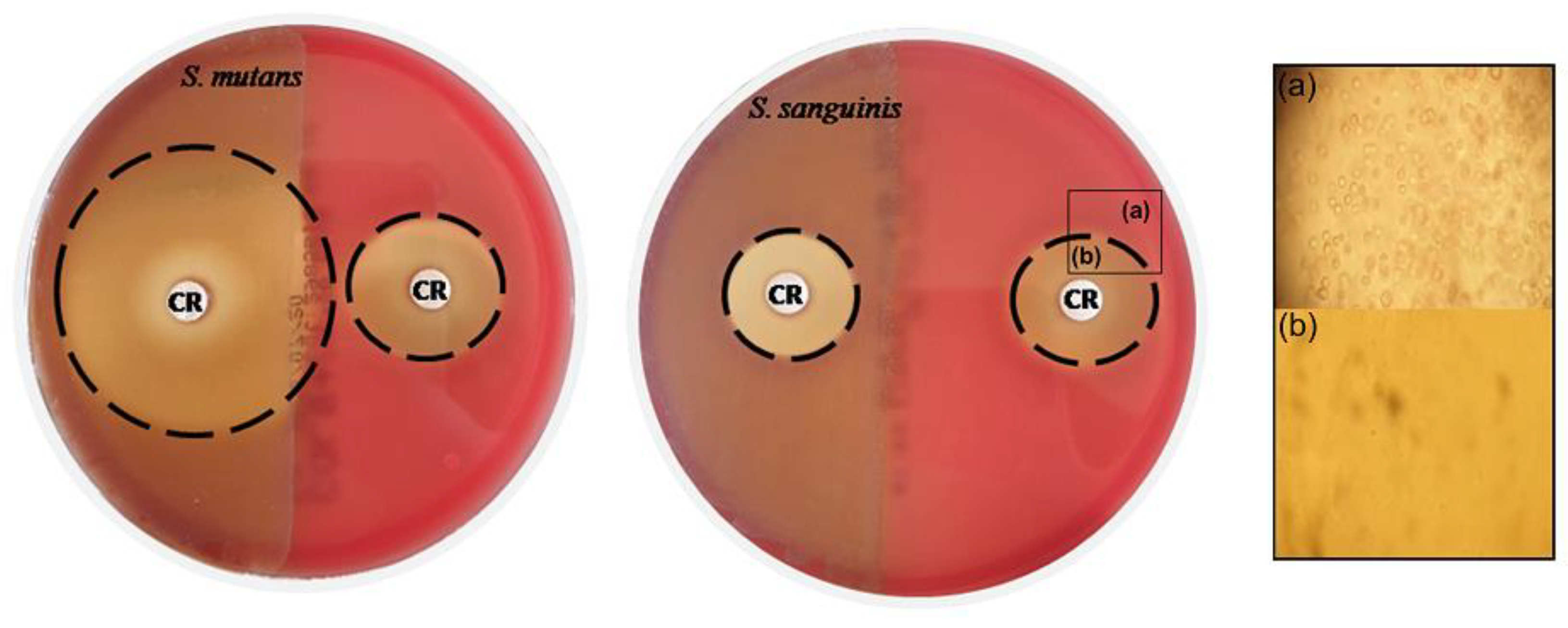

| Concentration CAR | S. mutans | S. sanguinis |
|---|---|---|
| ½ MIC (0.05% = 46.7 µg/mL) | 70.21% ± 43.81 | 189.52% ± 35.68 |
| ¼ MIC (0.02% = 23.3 µg/mL) | 95.53% ± 19.01 | 143.75% ± 32.46 * |
| ⅛ MIC (0.01% = 11.7 µg/mL) | 114.35% ± 21.16 | 114.97% ± 50.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Babiano, I.; Navarro-Pérez, M.L.; Pérez-Giraldo, C.; Fernández-Calderón, M.C. Antibacterial and Antibiofilm Activity of Carvacrol against Oral Pathogenic Bacteria. Metabolites 2022, 12, 1255. https://doi.org/10.3390/metabo12121255
Fernández-Babiano I, Navarro-Pérez ML, Pérez-Giraldo C, Fernández-Calderón MC. Antibacterial and Antibiofilm Activity of Carvacrol against Oral Pathogenic Bacteria. Metabolites. 2022; 12(12):1255. https://doi.org/10.3390/metabo12121255
Chicago/Turabian StyleFernández-Babiano, Irene, María Luisa Navarro-Pérez, Ciro Pérez-Giraldo, and María Coronada Fernández-Calderón. 2022. "Antibacterial and Antibiofilm Activity of Carvacrol against Oral Pathogenic Bacteria" Metabolites 12, no. 12: 1255. https://doi.org/10.3390/metabo12121255
APA StyleFernández-Babiano, I., Navarro-Pérez, M. L., Pérez-Giraldo, C., & Fernández-Calderón, M. C. (2022). Antibacterial and Antibiofilm Activity of Carvacrol against Oral Pathogenic Bacteria. Metabolites, 12(12), 1255. https://doi.org/10.3390/metabo12121255








