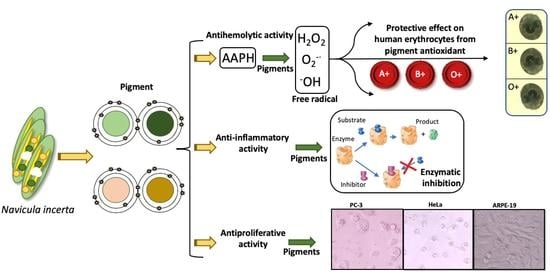
Association of Different ABO and Rh Blood Groups with the Erythroprotective Effect of Extracts from Navicula incerta and Their Anti-Inflammatory and Antiproliferative Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Biological Material
2.3. Extraction of Pigments from N. incerta
2.4. Acute Toxicity Bioassay on Artemia Salina
2.5. LC50 Determination
2.6. Blood Biocompatibility Assay of N. incerta Extracts on ABO and Rh Blood Types
2.7. Evaluation of the Erythroprotective Effect on Different ABO and Rh Blood Types of Groups
2.8. In Vitro Anti-Inflammatory Activity Screening
2.8.1. Inhibition of Enzyme Porcine Pancreatic Elastase (PPE) Activity
2.8.2. Inhibition of Albumin Denaturation Assay (Antiarthritic Activity)
2.8.3. Heat-Induced Hemolysis and Hypotonicity-Induced Hemolysis Assays
2.9. Antiproliferative Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Acute Toxicity Bioassay on Artemia Salina
3.2. Blood Biocompatibility Assay of N. incerta Extracts on Different ABO and Rh Blood Groups
3.3. Association of Different ABO and Rh Blood Groups with the Erythroprotective Effect against Oxidative Stress
3.3.1. Antihemolytic Activity
3.3.2. Association Study by Univariate Logistic Regression
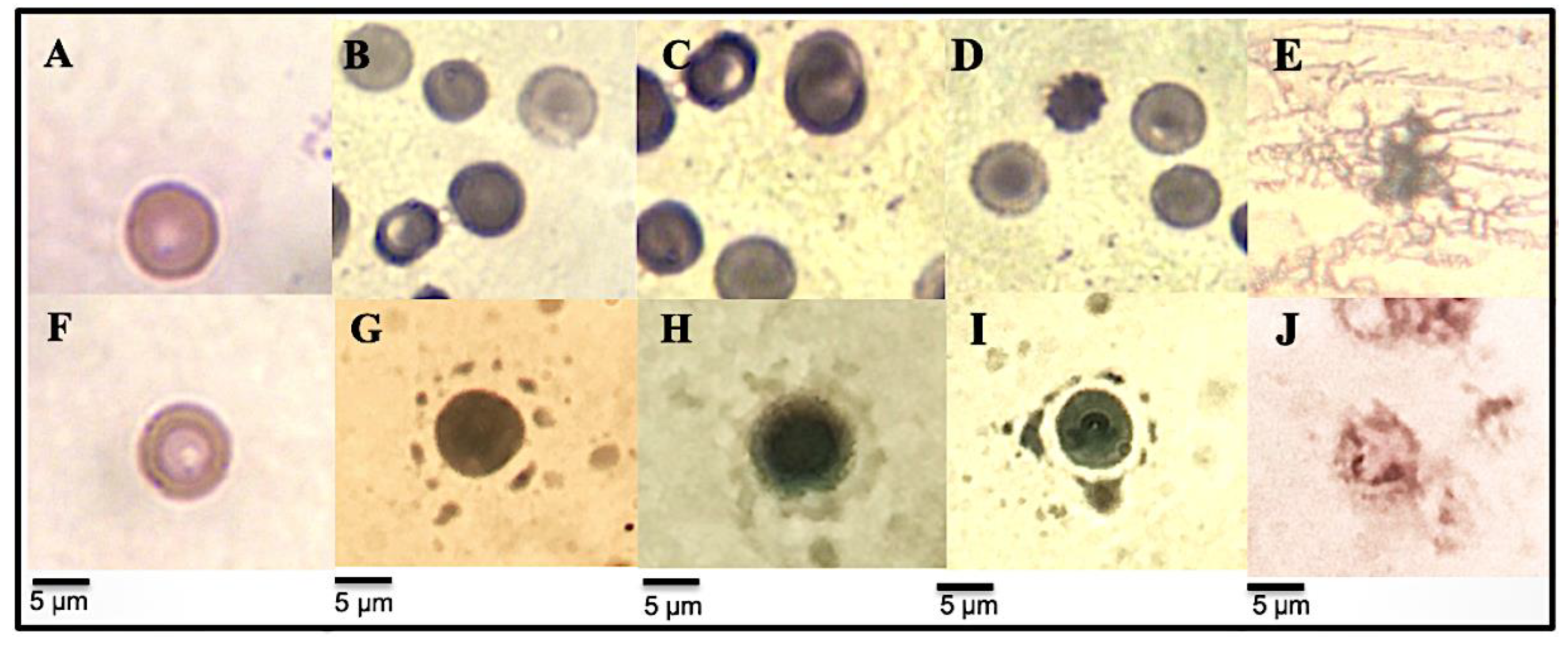
3.3.3. Micrographs of Human Erythrocytes after Erythroprotective Effect
3.4. In Vitro Anti-Inflammatory Activity Screening
3.4.1. Inhibition of PPE Enzyme Assay
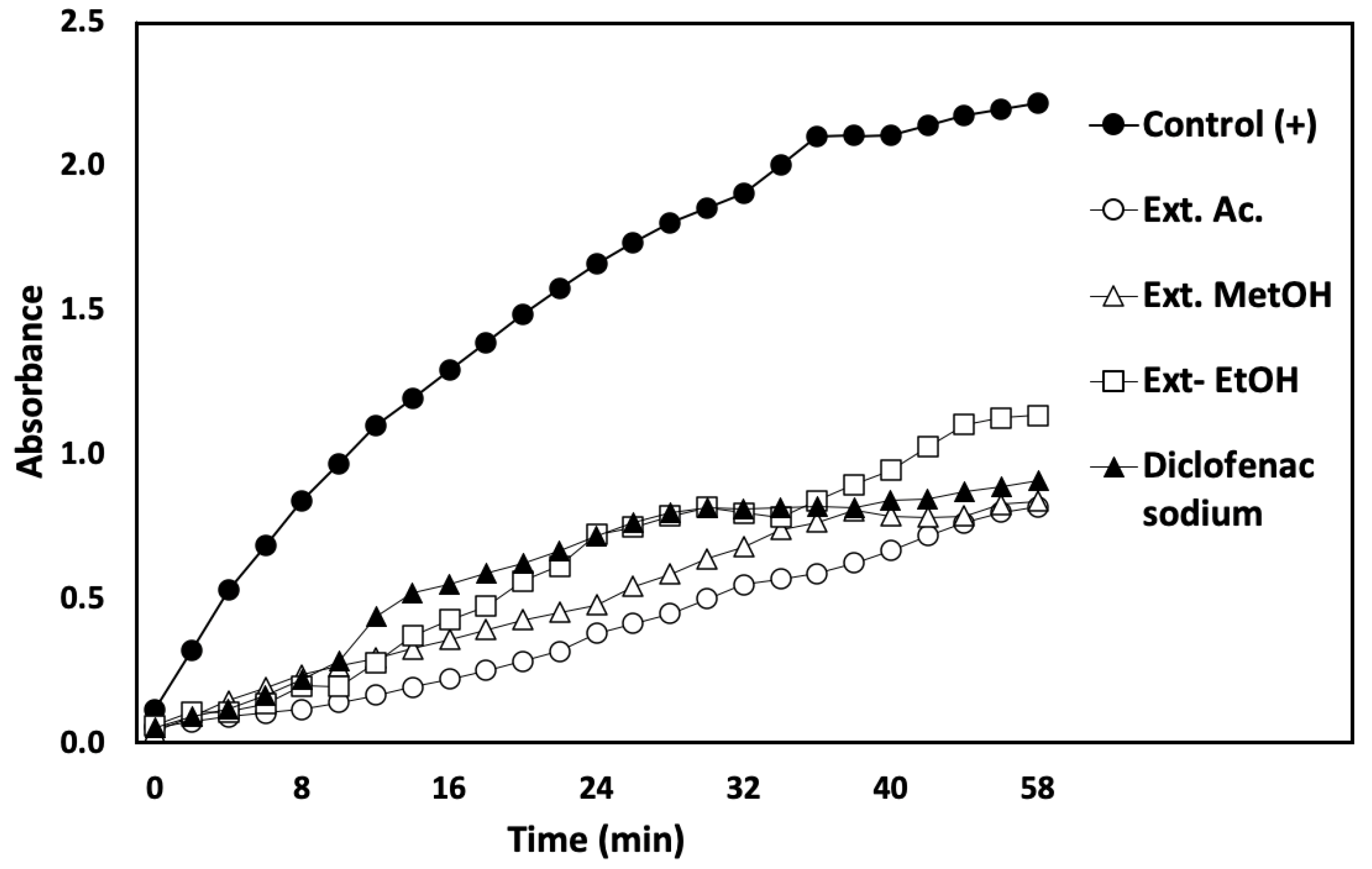
3.4.2. Inhibition of Albumin Denaturation Assay (Antarthritic Activity)
3.4.3. Membrane-Stabilizing Capacity Assay
3.4.4. RBC Morphological Changes Induced by Heat and Hypotonicity Damage by Optical Microscopy
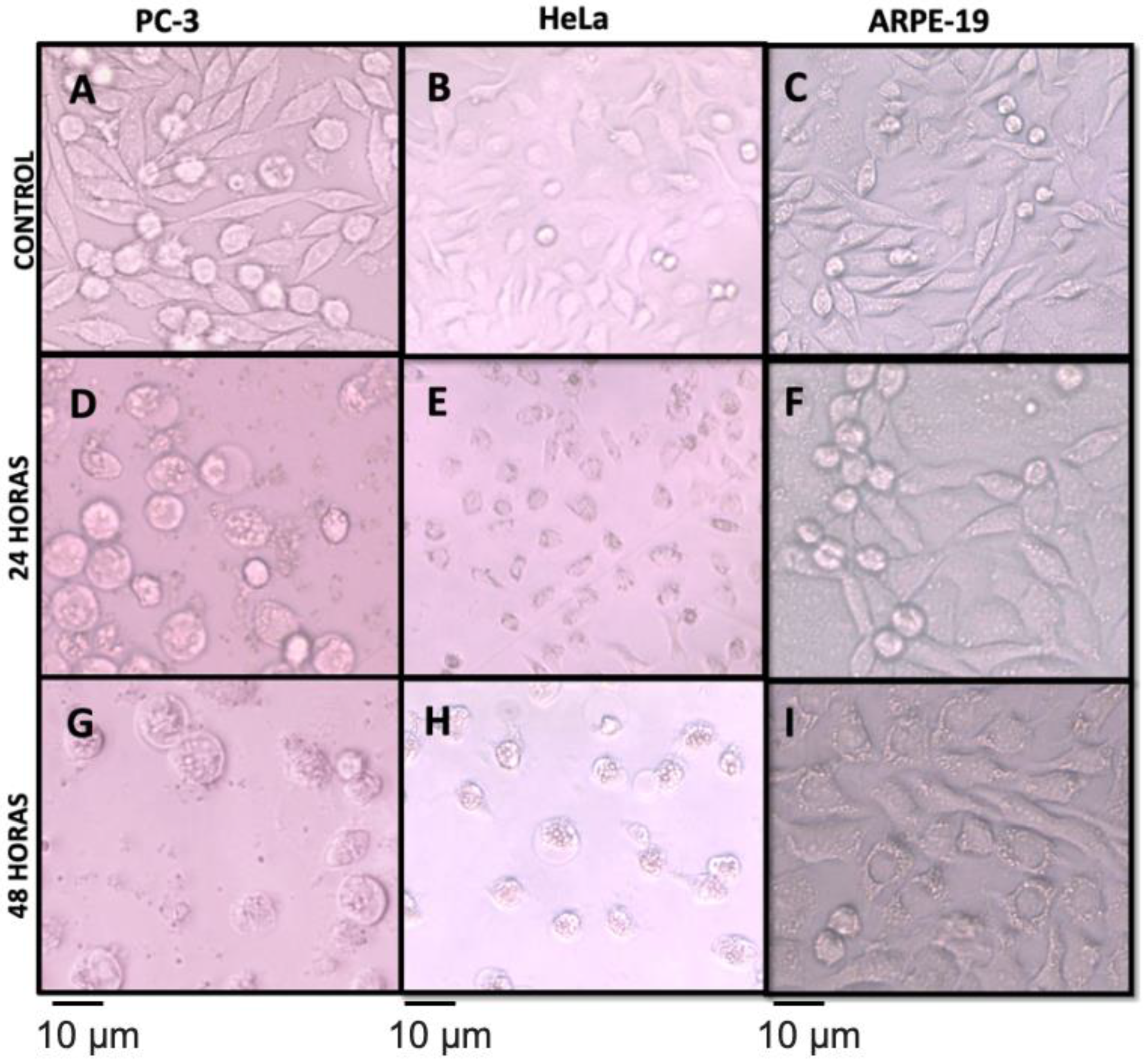
3.5. Antiproliferative Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bidgoli, S.A.; Azarshab, H. Role of vitamin D deficiency and lack of sun exposure in the incidence of premenopausal breast cancer: A case control study in Sabzevar, Iran. Asian Pac. J. Cancer Prev. 2014, 15, 3391–3396. [Google Scholar] [CrossRef] [Green Version]
- Anstee, D.J. The relationships between blood groups and disease. Blood 2010, 115, 4635–4643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, R.; Yousef, A.; Elbably, O. Association of ABO blood group and risk of breast cancer. J. Blood Disord. Transfuse 2014, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- Meo, S.A.; Rouq, F.A.; Suraya, F.; Zaidi, S.Z. Association of ABO and Rh blood groups with type 2 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 237–242. [Google Scholar] [CrossRef]
- Gates, M.A.; Wolpin, B.M.; Cramer, D.W.; Hankinson, S.E.; Tworoger, S.S. ABO blood group and incidence of epithelial ovarian cancer. Int. J. Cancer 2011, 128, 482–486. [Google Scholar] [CrossRef]
- Nozoe, T.; Ezaki, T.; Baba, H.; Kakeji, Y.; Maehara, Y. Correlation of ABO blood group with clinicopathologic characteristics of patients with esophageal squamous cell carcinoma. Dis. Esophagus 2004, 17, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Vadivelu, M.K.; Damodaran, S.; Solomon, J.; Rajaseharan, A. Distribution of ABO blood groups in acute leukaemias and lymphomas. Ann. Hematol. 2004, 83, 584–587. [Google Scholar] [CrossRef]
- Rasmi, Y.; Sadreddini, M.; Peirovi, T.; Jamali, M.; Khosravifa, F.; Dadkhah, A.; Fatemi, F.; Rahmati, M.; Zargari, M.; Sharifi, R. Frequency of ABO blood group in peptic ulcer disease in Iranian subjects. Pak. J. Biol. Sci. 2009, 12, 991–993. [Google Scholar] [CrossRef] [Green Version]
- The Severe COVID-19 GWAS Group. Genomewide Association Study of Severe COVID-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- Karakousis, C.P.; Evlogimenos, E.; Suh, O. Blood groups and malignant melanoma. J. Surg. Oncol. 1986, 33, 24–26. [Google Scholar] [CrossRef]
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). DARU J. Pharm. Sci. 2012, 20, 76. [Google Scholar] [CrossRef] [Green Version]
- Govindappa, M.; Channabasava, R.; Sowmya, D.V.; Meenakshi, J.; Shreevidya, M.R.; Lavanya, A.; Santoyo, G.; Sadananda, T.S. Phytochemical screening, antimicrobial and in vitro anti-inflammatory activity of endophytic extracts from Loranthus sp. Pharm. J. 2011, 3, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Divya, M.; Vijayakumar, S.; Chen, J.; Vaseeharan, B.; Durán-Lara, E.F. South Indian medicinal plants can combat deadly viruses along with COVID-19?—A review. Microb. Pathog. 2020, 148, 104277. [Google Scholar] [CrossRef] [PubMed]
- Piironen, V.; Toivo, J.; Lampi, A.M. Natural sources of dietary plant sterols. J. Food. Compos. Anal. 2000, 13, 619–624. [Google Scholar] [CrossRef]
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytosterols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid. Res. 2002, 41, 457–500. [Google Scholar] [CrossRef] [PubMed]
- Affan, A.; Karawita, R.; You-Jin, J.; Joan-Baek, L. Growth characteristics and antioxidant properties of the benthic diatom N. incerta (Bacillariophyceae) from Jeju Island, Korea. J. Phycol. 2007, 43, 823–832. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential Effects of Carotenoids on Lipid Peroxidation Due to Membrane Interactions: X-ray Diffraction Analysis. Chim. Biophys. Acta 2007, 1768, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef] [Green Version]
- González-Vega, R.I.; Cárdenas-López, J.C.; López-Elías, J.A.; Ruiz-Cruz, S.; Reyes-Díaz, A.; Perez-Perez, L.M.; Cinco-Moroyoqui, F.J.; Robles-Zepeda, R.E.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Optimization of growing conditions for pigments production from microalga Navicula incerta using response surface methodology and its antioxidant capacity. Saudi J. Biol. Sci. 2021, 28, 1401–1416. [Google Scholar] [CrossRef]
- Alghazwia, M.; Smidd, S.; Musgraved, I. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aà1-42) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, H.; Shanmugam, V.K. Anti-inflammatory activity screening of Kalanchoe pinnata methanol extract T and its validation using a computational simulation approach. Inform. Med. Unlocked. 2019, 14, 6–14. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Said-Fernández, S.L.; Nuevo, M. A modified microplate cytotoxicity assay with brine shrimp larvae (Artemia salina). Pharm. Online 2006, 3, 633–638. [Google Scholar]
- Leos-Rivas. Estudio de la Actividad Biológica de los Extractos de Tres Plantas de la Familia Boraginaceae. Ph.D. Thesis, Universidad Autónoma de Nuevo León Facultad de Ciencias Biológicas, Monterrey, Mexican, 2010. [Google Scholar]
- Robles-García, M.A.; Aguilar, A.J.; Gutiérrez-Lomelí, M.; Rodríguez-Félix, F.; Morales-Del-Río, J.A.; Guerrero-Medina, P.J.; Madrigal-Pulido, J.A.; Del-Toro-Sánchez, C.L. Qualitative identification of secondary metabolites and cytotoxicity determination of tempisque extracts (Sideroxylum capiri PITTIER). Biotecnia 2016, 18, 3–8. [Google Scholar] [CrossRef]
- Belokoneva, O.S.; Villegas, E.; Corzo, G.; Dai, L.; Nakajima, T. The hemolytic activity of six arachnid cationic peptides is affected by the phosphatidylcholine- to-sphingomyelin ratio in lipid bilayers. Biochim. Biophys. Acta 2003, 1617, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.J.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Rodríguez-Félix, F. Evaluation of Antioxidant Capacity, Protective Effect on Human Erythrocytes and Phenolic Compound Identification in Two Varieties of Plum Fruit (Spondias spp.) by UPLC-MS. Molecules 2018, 23, 3200. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.K.; Kim, J.H.; Cho, J.J.; Choi, J.D. Inhibitory effects of 150 plant extracts on elastase activity, and their anti-inflammatory effects. Int. J. Cosmet. Sci. 1999, 21, 71–82. [Google Scholar] [CrossRef]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of anti-inflammatory effect of Ashwagandha: A preliminary study in vitro. Pharm. J. 2012, 4, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Meerloo, J.V.; Kasper, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Cancer Cell Culture 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Barahona-Gomariz, M.V.; Sanz-Barrera, F.; Sánchez-Fortún, S. Acute toxicity of organic solvents on Artemia salina. Bull. Environ. Contam. Toxicol. 1994, 52, 766–771. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Fernandes, T.; Santos, L.N.D. Nascimento SM Toxicity of benthic dinoflagellates on grazing, behavior, and survival of the brine shrimp Artemia salina. PLoS ONE 2017, 12, e0175168. [Google Scholar] [CrossRef] [Green Version]
- Chisté, R.C.; Freitas, M.; Mercadante, A.Z.; Fernandes, E. Carotenoids are Effective Inhibitors of in vitro Hemolysis of Human Erythrocytes, as Determined by a Practical and Optimized Cellular Antioxidant Assay. J. Food Sci. 2014, 79, 9. [Google Scholar] [CrossRef]
- Jilani, K.; Lang, F. Carmustine-induced phosphatidylserine translocation in the erythrocyte membrane. Toxins 2013, 5, 703–716. [Google Scholar] [CrossRef]
- Abed, M.; Towhid, S.T.; Shaik, N.; Lang, F. Stimulation of suicidal death of erythrocytes by rifampicina. Toxicology 2012, 302, 123–128. [Google Scholar] [CrossRef]
- Adem, S.; Comakli, V.; Kuzu, M.; Demirdag, R. Investigation of the effects of some phenolic compounds on the activities of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase from human erythrocytes. J. Biochem. Mol. Toxicol. 2014, 28, 510–514. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Hatade, K.; Asai, A.; Kimura, F.; Sookwong, P.; Tsuduki, T.; Arai, H.; Miyazawa, T. Development of a high-performance liquid chromatography-based as- say for carotenoids in human red blood cells: Application to clinical studies. Anal. Biochem. 2008, 381, 129–134. [Google Scholar] [CrossRef]
- Merino, A. Alteraciones Morfológicas de Los Eritrocitos. Educ. Contin. En El Lab. Clin. 2015, 20, 41–64. Available online: http://www.seqc.es/download/tema/3/2767/7982539/2987076/cms/tema-5-alteraciones-morfologicas-de-los-eritrocitos.pdf/ (accessed on 18 July 2022).
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Zhang, G.; Xiao, H.; Mcclements, D.J. Influence of lipid phase composition of excipient emulsions on curcumin solubility, stability, and bioaccessibility. Food Biophys. 2016, 11, 213–225. [Google Scholar] [CrossRef]
- Babu, B.H.; Shylesh, B.S.; Padikkala, J. Antioxidant and hepatoprotective effect of Alanthus icicifocus. Fitoterapia 2001, 72, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, C.M. Inmunohematology. Eherd Edition; Appleton Century Crojts: New York, NY, USA, 1978. [Google Scholar]
- Hou, J.; Cui, H.L. In Vitro Antioxidant, Antihemolytic, and Anticancer Activity of the Carotenoids from Halophilic Archaea. Curr. Microbiol. 2018, 75, 266–271. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Azizan, A.; Safwan, M.; Bustamam, A.; Maulidiani, M.; Shaari, K.; Safinar, I.; Nagao, N.; Abas, F. Metabolite Profiling of the Microalgal Diatom Chaetoceros Calcitrans and Correlation with Antioxidant and Nitric Oxide Inhibitory Activities via 1H NMR-Based Metabolomics. Mar. Drugs 2018, 16, 154. [Google Scholar] [CrossRef] [Green Version]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Jomova, K.; Kysel, O.; Madden, J.C.; Morris, H.; Enoch, S.J.; Budzak, S.; Young, A.J.; Cronin, M.T.D.; Mazur, M.; Valko, M. Electron transfer from all-trans- β-carotene to the t-butyl peroxyl radical at low oxygen pressure (an EPR spectroscopy and computational study). Chem. Phys. Lett. 2009, 478, 266–270. [Google Scholar] [CrossRef]
- Rodrigues, E.; Mariutti, L.R.B.; Chiste, R.C.; Mercadante, A.Z. Development of a novel micro-assay for evaluation of peroxyl radical scavenger capacity: Application to carotenoids and structure-activity relationship. Food Chem. 2012, 135, 2103–2111. [Google Scholar] [CrossRef] [Green Version]
- Hosen, S.M.; Yusuff, S.; Barua, A. Chowdhury: ABO Blood Type and threat of git cancer and liver cancer in Bangladeshi populations. J. Med. Biomed. Appl. Sci. 2018, 10, 15520-105. [Google Scholar]
- Yu, H.; Xu, N.; Li, Z.K.; Xia, H.; Ren, H.T.; Li, N.; Wei, J.B.; Bao, H.Z. Association of ABO Blood Groups and Risk of Gastric Cancer. Scand. J. Surg. 2020, 109, 309–313. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Huang, Y.; Zheng, H.; Li, L. Association of ABO polymorphisms and pancreatic Cancer/ Cardiocerebrovascular disease: A meta-analysis. BMC Med. Genet. 2020, 21, 41. [Google Scholar] [CrossRef] [Green Version]
- Barbalic, M.; Dupuis, J.; Dehghan, A.; Bis, J.C.; Hoogeveen, R.C.; Schnabel, R.B.; Nambi, V.; Bretler, M.; Smith, N.L.; Peters, A.; et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum. Mol. Genet. 2010, 19, 1863–1872. [Google Scholar] [CrossRef] [Green Version]
- Siransy, L.K.; Nanga, Z.Y.; Zaba, F.S.; Tufa, N.Y.; Dasse, S.R. ABO/Rh Blood Groups and Risk of HIV Infection and Hepatitis B Among Blood Donors of Abidjan, Côte D’ivoire. Eur. J. Microbiol. Immunol. 2015, 5, 205–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, S.; Dong, Q.; Dang, J.; Liu, Z.; Han, H.; Tao, Y.; Yue, H. Anti-rheumatoid arthritis effects of iridoid glucosides from Lamiophlomis rotata (Benth.) kudo on adjuvant-induced arthritis in rats by OPG/RANKL/ NF-κB signaling pathways. J. Ethnopharmacol. 2021, 266, 113402. [Google Scholar] [CrossRef] [PubMed]
- Zietz, M.; Tatonetti, N.P. Testing the association between blood type and COVID-19 infection, intubation, and death. Nat. Commun. 2020, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, A.; Mahmoudi-Aliabadi, M.; Mehrtash, V.; Jafarzadeh, B.; Salehi, M. The Novel Coronavirus SARS-CoV-2 Vulnerability Association with ABO/Rh Blood Types. Iran J. Pathol. 2020, 15, 156–160. [Google Scholar] [CrossRef]
- Dai, X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur. J. Prev. Cardiol. 2020, 27, 1436–1437. [Google Scholar] [CrossRef]
- Brás, N.F.; Gonçalves, R.; Mateus, N.; Fernandes, P.A.; Ramos, M.J.; Freitas, V. Inhibition of Pancreatic Elastase by Polyphenolic Compounds. J. Agric. Food Chem. 2010, 58, 10668–10676. [Google Scholar] [CrossRef] [PubMed]
- Ololade, Z.S.; Kuyooro, S.E.; Ogunmola, O.O.; Abiona, O.O. Phytochemical, Antioxidant, Anti-Arthritic, Anti-Inflammatory, and Bactericidal Potentials of the Leaf Extract of Lactuca teraxacifolia. Glob. J. Med. Res. 2017, 17, 19–28. [Google Scholar]
- Gan, T.J. Diclofenac: An update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, G.; Moovendhan, M.; Arumugam, A.; Matharasi, A.; Dineshkumar, R.; Sampathkumar, P. Evaluation of Chemical Composition and In Vitro Anti-inflammatory Effect of Marine Microalgae Chlorella vulgaris. Waste Biomass Valori. 2018, 10, 3263–3270. [Google Scholar] [CrossRef]
- Elisha, I.L.; Dzoyem, J.; Mcgaw, L.J.; Botha, F.S.; Eloff, J.N. The anti-arthritic, anti-inflammatory, antioxidant activity and relationships with total phenolics and total flavonoids of nine South African plants used traditionally to treat arthritis. BMC Complement. Altern. Med. 2016, 6, 307. [Google Scholar] [CrossRef] [Green Version]
- Moualek, I.; Iratni Aiche, G.; Mestar Guechaoui, N.; Lahcene, S.; Houali, K. Antioxidant and anti-inflammatory activities of Arbutus unedo aqueous extract. Asian Pac. J. Trop. Biomed. 2016, 6, 937–944. [Google Scholar] [CrossRef] [Green Version]
- Ananthi, S.; Raghavendran, H.R.B.; Sunil, A.G.; Gayathri, V.; Ramakhrisnan, G.; Vasanthi, H.R. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornate (Marine Brown Alga). Food Chem. Toxicol. 2010, 48, 187–192. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Alves, M.G.C.F.; Will, L.S.E.P.; Costa, T.G.; Sabry, D.A.; Rêgo, L.A.R.S.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A. Elsayed FA. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Ikeda, M.; Prachasilchai, W.; Burner-Taney, M.J.; Rabb, H.; Yokota-Ikeda, N. Ischemic acute tubular necrosis models and drug discovery: A focus on cellular inflammation. Drug Discov. Today 2006, 11, 364–370. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary Phytochemicals and Cancer Chemoprevention: A Review of the Clinical Evidence; Department of Medicine, Beth Israel Deaconess Medical Center: Boston, MA, USA, 2016. [Google Scholar]
- Leyva-Peralta, M.A.; Robles-Zepeda, R.E.; Garibay-Escobar, A.; Ruiz-Bustos, E.; Alvarez-Berber, L.P.; Gálvez-Ruiz, J.C. In vitro anti-proliferative activity of Argemone gracilenta and identification of some active components. BMC Complement. Altern. Med. 2015, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Se-Kwon, K. Biological Activities and Health Benefit Effects of Natural Pigments Derived from Marine Algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- San Millan, C.S.; Soldevilla, B.; Martín, P.; Gil-Calderon, B.; Compte, M.; Perez-Sacristan, B.; Donoso, E.; Pena, C.; Romero, J.; Granado-Lorencio, F.; et al. β-Cryptoxanthin synergistically enhances the antitumoral activity of Oxaliplatin through ∆NP73 negative regulation in colon cancer. Clin. Cancer Res. 2015, 21, 4398–4409. [Google Scholar] [CrossRef] [Green Version]
- Baudelet, P.H.; Anne-Laure, G.; Jean-Baptiste, B.; Camille, J.; Nicolas, B.; Kaas, R.; Thiéry, V.; Cadoret, J.P.; Laurent, P. Antiproliferative Activity of Cyanophora paradoxa Pigments in Melanoma, Breast and Lung Cancer Cells. Mar. Drugs 2013, 11, 4390–4406. [Google Scholar] [CrossRef] [Green Version]
- Jun-Xia, X.; Guo-Qing, H.; Cai-Ping, Z.; Dan-Dan, R.; Sheng-Hua, Z. Morphological study on apoptosis Hela cells induced by soyasaponins. Toxicol. Vitr. 2007, 21, 820–826. [Google Scholar] [CrossRef]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, F.L. ARPE-19, A Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef]
- Hazim, R.A.; Volland, S.; Yen, A.; Burgess, B.L.; Williams, D.L. Rapid differentiation of the human RPE cell line, ARPE-19, induced by nicotinamide. Exp. Eye Res. 2019, 179, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.; Cookson, B. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Terasaki, M.; Nagao, A. Characterization of apoptosis induced by fucoxanthin in human promyelocytic leukemia cells. Biosci. Biotechnol. Biochem. 2005, 69, 224–227. [Google Scholar] [CrossRef]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, P.; Hamada, M.; Takahashi, S.; Xing, G.; Liu, J.; Sugiura, N. Potential chemoprevention effect of dietary fucoxanthin on urinary bladder cancer EJ-1 cell line. Oncol. Rep. 2008, 20, 1099–1103. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef]


| * Classification | μg·mL−1 |
|---|---|
| (E) Extremely toxic | 1–10 |
| (H) Highly toxic | 10–100 |
| (M) Moderately toxic | 100–500 |
| (S) Slightly toxic | 500–1000 |
| (V) Virtually non-toxic | 1000–1500 |
| (R) Relatively innocuous | >1500 |
| Concentration (µg/mL) | Live Nauplii | Dead Nauplii | Toxicity Degree (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ex. Acet. | Ex. MetOH | Ex. EtOH | Ex. Acet. | Ex. MetOH | Ex. EtOH | Ex. Acet. | Ex. MetOH | Ex. EtOH | |
| 5 | 9.7 ± 0.58 | 9.7 ± 0.58 | 9.3 ± 1.15 | 0.33 ± 0.58 | 0.33 ± 0.58 | 0 ± 0 | 3.33 ±0.58 | 3.33 ± 0.58 | 0 ± 0 |
| 50 | 9.7 ± 0.58 | 10 ± 0.00 | 10 ± 0.00 | 0.33 ± 0.58 | 0 ± 0 | 0.67 ± 1.15 | 3.33 ± 0.58 | 0 ± 0 | 6.67 ± 1.15 |
| 250 | 10 ± 0.00 | 9.7 ± 0.58 | 7 ± 2.00 | 0 ± 0 | 0.33 ± 0.58 | 1.67 ± 0.58 | 0 ± 0 | 3.33 ± 0.58 | 16.67 ± 0.58 |
| 750 | 8.7 ± 0.53 | 8.7 ± 0.58 | 8.3 ± 0.58 | 1.33 ± 1.53 | 0.33 ± 0.58 | 3.00 ± 2.00 | 13.33 ± 1.53 | 13.33± 0.58 | 30.00 ± 2.00 |
| 1250 | 6.7 ± 1.15 | 5 ± 3.00 | 6 ± 1.00 | 3.33 ± 1.15 | 5.00 ± 3.00 | 3.67 ± 0.58 | 33.33 ± 1.15 | 50.00 ± 3.00 * | 36.67 ± 0.58 |
| 1500 | 5 ± 1.73 | 3.3 ± 2.52 | 6.3 ± 0.58 | 5.00 ± 1.73 | 6.67 ± 2.52 | 4.67 ± 1.53 | 50.00 ± 1.73 * | 66.67 ± 2.52 | 46.67 ± 1.53 ** |
| Blood Group | Hemolysis (%) | ||
|---|---|---|---|
| Ex. Ac. | Ex. MetOH | Ex. EtOH | |
| A | 10.77 b ± 3.12 | 2.34 a ± 3.39 | 6.76 b ± 1.86 |
| B | 14.22 c ± 3.12 | 1.56 a ± 1.42 | 2.34 a ± 1.75 |
| O | 1.28 a ± 1.26 | 3.25 a ± 3.39 | 14.73 c ± 1.79 |
| Blood Group | Hemolysis (%) | ||
|---|---|---|---|
| Ex. Ac. | Ex. MetOH | Ex. EtOH | |
| O RhD+ve | 1.35 a ± 1.23 | 4.13 b ± 1.08 | 12.58 b ± 3.48 |
| O RhD-ve | 0.67 a ± 0.15 | 1.02 a ± 1.27 | 5.91 c ± 2.87 |
| Blood Group | Hemolysis Inhibition (%) | ||
|---|---|---|---|
| Ex. Ac. | Ex. MetOH | Ex. EtOH | |
| A | 88.33 b ± 2.98 | 68.67 b ± 5.36 | 83.52 a ± 2.06 |
| B | 98.61 a ± 3.03 | 76.33 a ± 3.51 | 62.18 b ± 7.61 |
| O | 83.78 c ± 2.78 | 77.73 a ± 4.03 | 81.21 a ± 3.09 |
| Blood Group | Hemolysis Inhibition (%) | ||
|---|---|---|---|
| Ex. Ac. | Ex. MetOH | Ex. EtOH | |
| O RhD+ve | 88.16 a ± 0.69 | 69.09 a ± 2.44 | 86.08 a ± 3.23 |
| O RhD-ve | 68.21 b ± 4.93 | 68.18 a ± 3.21 | 80.51 b ± 2.51 |
| Ex-Acet. | Ex-MetOH | Ex-EtOH | |
|---|---|---|---|
| Blood Groups | OR (95% Cl) | OR (95% Cl) | OR (95% Cl) |
| O | 0.29 (0.14–0.46) | 0.45 (0.43–0.47) | 0.91 (0.83–0.98) |
| A | 1.19 * (0.17–2.22) | 0.43 (0.42–0.45) | 2.60 * (0.68–1.77) |
| B | 0.51 (0.20–0.81) | 0.52 (0.48–0.54) | 0.73 (0.62–0.83) |
| RhD+ve | 2.61 * (1.38–3.61) | 2.17 * (1.51–2.83) | 1.47 * (0.61–2.33) |
| RhD-ve | 0.56 (0.17–1.0) | 0.51 (0.47–0.53) | 1.28 * (1.25–1.6) |
| Samples | Inhibition of PPE (%) | IC50 to PPE (µg·mL−1) | Inhibition of BSA Denaturation (%) | Membrane Stabilization (%) | |
|---|---|---|---|---|---|
| Inhibition of Heat-Induced Hemolysis | Inhibition of Hypotonicity-Induced Hemolysis | ||||
| Ex. Acet. | 96.64 a ± 4.82 | 10.82 a ± 3.24 | 43.92 b ± 3.23 | 100.12 a ± 1.23 | 87.91 b ± 1.35 |
| Ex. MetOH | 72.57 b ± 2.92 | 34.69 b ± 4.14 | 38.12 b ± 3.96 | 100.91 a ± 1.18 | 87.32 b ± 1.34 |
| Ex. EtOH | 62.39 c ± 1.51 | 35.39 b ± 2.56 | 39.77 b ± 1.64 | 100.74 a ± 1.68 | 91.74 a ± 0.51 |
| DS | 61.18 c ± 2.23 | 35.75 b ± 4.12 | 94.67 a ± 2.61 | 92.32 b ± 8.06 | 93.80 a ± 0.88 |
| Extracts | IC50 μg·mL−1 ± SD | ||||
|---|---|---|---|---|---|
| A549 | HeLa | PC-3 | LS-180 | ARPE-19 | |
| Acetonic | >200 * | 75.91 b ± 2.47 | 122.58 b ± 2.86 | >200 * | >200 * |
| Methanolic | 141.99 b ± 3.23 | 61.93 a ± 3.10 | 107.01 a ± 2.49 | >200 * | >200 * |
| Ethanolic | 123.73 a ± 2.59 | 59.28 a ± 2.58 | 96.05 a ± 3.48 | >200 * | >200 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Cruz, S.; González-Vega, R.I.; Robles-Zepeda, R.E.; Reyes-Díaz, A.; López-Elías, J.A.; Álvarez-Ainza, M.L.; Cinco-Moroyoqui, F.J.; Moreno-Corral, R.A.; Wong-Corral, F.J.; Borboa-Flores, J.; et al. Association of Different ABO and Rh Blood Groups with the Erythroprotective Effect of Extracts from Navicula incerta and Their Anti-Inflammatory and Antiproliferative Properties. Metabolites 2022, 12, 1203. https://doi.org/10.3390/metabo12121203
Ruiz-Cruz S, González-Vega RI, Robles-Zepeda RE, Reyes-Díaz A, López-Elías JA, Álvarez-Ainza ML, Cinco-Moroyoqui FJ, Moreno-Corral RA, Wong-Corral FJ, Borboa-Flores J, et al. Association of Different ABO and Rh Blood Groups with the Erythroprotective Effect of Extracts from Navicula incerta and Their Anti-Inflammatory and Antiproliferative Properties. Metabolites. 2022; 12(12):1203. https://doi.org/10.3390/metabo12121203
Chicago/Turabian StyleRuiz-Cruz, Saúl, Ricardo Iván González-Vega, Ramón Enrique Robles-Zepeda, Aline Reyes-Díaz, José Antonio López-Elías, Maritza Lizeth Álvarez-Ainza, Francisco Javier Cinco-Moroyoqui, Ramón Alfonso Moreno-Corral, Francisco Javier Wong-Corral, Jesús Borboa-Flores, and et al. 2022. "Association of Different ABO and Rh Blood Groups with the Erythroprotective Effect of Extracts from Navicula incerta and Their Anti-Inflammatory and Antiproliferative Properties" Metabolites 12, no. 12: 1203. https://doi.org/10.3390/metabo12121203
APA StyleRuiz-Cruz, S., González-Vega, R. I., Robles-Zepeda, R. E., Reyes-Díaz, A., López-Elías, J. A., Álvarez-Ainza, M. L., Cinco-Moroyoqui, F. J., Moreno-Corral, R. A., Wong-Corral, F. J., Borboa-Flores, J., Cornejo-Ramírez, Y. I., & Del-Toro-Sánchez, C. L. (2022). Association of Different ABO and Rh Blood Groups with the Erythroprotective Effect of Extracts from Navicula incerta and Their Anti-Inflammatory and Antiproliferative Properties. Metabolites, 12(12), 1203. https://doi.org/10.3390/metabo12121203