GlycA, a Biomarker of Low-Grade Inflammation, Is Increased in Male Night Shift Workers
Abstract
1. Introduction
2. Material and Methods
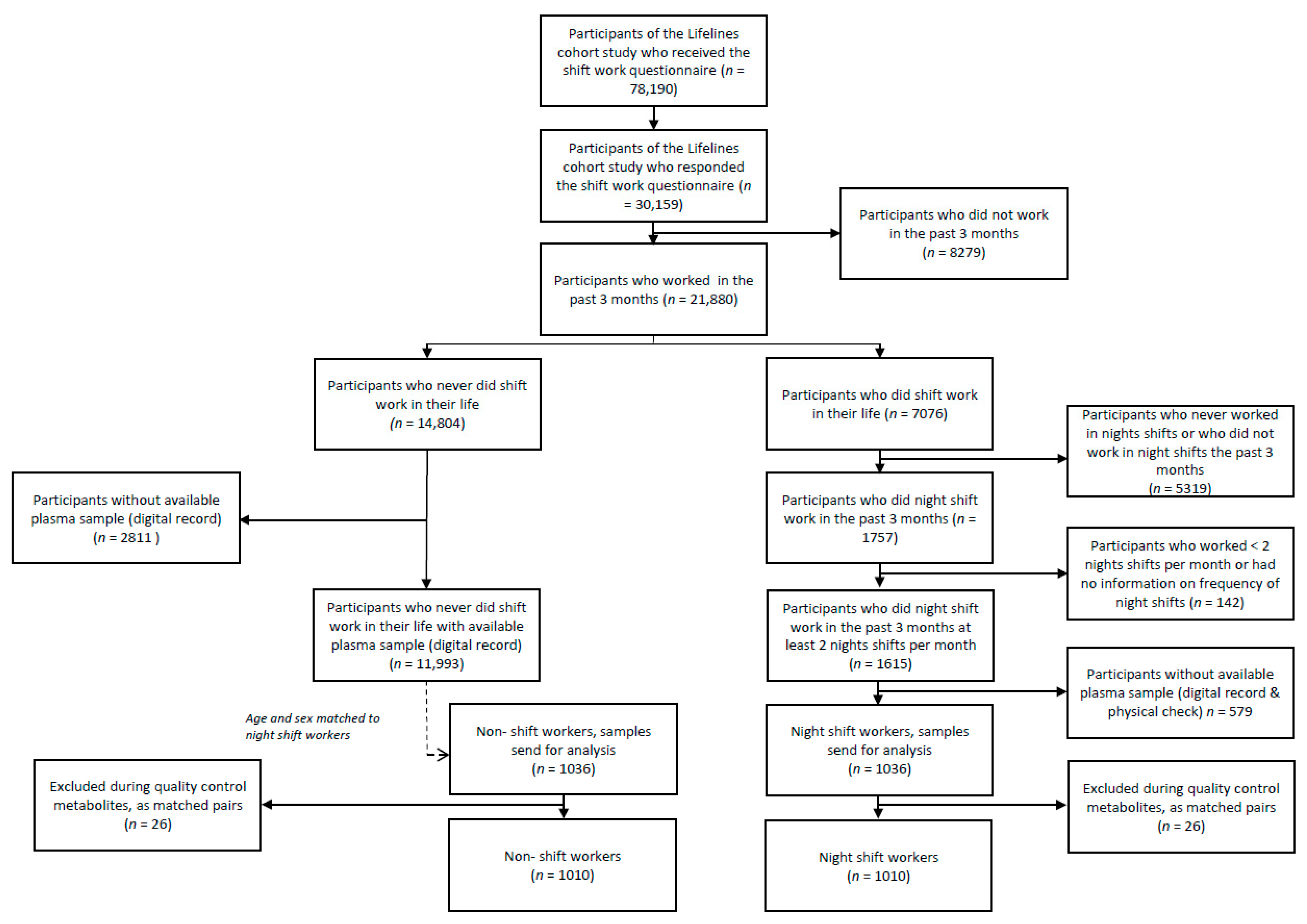
2.1. Study Population and Design
2.2. Data and Sample Collection
2.3. Covariates
2.4. Metabolomics Measurements
2.5. Statistical Methods
3. Results
3.1. Description of the Study Population and Dataset
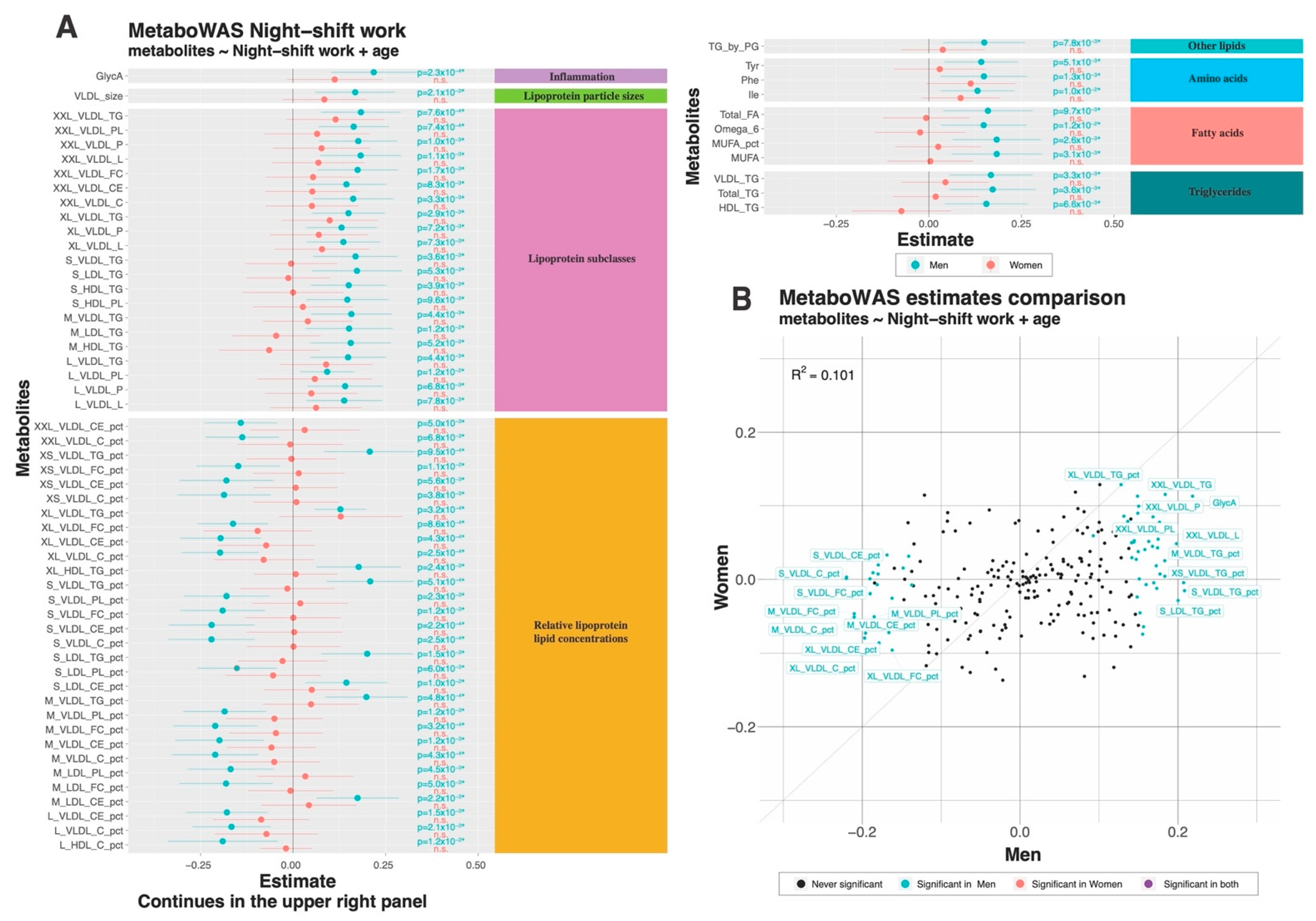
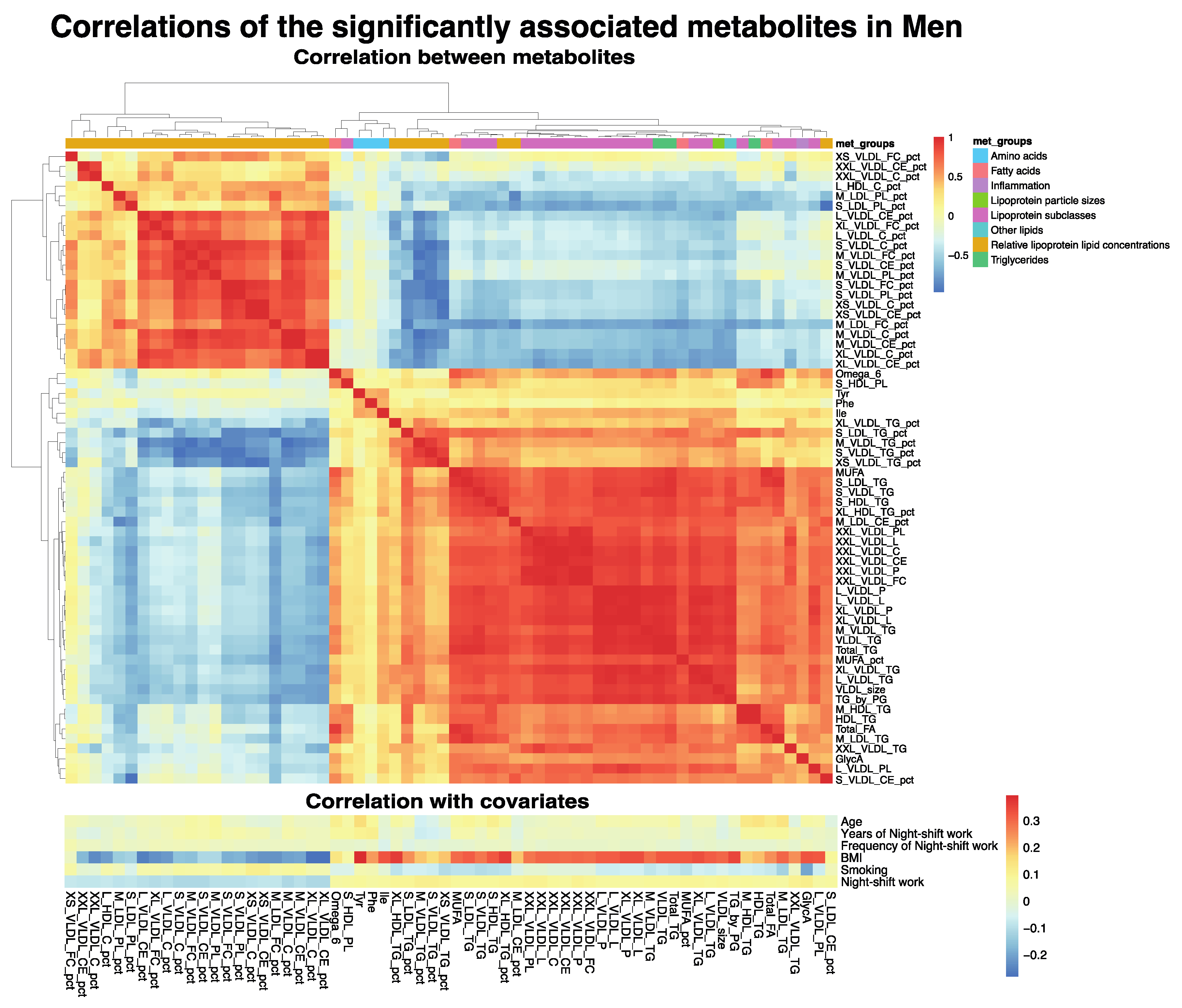
3.2. Associations between Metabolite Markers and Night Shift Work
3.3. Multi-Biomarker Scores
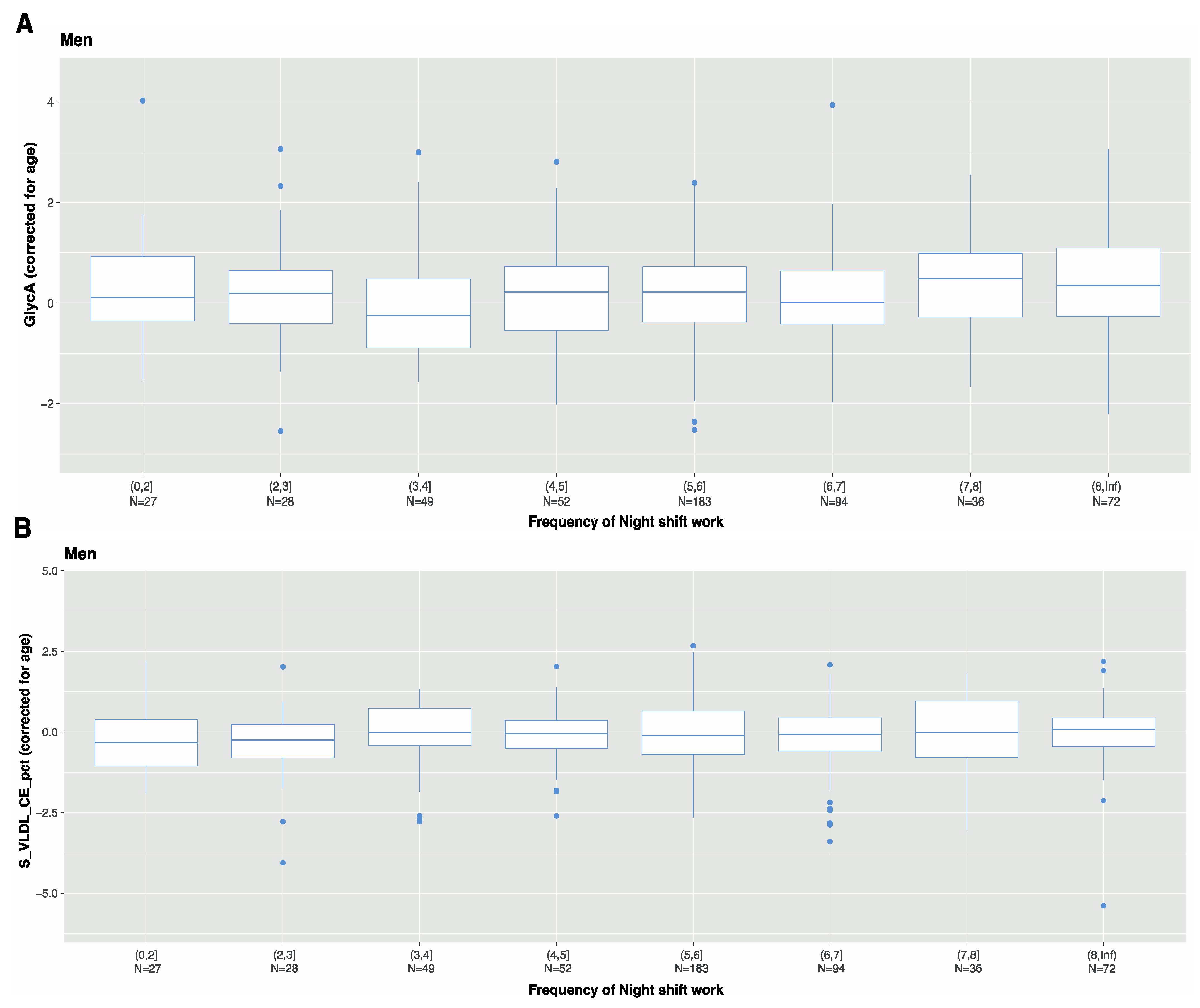
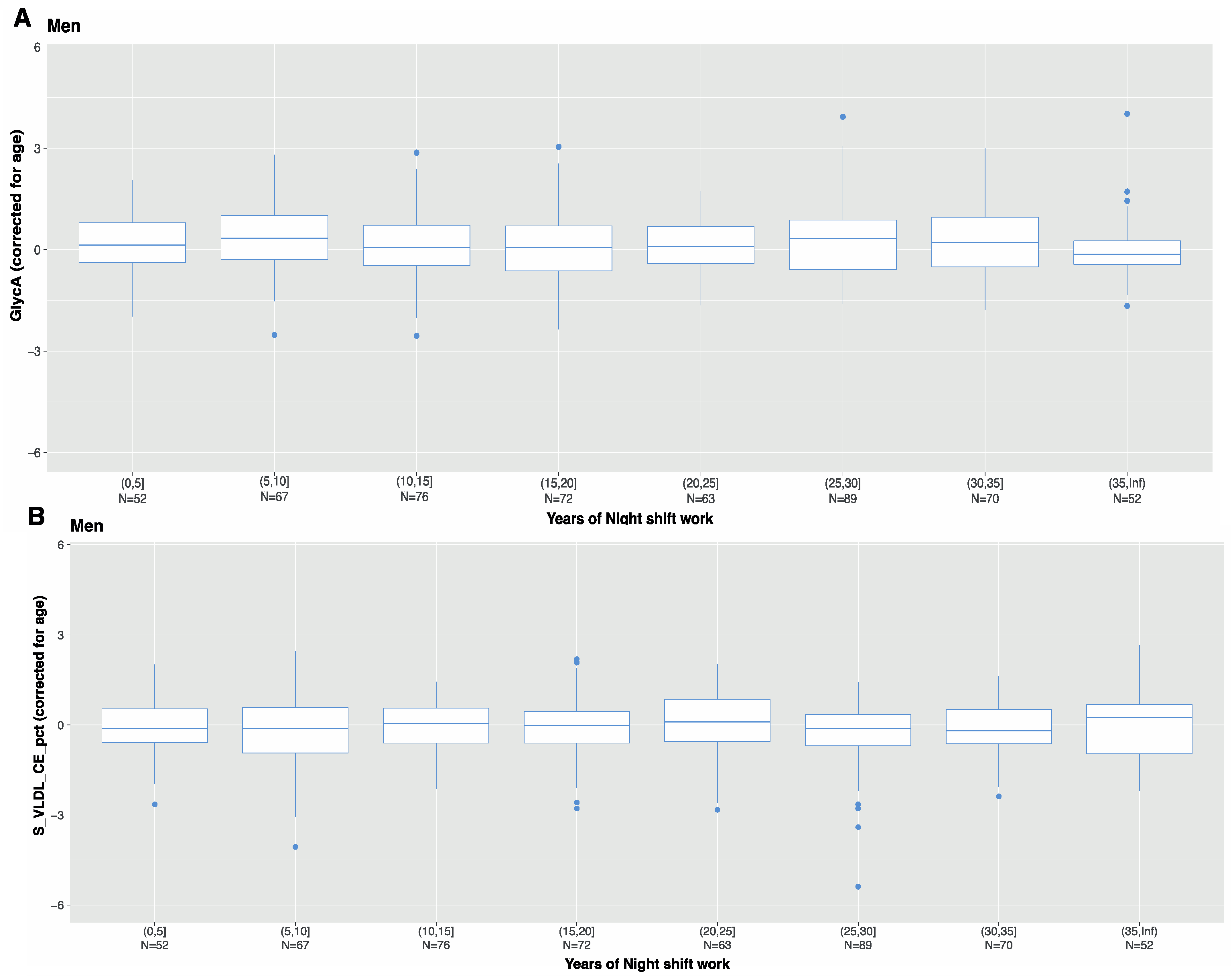
3.4. Characteristics of Night Shift Work
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurofound. European Working Conditions Survey. 2015. Available online: https://www.eurofound.europa.eu/data/european-working-conditions-survey (accessed on 7 January 2021).
- IARC Working Group. Carcinogenicity of night shift work. Lancet Oncol. 2019, 20, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Torquati, L.; Mielke, G.I.; Brown, W.J.; Kolbe-Alexander, T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand. J. Work Environ. Health 2018, 44, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Armstrong, M.; Cairns, B.; Key, T.J.; Travis, R.C. Shift work and chronic disease: The epidemiological evidence. Occup. Med. 2011, 61, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-J.; Sun, M.H.; Wen, M.Z.-Y.; Zhang, M.M.; Wang, M.H.-Y.; He, M.X.-H.; Jiang, M.Y.-T.; Zhao, Y.-H. Shift work and health outcomes: An umbrella review of systematic reviews and meta-analyses of epidemiological studies. J. Clin. Sleep Med. 2021, 18, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Loef, B.; van Baarle, D.; van der Beek, A.J.; Sanders, E.A.M.; Bruijning-Verhagen, P.; Proper, I.K. Shift Work and Respiratory Infections in Health-Care Workers. Am. J. Epidemiol. 2019, 188, 509–517. [Google Scholar] [CrossRef]
- Wei, F.; Chen, W.; Lin, X. Night-shift work, breast cancer incidence, and all-cause mortality: An updated meta-analysis of prospective cohort studies. Sleep Breath. 2021, 26, 1509–1526. [Google Scholar] [CrossRef]
- Nätti, J.; Anttila, T.; Oinas, T.; Mustosmäki, A. Night Work and Mortality: Prospective Study Among Finnish Employees Over the Time Span 1984 to 2008. Chronobiol. Int. 2012, 29, 601–609. [Google Scholar] [CrossRef]
- Trudel-Fitzgerald, C.; Zhou, E.; Poole, E.M.; Zhang, X.; Michels, K.B.; Eliassen, A.H.; Chen, W.Y.; Holmes, M.D.; Tworoger, S.S.; Schernhammer, E. Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. Br. J. Cancer 2017, 116, 1239–1246. [Google Scholar] [CrossRef]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef]
- Leung, G.K.W.; Huggins, C.E.; Ware, R.S.; Bonham, M.P. Time of day difference in postprandial glucose and insulin responses: Systematic review and meta-analysis of acute postprandial studies. Chronobiol. Int. 2019, 37, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, E.J.; Wopereis, S.; Kalsbeek, A. The Circadian Clock, Shift Work, and Tissue-Specific Insulin Resistance. Endocrinology 2020, 161, bqaa180. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.V.D.; Mook-Kanamori, D.O.; Donga, E.; van Dijk, M.; van Dijk, J.G.; Lammers, G.-J.; van Kralingen, K.W.; Prehn, C.; Adamski, J.; Romijn, J.A.; et al. A single night of sleep curtailment increases plasma acylcarnitines: Novel insights in the relationship between sleep and insulin resistance. Arch. Biochem. Biophys. 2016, 589, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Skornyakov, E.; Chowdhury, N.R.; Gajula, R.P.; Middleton, B.; Satterfield, B.C.; Porter, K.I.; Van Dongen, H.P.A.; Gaddameedhi, S. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 7825–7830. [Google Scholar] [CrossRef]
- Würtz, P.; Kangas, A.J.; Soininen, P.; Lawlor, A.D.; Smith, G.D.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2017, 186, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Streng, A.A.; Loef, B.; Dollé, M.E.T.; van der Horst, G.T.J.; Chaves, I.; Proper, K.I.; van Kerkhof, L.W.M. Night shift work characteristics are associated with several elevated metabolic risk factors and immune cell counts in a cross-sectional study. Sci. Rep. 2022, 12, 2022. [Google Scholar] [CrossRef]
- Stolk, R.P.; Rosmalen, J.G.M.; Postma, D.S.; de Boer, R.A.; Navis, G.; Slaets, J.P.J.; Ormel, J.; Wolffenbuttel, B.H.R. Universal risk factors for multifactorial diseases. Eur. J. Epidemiol. 2008, 23, 67–74. [Google Scholar] [CrossRef]
- Scholtens, S.; Smidt, N.; Swertz, M.A.; Bakker, S.J.L.; Dotinga, A.; Vonk, J.M.; Van Dijk, F.; Van Zon, S.K.R.; Wijmenga, C.; Wolffenbuttel, B.H.R.; et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2015, 44, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Sijtsma, A.; Rienks, J.; van der Harst, P.; Navis, G.; Rosmalen, J.G.M.; Dotinga, A. Cohort Profile Update: Lifelines, a three-generation cohort study and biobank. Int. J. Epidemiol. 2021, 51, e295–e302. [Google Scholar] [CrossRef]
- Stevens, R.G.; Hansen, J.; Costa, G.; Haus, E.; Kauppinen, T.; Aronson, K.J.; Castaño-Vinyals, G.; Davis, S.; Frings-Dresen, M.H.W.; Fritschi, L.; et al. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup. Environ. Med. 2011, 68, 154–162. [Google Scholar] [CrossRef]
- Garde, A.H.; Begtrup, L.; Bjorvatn, B.; Bonde, J.P.; Hansen, J.; Hansen, M.; Härmä, M.; Jensen, M.A.; Kecklund, G.; Kolstad, A.H.; et al. How to schedule night shift work in order to reduce health and safety risks. Scand. J. Work. Environ. Health 2020, 46, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Gibbons, H. Sex matters: A focus on the impact of biological sex on metabolomic profiles and dietary interventions. Proc. Nutr. Soc. 2020, 79, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A. Disturbance of the Circadian System in Shift Work and Its Health Impact. J. Biol. Rhythm. 2022, 37, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Krumsiek, J.; Mittelstrass, K.; Do, K.T.; Stückler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics 2015, 11, 1815–1833. [Google Scholar] [CrossRef]
- Proper, K.I.; Jaarsma, E.; Robroek, S.J.W.; Schram, J.L.D.; Boshuizen, H.; Picavet, H.S.J.; Verschuren, W.M.M.; van Oostrom, S.H. The mediating role of unhealthy behavior in the relationship between shift work and perceived health. BMC Public Health 2021, 21, 1300. [Google Scholar] [CrossRef]
- Hu, X.; Fan, Y.; Li, H.; Zhou, R.; Zhao, X.; Sun, Y.; Zhang, S. Impacts of Cigarette Smoking Status on Metabolomic and Gut Microbiota Profile in Male Patients With Coronary Artery Disease: A Multi-Omics Study. Front. Cardiovasc. Med. 2021, 8, 766739. [Google Scholar] [CrossRef]
- Stacklies, W.; Redestig, H.; Scholz, M.; Walther, D.; Selbig, J. pcaMethods a bioconductor package providing PCA methods for incomplete data. Bioinformatics 2007, 23, 1164–1167. [Google Scholar] [CrossRef]
- Deelen, J.; Kettunen, J.; Fischer, K.; van der Spek, A.; Trompet, S.; Kastenmüller, G.; Boyd, A.; Zierer, J.; Akker, E.B.V.D.; Ala-Korpela, M.; et al. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat. Commun. 2019, 10, 3346. [Google Scholar] [CrossRef]
- Hart, L.M.; Vogelzangs, N.; Mook-Kanamori, O.D.; Brahimaj, A.; Nano, J.; van der Heijden, A.A.W.A.; Van Dijk, K.W.; Slieker, R.C.; Steyerberg, E.W.; Ikram, M.A.; et al. Blood Metabolomic Measures Associate With Present and Future Glycemic Control in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 4569–4579. [Google Scholar] [CrossRef]
- Akker, E.B.V.D.; Trompet, S.; Wolf, J.J.B.; Beekman, M.; Suchiman, H.E.D.; Deelen, J.; Asselbergs, F.W.; Boersma, E.; Cats, D.; Elders, P.M.; et al. Metabolic Age Based on the BBMRI-NL 1H-NMR Metabolomics Repository as Biomarker of Age-related Disease. Circ. Genom. Precis. Med. 2020, 13, 541–547. [Google Scholar] [CrossRef]
- Lawler, P.R.; Mora, S. Glycosylation Signatures of Inflammation Identify Cardiovascular Risk: Some Glyc It Hot. Circ. Res. 2016, 119, 1154–1156. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Shalaurova, I.; Wolak-Dinsmore, J.; Connelly, M.A.; Mackey, R.H.; Stein, J.H.; Tracy, R.P. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin. Chem. 2015, 61, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Akinkuolie, A.O.; Pradhan, A.D.; Buring, J.E.; Ridker, P.M.; Mora, S. Novel Protein Glycan Side-Chain Biomarker and Risk of Incident Type 2 Diabetes Mellitus. Arter. Thromb. Vasc. Biol. 2015, 35, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.T.; Charakida, M.; Georgiopoulos, G.; Roberts, J.D.; Stafford, S.J.; Park, C.; Mykkänen, J.; Kähönen, M.; Lehtimäki, T.; Ala-Korpela, M.; et al. Glycoprotein Acetyls: A Novel Inflammatory Biomarker of Early Cardiovascular Risk in the Young. J. Am. Heart Assoc. 2022, 11, e024380. [Google Scholar] [CrossRef]
- Lawler, P.R.; Akinkuolie, A.O.; Chandler, P.D.; Moorthy, M.V.; Vandenburgh, M.J.; Schaumberg, D.A.; Lee, I.-M.; Glynn, R.J.; Ridker, P.M.; Buring, J.E.; et al. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circ. Res. 2016, 118, 1106–1115. [Google Scholar] [CrossRef]
- Ritchie, S.C.; Würtz, P.; Nath, A.P.; Abraham, G.; Havulinna, A.S.; Fearnley, L.G.; Sarin, A.-P.; Kangas, A.J.; Soininen, P.; Aalto, K.; et al. The Biomarker GlycA Is Associated with Chronic Inflammation and Predicts Long-Term Risk of Severe Infection. Cell Syst. 2015, 1, 293–301. [Google Scholar] [CrossRef]
- Dutheil, F.; Baker, J.S.; Mermillod, M.; De Cesare, M.; Vidal, A.; Moustafa, F.; Pereira, B.; Navel, V. Shift work, and particularly permanent night shifts, promote dyslipidaemia: A systematic review and meta-analysis. Atherosclerosis 2020, 313, 156–169. [Google Scholar] [CrossRef]
- Slade, E.; Irvin, M.R.; Xie, K.; Arnett, D.K.; Claas, S.A.; Kind, T.; Fardo, D.W.; Graf, G.A. Age and sex are associated with the plasma lipidome: Findings from the GOLDN study. Lipids Health Dis. 2021, 20, 30. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Zhao, S.; Hou, J.; Huang, L.; Xu, J.; Wang, W.; He, M.; Shen, O.; Zhang, J. Metabolomic Profiles of Shift Workers and Day Workers: A Cross-Sectional Study. Obesity 2021, 29, 1074–1082. [Google Scholar] [CrossRef]
- Rotter, M.; Brandmaier, S.; Covic, M.; Burek, K.; Hertel, J.; Troll, M.; Bader, E.; Adam, J.; Prehn, C.; Rathkolb, B.; et al. Night Shift Work Affects Urine Metabolite Profiles of Nurses with Early Chronotype. Metabolites 2018, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Chaby, L.E.; Lasseter, H.C.; Contrepois, K.; Salek, R.M.; Turck, C.W.; Thompson, A.; Vaughan, T.; Haas, M.; Jeromin, A. Cross-Platform Evaluation of Commercially Targeted and Untargeted Metabolomics Approaches to Optimize the Investigation of Psychiatric Disease. Metabolites 2021, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Manmadhan, A.; Lin, B.-X.; Zhong, J.; Parikh, M.; Berger, J.S.; Fisher, E.A.; Heffron, S.P.; Parikh, M.M.; Fisher, M.E.A. Elevated GlycA in severe obesity is normalized by bariatric surgery. Diabetes Obes. Metab. 2018, 21, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Gommans, F.; Jansen, N.; Stynen, D.; De Grip, A.; Kant, I. The ageing shift worker: A prospective cohort study on need for recovery, disability, and retirement intentions. Scand. J. Work. Environ. Health 2015, 41, 356–367. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costa, G. Shift work and occupational medicine: An overview. Occup. Med. 2003, 53, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Loef, B.; Dollé, M.E.; Proper, K.I.; van Baarle, D.; Initiative, L.C.R.; van Kerkhof, L.W. Night-shift work is associated with increased susceptibility to SARS-CoV-2 infection. Chronobiol. Int. 2022, 39, 1100–1109. [Google Scholar] [CrossRef]
- Kervezee, L.; Cermakian, N.; Boivin, D.B. Individual metabolomic signatures of circadian misalignment during simulated night shifts in humans. PLoS Biol. 2019, 17, e3000303. [Google Scholar] [CrossRef]
| Study Population (n = 2020) | Non-Shift Workers (n = 1010) | Night Shift Workers (n = 1010) |
|---|---|---|
| Age (in years, mean (SD)) | 46.4 (8.5) | 46.4 (8.5) |
| Sex (% male) | 53.6 | 53.6 |
| BMI (in kg/M2, mean (SD)) | 25.9 (4.0) | 26.6 (4.4) * |
| Smoking (current/former/never, %) (n = 1922) | ||
| Frequency of night shifts/month (mean, SD) | n.a. | 5.9 (3.7) |
| Duration of night shifts in years (mean, SD) | n.a. | 18.3 (10.5) |
| Men (n = 1082) | Non-shift workers (n = 541) | Night shift workers (n = 541) |
| Age (in years, mean (SD)) | 47.7 (8.1) | 47.7 (8.1) |
| BMI (in kg/M2, mean (SD)) | 26.1 (3.6) | 26.9 (3.9) * |
| Smoking (current/former/never, %) (n = 1030) | 19/27/54% | 21/34/46% * |
| Frequency of night shifts/ month (mean, SD) | n.a. | 6.8 (3.8) |
| Duration of night shifts in years (mean, SD) | n.a. | 18.9 (10.7) |
| Women (n = 938) | Non-shift workers (n = 469) | Night shift workers (n = 469) |
| Age (in years, mean (SD)) | 45.0 (8.8) | 45.0 (8.8) |
| BMI (in kg/M2, mean (SD)) | 25.5 (4.4) | 26.2 (4.8) * |
| Smoking (current/former/never, %) (n = 892) | 11/28/61% | 15/32/55% |
| Frequency of night shifts/ month (mean, SD) | n.a. | 4.8 (3.1) |
| Duration of night shifts in years (mean, SD) | n.a. | 17.6 (10.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizzarri, D.; Dollé, M.E.T.; Loef, B.; van den Akker, E.B.; van Kerkhof, L.W.M. GlycA, a Biomarker of Low-Grade Inflammation, Is Increased in Male Night Shift Workers. Metabolites 2022, 12, 1172. https://doi.org/10.3390/metabo12121172
Bizzarri D, Dollé MET, Loef B, van den Akker EB, van Kerkhof LWM. GlycA, a Biomarker of Low-Grade Inflammation, Is Increased in Male Night Shift Workers. Metabolites. 2022; 12(12):1172. https://doi.org/10.3390/metabo12121172
Chicago/Turabian StyleBizzarri, Daniele, Martijn E. T. Dollé, Bette Loef, Erik B. van den Akker, and Linda W. M. van Kerkhof. 2022. "GlycA, a Biomarker of Low-Grade Inflammation, Is Increased in Male Night Shift Workers" Metabolites 12, no. 12: 1172. https://doi.org/10.3390/metabo12121172
APA StyleBizzarri, D., Dollé, M. E. T., Loef, B., van den Akker, E. B., & van Kerkhof, L. W. M. (2022). GlycA, a Biomarker of Low-Grade Inflammation, Is Increased in Male Night Shift Workers. Metabolites, 12(12), 1172. https://doi.org/10.3390/metabo12121172








