Dolutegravir Discontinuation for Neuropsychiatric Symptoms in People Living with HIV and Their Outcomes after Treatment Change: A Pharmacogenetic Study
Abstract
:1. Introduction
2. Experimental Design
3. Procedure
4. Results
4.1. Participants Characteristics
4.2. Incidence and Reasons for DTG Discontinuation
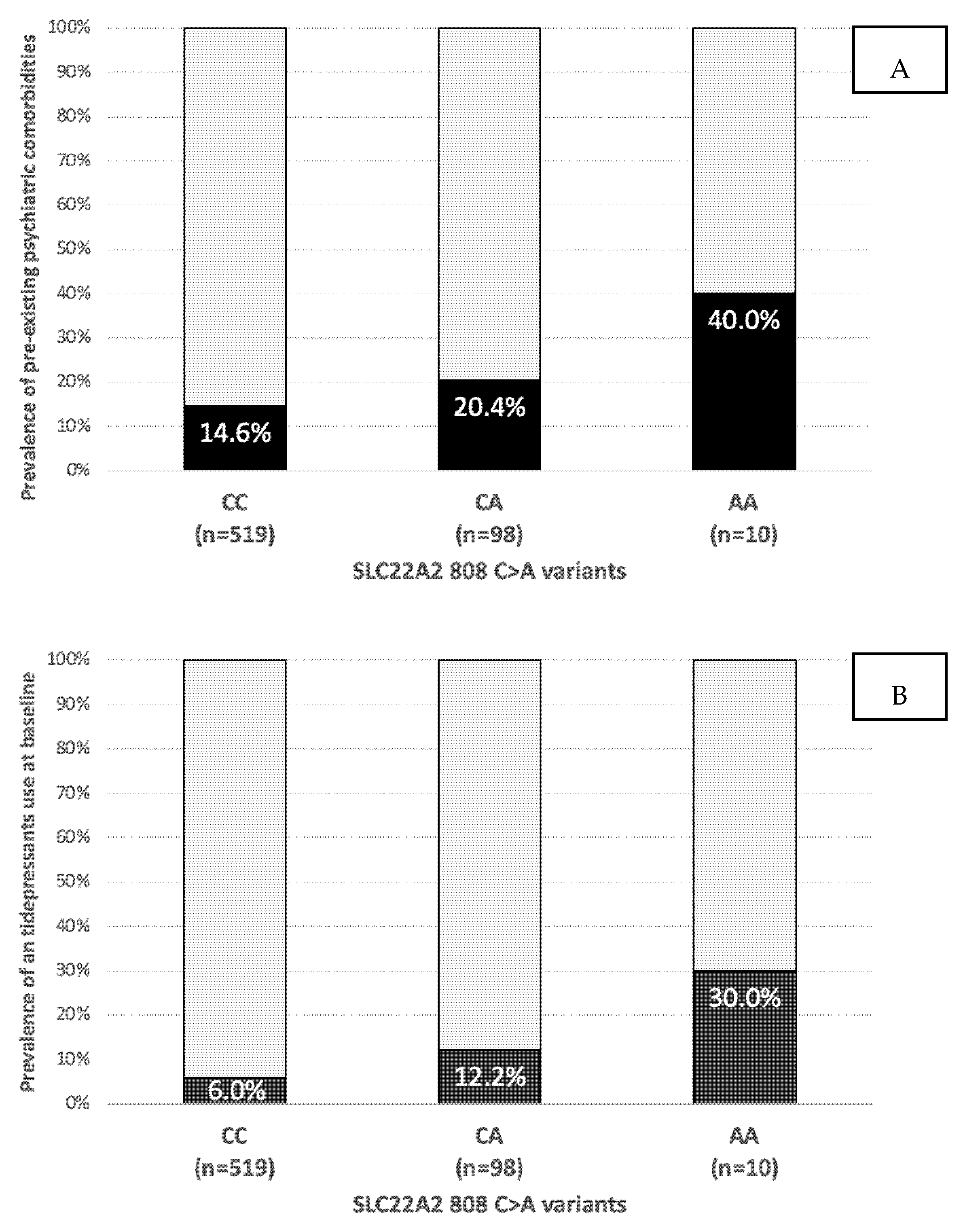
4.3. Factors Associated with DTG Discontinuation
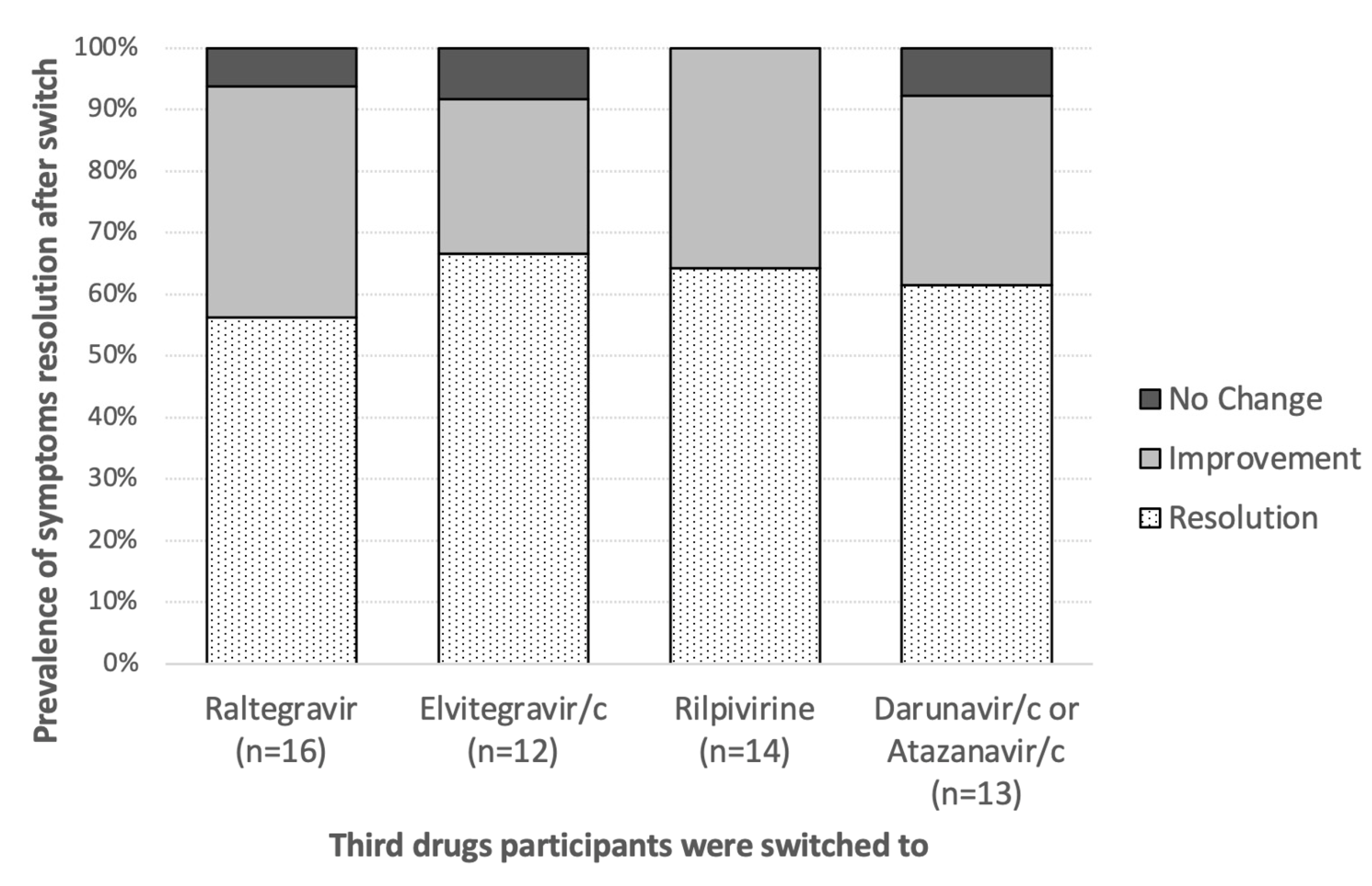
4.4. Follow Up of Participants after DTG Discontinuation
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nickel, K.; Halfpenny, N.J.A.; Snedecor, S.J.; Punekar, Y.S. Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naive patients infected with HIV-1: An update on a systematic review and network meta-analysis. BMC Infect. Dis. 2021, 21, 222. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.E.; Trevillyan, J. Impact of Integrase inhibitors and tenofovir alafenamide on weight gain in people with HIV. Curr. Opin. HIV AIDS 2021, 16, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Schewe, K.; Fenske, S.; Buhk, T.; Sabranski, M.; Adam, A.; Hansen, S.; Stellbrink, H.J. Short-term neuropsychiatric tolerability of bictegravir combined with emtricitabine/tenofovir alafenamide in clinical practice. Antivir. Ther. 2020, 25, 83–90. [Google Scholar] [CrossRef]
- Cuzin, L.; Pugliese, P.; Katlama, C.; Bani-Sadr, F.; Ferry, T.; Rey, D.; Lourenco, J.; Bregigeon, S.; Allavena, C.; Reynes, J.; et al. Integrase strand transfer inhibitors and neuropsychiatric adverse events in a large prospective cohort. J. Antimicrob. Chemother. 2018, 74, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Welz, T.; Sabranski, M.; Kolb, M.; Wolf, E.; Stellbrink, H.J.; Wyen, C. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2016, 18, 56–63. [Google Scholar] [CrossRef]
- Llibre, J.M.; Montoliu, A.; Miro, J.M.; Domingo, P.; Riera, M.; Tiraboschi, J.; Curran, A.; Homar, F.; Ambrosioni, J.; Abdulghani, N.; et al. Discontinuation of dolutegravir, elvitegravir/cobicistat and raltegravir because of toxicity in a prospective cohort. HIV Med. 2019, 20, 237–247. [Google Scholar] [CrossRef]
- Bonfanti, P.; Madeddu, G.; Gulminetti, R.; Squillace, N.; Orofino, G.; Vitiello, P.; Rusconi, S.; Celesia, B.M.; Maggi, P.; Ricci, E. Discontinuation of treatment and adverse events in an Italian cohort of patients on dolutegravir. AIDS 2017, 31, 455–457. [Google Scholar] [CrossRef]
- Venter, W.D.F.; Sokhela, S.; Simmons, B.; Moorhouse, M.; Fairlie, L.; Mashabane, N.; Serenata, C.; Akpomiemie, G.; Masenya, M.; Qavi, A.; et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): Week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV 2020, 7, e666–e676. [Google Scholar] [CrossRef]
- Elliot, E.R.; Wang, X.; Singh, S.; Simmons, B.; Vera, J.H.; Miller, R.F.; Fitzpatrick, C.; Moyle, G.; McClure, M.; Boffito, M. Increased Dolutegravir Peak Concentrations in People Living with Human Immunodeficiency Virus Aged 60 and Over, and Analysis of Sleep Quality and Cognition. Clin. Infect. Dis. 2018, 68, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Calcagno, A.; Cusato, J.; D’Avolio, A.; Bonora, S. Genetic Polymorphisms Affecting the Pharmacokinetics of Antiretroviral Drugs. Clin. Pharmacokinet. 2016, 56, 355–369. [Google Scholar] [CrossRef]
- Yagura, H.; Watanabe, D.; Kushida, H.; Tomishima, K.; Togami, H.; Hirano, A.; Takahashi, M.; Hirota, K.; Ikuma, M.; Kasai, D.; et al. Impact of UGT1A1 gene polymorphisms on plasma dolutegravir trough concentrations and neuropsychiatric adverse events in Japanese individuals infected with HIV-1. BMC Infect. Dis. 2017, 17, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, A.E.; Karbach, U.; Miska, D.; Gorboulev, V.; Akhoundova, A.; Volk, C.; Arndt, P.; Ulzheimer, J.C.; Sonders, M.S.; Baumann, C.; et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol. Pharmacol. 1998, 54, 342–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, T.; Nakata, T.; Kobayashi, Y. Localization of organic cation transporter 2 (OCT2) in monoaminergic and cholinergic axon terminals of the mouse brain. Neurosci. Lett. 2016, 633, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Nakata, T.; Matsui, T.; Kobayashi, K.; Kobayashi, Y.; Anzai, N. Organic cation transporter 2 (SLC22A2), a low-affinity and high-capacity choline transporter, is preferentially enriched on synaptic vesicles in cholinergic neurons. Neuroscience 2013, 252, 212–221. [Google Scholar] [CrossRef]
- Bacq, A.; Balasse, L.; Biala, G.; Guiard, B.; Gardier, A.M.; Schinkel, A.; Louis, F.; Vialou, V.; Martres, M.P.; Chevarin, C.; et al. Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol. Psychiatry 2011, 17, 926–939. [Google Scholar] [CrossRef] [Green Version]
- Borghetti, A.; Calcagno, A.; Lombardi, F.; Cusato, J.; Belmonti, S.; D’Avolio, A.; Ciccarelli, N.; La Monica, S.; Colafigli, M.; Delle Donne, V.; et al. SLC22A2 variants and dolutegravir levels correlate with psychiatric symptoms in persons with HIV. J. Antimicrob. Chemother. 2018, 74, 1035–1043. [Google Scholar] [CrossRef]
- Fernandez-Bargiela, N.; Rotea-Salvo, S.; Margusino-Framinan, L.; Balboa-Barreiro, V.; Martin-Herranz, I.; Castro-Iglesias, A.; Mena-De-Cea, A.; Lopez-Calvo, S.; Vazquez-Rodriguez, P.; Miguez-Rey, E.; et al. Discontinuation due to neuropsychiatric adverse events with efavirenz- and dolutegravir-based antiretroviral therapy: A comparative real-life study. Eur. J. Hosp. Pharm. 2020, 29, 207–211. [Google Scholar] [CrossRef]
- Greenberg, L.; Ryom, L.; Wandeler, G.; Grabmeier-Pfistershammer, K.; Ollinger, A.; Neesgaard, B.; Stephan, C.; Calmy, A.; Rauch, A.; Castagna, A.; et al. Uptake and Discontinuation of Integrase Inhibitors (INSTIs) in a Large Cohort Setting. J. Acquir. Immune Defic. Syndr. 2020, 83, 240–250. [Google Scholar] [CrossRef]
- Brehm, T.T.; Franz, M.; Hufner, A.; Hertling, S.; Schmiedel, S.; Degen, O.; Kreuels, B.; Zur Wiesch, J.S. Safety and efficacy of elvitegravir, dolutegravir, and raltegravir in a real-world cohort of treatment-naive and -experienced patients. Medicine 2019, 98, e16721. [Google Scholar] [CrossRef]
- Mondi, A.; Cozzi-Lepri, A.; Tavelli, A.; Rusconi, S.; Vichi, F.; Ceccherini-Silberstein, F.; Calcagno, A.; De Luca, A.; Maggiolo, F.; Marchetti, G.; et al. Effectiveness of dolutegravir-based regimens as either first-line or switch antiretroviral therapy: Data from the Icona cohort. J. Int. AIDS Soc. 2019, 22, e25227. [Google Scholar] [CrossRef]
- Povar-Echeverria, M.; Comet-Bernad, M.; Gasso-Sanchez, A.; Ger-Buil, A.; Navarro-Aznarez, H.; Martinez-Alvarez, R.; Arazo-Garces, P. Neuropsychiatric adverse effects of dolutegravir in real-life clinical practice. Enferm. Infecc. Microbiol. Clin. 2020, 39, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, R.; Florence, E.; Vandercam, B.; Moutschen, M.; Goffard, J.C.; De Munter, P.; Delforge, M.; Marinus, W.; De Wit, S. Effectiveness of dolutegravir-based antiretroviral therapy in a real-world setting in a Belgian cohort of 4101 HIV patients. AIDS 2020, 34, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Penafiel, J.; de Lazzari, E.; Padilla, M.; Rojas, J.; Gonzalez-Cordon, A.; Blanco, J.L.; Blanch, J.; Marcos, M.A.; Lonca, M.; Martinez-Rebollar, M.; et al. Tolerability of integrase inhibitors in a real-life setting. J. Antimicrob. Chemother. 2017, 72, 1752–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepik, K.J.; Yip, B.; Ulloa, A.C.; Wang, L.; Toy, J.; Akagi, L.; Lima, V.D.; Guillemi, S.; Montaner, J.S.G.; Barrios, R. Adverse drug reactions to integrase strand transfer inhibitors. AIDS 2018, 32, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Menard, A.; Montagnac, C.; Solas, C.; Meddeb, L.; Dhiver, C.; Tomei, C.; Ravaux, I.; Tissot-Dupont, H.; Mokhtari, S.; Colson, P.; et al. Neuropsychiatric adverse effects on dolutegravir: An emerging concern in Europe. AIDS 2017, 31, 1201–1203. [Google Scholar] [CrossRef] [PubMed]
- Parant, F.; Miailhes, P.; Brunel, F.; Gagnieu, M.C. Dolutegravir-Related Neurological Adverse Events: A Case Report of Successful Management with Therapeutic Drug Monitoring. Curr. Drug. Saf. 2018, 13, 69–71. [Google Scholar] [CrossRef]
- Calcagno, A.; Molto, J.; Borghetti, A.; Gervasoni, C.; Milesi, M.; Valle, M.; Avataneo, V.; Alcantarini, C.; Pla-Junca, F.; Trunfio, M.; et al. Older Age is Associated with Higher Dolutegravir Exposure in Plasma and Cerebrospinal Fluid of People Living with HIV. Clin. Pharmacokinet. 2020, 60, 103–109. [Google Scholar] [CrossRef]
- Fettiplace, A.; Stainsby, C.; Winston, A.; Givens, N.; Puccini, S.; Vannappagari, V.; Hsu, R.; Fusco, J.; Quercia, R.; Aboud, M.; et al. Psychiatric Symptoms in Patients Receiving Dolutegravir. J. Acquir. Immune Defic. Syndr. 2016, 74, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Kohli, M.A.; Lucae, S.; Saemann, P.G.; Schmidt, M.V.; Demirkan, A.; Hek, K.; Czamara, D.; Alexander, M.; Salyakina, D.; Ripke, S.; et al. The neuronal transporter gene SLC6A15 confers risk to major depression. Neuron 2011, 70, 252–265. [Google Scholar] [CrossRef] [Green Version]
- Sanwald, S.; Widenhorn-Muller, K.; Schonfeldt-Lecuona, C.; Montag, C.; Kiefer, M. Factors related to age at depression onset: The role of SLC6A4 methylation, sex, exposure to stressful life events and personality in a sample of inpatients suffering from major depression. BMC Psychiatry 2021, 21, 167. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]
- Courousse, T.; Gautron, S. Role of organic cation transporters (OCTs) in the brain. Pharmacol. Ther. 2014, 146, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Song, I.S.; Shin, H.J.; Shin, J.G. Genetic variants of organic cation transporter 2 (OCT2) significantly reduce metformin uptake in oocytes. Xenobiotica 2008, 38, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.M.; Leblanc, A.F.; Uddin, M.E.; Kim, J.Y.; Chen, M.; Eisenmann, E.D.; Gibson, A.A.; Li, Y.; Hong, K.W.; DiGiacomo, D.; et al. Neuronal uptake transporters contribute to oxaliplatin neurotoxicity in mice. J. Clin. Investig 2020, 130, 4601–4606. [Google Scholar] [CrossRef]

| Number (%) or Average (±Standard Deviation) | |
|---|---|
| European ancestry | 300 (84%) |
| Male sex at birth | 439 (70%) |
| Age (years) | 50.5 (±11.3) |
| BMI (Kg/m2) | 24.4 (±4.1) |
| CD4 cell count (cell/mm3) | 569 (±498) |
| Patients on treatment and with HIV RNA < 50 | 253 (40.3) |
| Positive serology for: | |
| HCV | 133 (21.7%) |
| HBV | 35 (5.6%) |
| HCV/HBV coinfected patients | 25 (4.0%) |
| Pre-existing psychiatric comorbidities | 100 (15.9%) |
| Antidepressant use | 46 (7.3%) |
| Anxiolytics use | 42 (6.7%) |
| Methadone/buprenorphine use | 28 (4.5%) |
| Three-drug based therapies | 377 (60.1%) |
| Lamivudine/emtricitabine | 355 (94.2%) |
| Abacavir | 169 (44.8%) |
| Tenofovir disoproxil fumarate | 86 (22.8%) |
| Tenofovir alafenamide | 105 (27.9%) |
| Two-drug based therapies | 230 (36.7%) |
| Lamivudine | 95 (41.3%) |
| Darunavir/c | 51 (22.2%) |
| Atazanavir or atazanavir/c | 46 (20%) |
| Rilpivirine | 32 (13.9%) |
| SLC22A2 808 C>A carriers: | |
| CC | 519 (82.8%) |
| CA | 98 (15.6%) |
| AA | 10 (1.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusato, J.; Borghetti, A.; Teti, E.; Milesi, M.; Tettoni, M.C.; Bonora, S.; Trunfio, M.; D’Avolio, A.; Compagno, M.; Di Giambenedetto, S.; et al. Dolutegravir Discontinuation for Neuropsychiatric Symptoms in People Living with HIV and Their Outcomes after Treatment Change: A Pharmacogenetic Study. Metabolites 2022, 12, 1202. https://doi.org/10.3390/metabo12121202
Cusato J, Borghetti A, Teti E, Milesi M, Tettoni MC, Bonora S, Trunfio M, D’Avolio A, Compagno M, Di Giambenedetto S, et al. Dolutegravir Discontinuation for Neuropsychiatric Symptoms in People Living with HIV and Their Outcomes after Treatment Change: A Pharmacogenetic Study. Metabolites. 2022; 12(12):1202. https://doi.org/10.3390/metabo12121202
Chicago/Turabian StyleCusato, Jessica, Alberto Borghetti, Elisabetta Teti, Maurizio Milesi, Maria Cristina Tettoni, Stefano Bonora, Mattia Trunfio, Antonio D’Avolio, Mirko Compagno, Simona Di Giambenedetto, and et al. 2022. "Dolutegravir Discontinuation for Neuropsychiatric Symptoms in People Living with HIV and Their Outcomes after Treatment Change: A Pharmacogenetic Study" Metabolites 12, no. 12: 1202. https://doi.org/10.3390/metabo12121202
APA StyleCusato, J., Borghetti, A., Teti, E., Milesi, M., Tettoni, M. C., Bonora, S., Trunfio, M., D’Avolio, A., Compagno, M., Di Giambenedetto, S., Di Perri, G., & Calcagno, A. (2022). Dolutegravir Discontinuation for Neuropsychiatric Symptoms in People Living with HIV and Their Outcomes after Treatment Change: A Pharmacogenetic Study. Metabolites, 12(12), 1202. https://doi.org/10.3390/metabo12121202










