Identification and Dissipation of Chlorpyrifos and Its Main Metabolite 3,5,6-TCP during Wheat Growth with UPLC-QTOF/MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Field Experiment Design
2.3. Sample Treatment
2.4. Instrumentation and Conditions
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
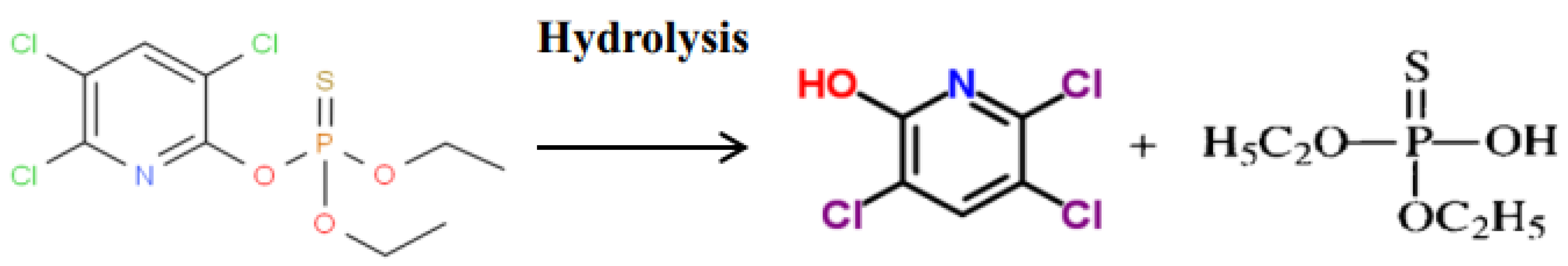
3.1. Identification and Confirmation of Chlorpyrifos and Its Metabolites with UPLC-QTOF/MS
3.2. Method Validation for Chlorpyrifos and 3,5,6-TCP in Different Parts of Wheat
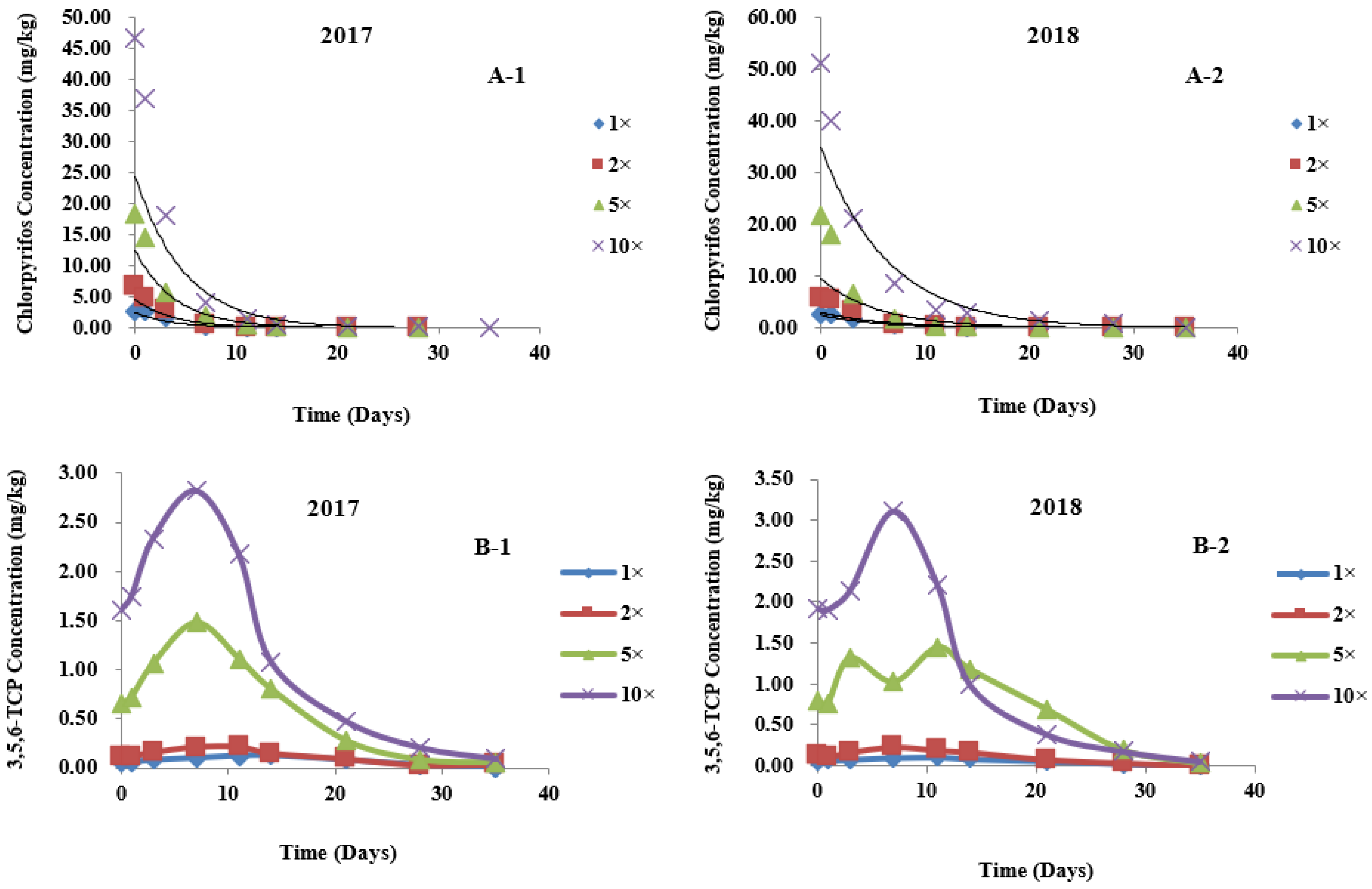
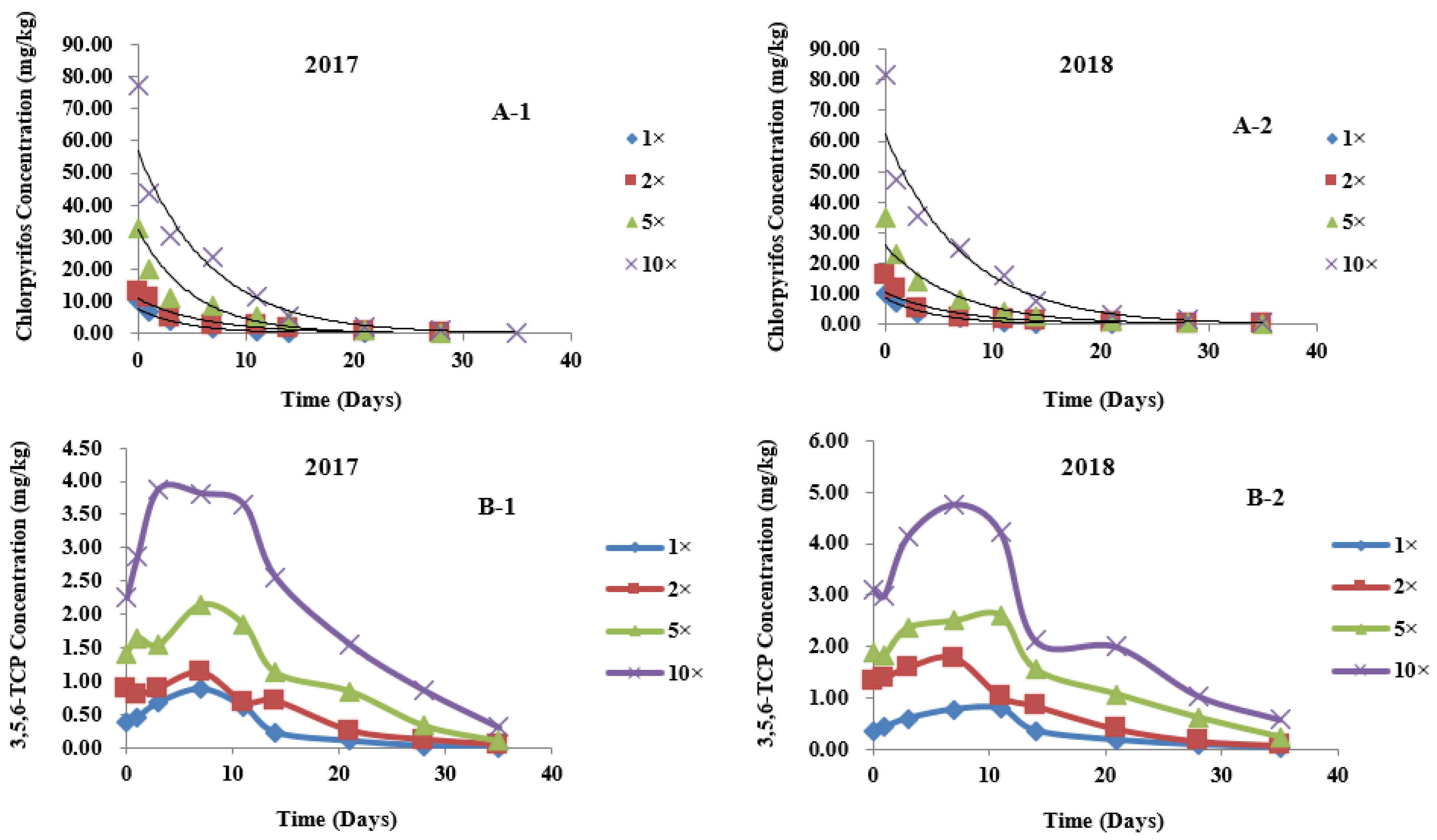
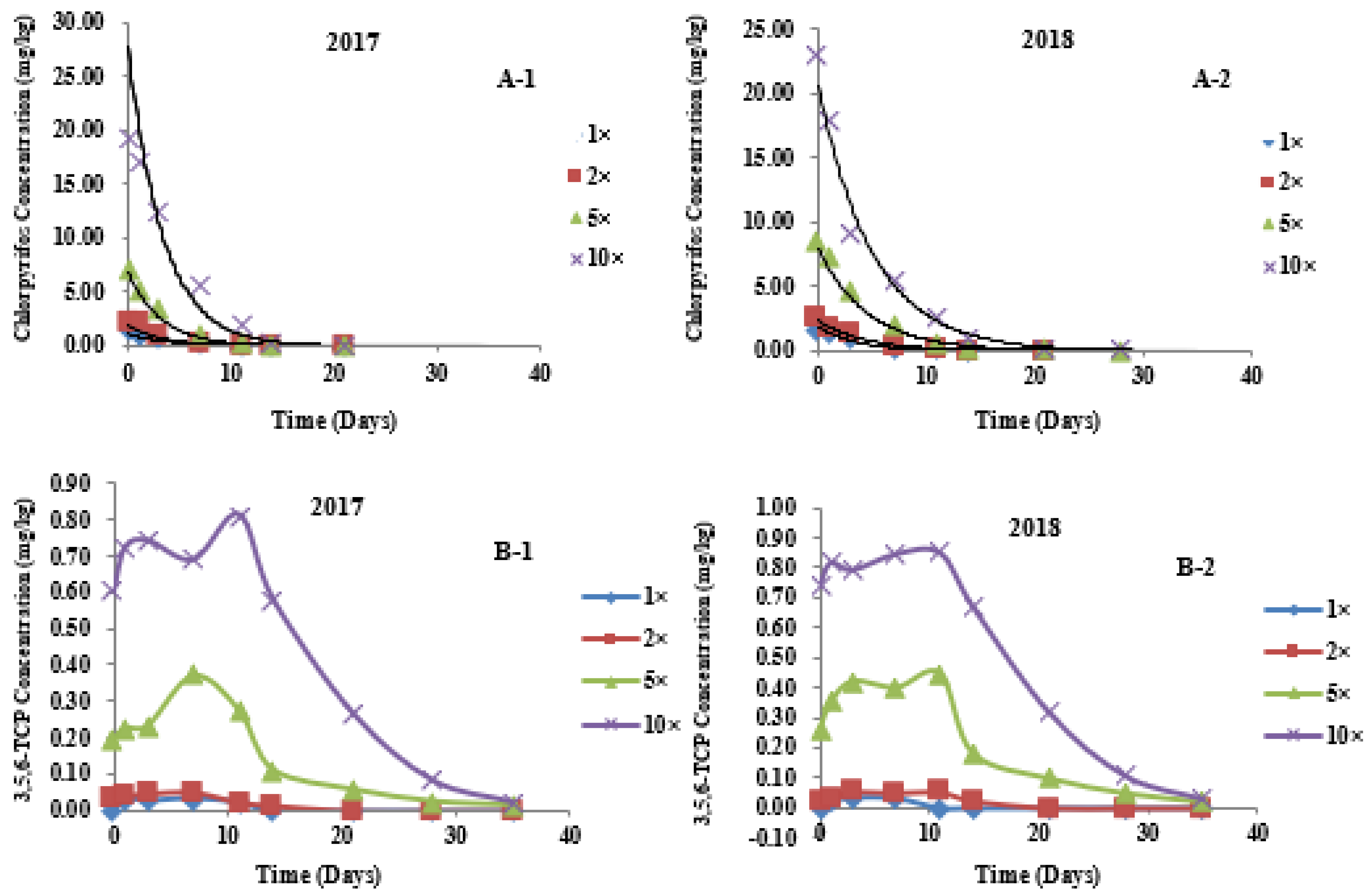
3.3. Dynamic Distribution of Chlorpyrifos in Different Parts of Wheat
3.4. Metabolic Kinetics of 3,5,6-TCP in Different Parts of Wheat
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bose, S.; Kumar, P.S.; Dai-Viet, N.V. A review on the microbial degradation of chlorpyrifos and its metabolite TCP. Chemosphere 2021, 283, 131447. [Google Scholar] [CrossRef] [PubMed]
- Sud, D.; Kumar, J.; Kaur, P.; Bansal, P. Toxicity, natural and induced degradation of chlorpyrifos. J. Chil. Chem. Soc. 2020, 65, 4807–4816. [Google Scholar] [CrossRef]
- Akbar, S.; Sultan, S. Soil bacteria showing a potential of chlorpyrifos degradation and plant growth enhancement. Braz. J. Microbiol. 2016, 47, 563–570. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Sandoval, M. Effects of chlorpyrifos on soil carboxylesterase activity at an aggregate-size scale. Ecotoxicol. Environ. Saf. 2017, 142, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y. Residue Characteristics and Degradation Mechanisms of Common Pesticides in Wheat [D]; University of Yangzhou: Yangzhou, China, 2007; pp. 51–57. [Google Scholar]
- Wu, Y.J.; Lv, C.Q.; Li, Y.; Chen, Y.P.; Yao, W.M.; Pan, Y.K.; Zhen, Y.K. Study on resides and degradation dynamics of chlorpyrifos in green bean. Chin. J. Insp. Quar. 2012, 6, 44–47. [Google Scholar]
- Cao, L.; Xu, J.H.; Wu, G.; Li, M.M.; Jiang, J.D.; He, J.; Li, S.P.; Hong, Q. Identification of two combined genes responsible for dechlorination of 3,5,6-trichloro-2-pyridinol (TCP) in Cupriavidus pauculus P2. J. Hazard. Mater. 2013, 260, 700–706. [Google Scholar] [CrossRef]
- Haque, M.A.; Hong, S.Y.; Hwang, C.E.; Kim, S.C.; Cho, K.M. Cloning of an organophosphorus hydrolase (opdD) gene of Lactobacillus sakei WCP904 isolated from chlorpyrifos-impregnated kimchi and hydrolysis activities of its gene product for organophosphorus pesticides. Appl. Biol. Chem. 2018, 61, 643–651. [Google Scholar] [CrossRef]
- Watts, M. Chlorpyrifos as a Possible Global Persistent Organic Pollutant; Pesticide Action Network North America: Berkeley, CA, USA, 2012; pp. 1–34. [Google Scholar]
- Furnes, B.; Schlenk, D. Extrahepatic metabolism of carbamate and organophosphate thioether compounds by the flavin-containing monooxygenase and cytochrome P450 systems. Drug. Metab. Dispos. Biol. Fate. Chem. 2005, 33, 214–218. [Google Scholar] [CrossRef]
- Forsyth, C.S.; Chambers, J.E. Activation and degradation of the phosphorothionate insecticides parathion and EPN by rat brain. Biochem. Pharmacol. 1989, 38, 1597–1603. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Wendling, L.A.; Wang, C.H.; Pei, Y.S. Behavior of chlorpyrifos and its major metabolite TCP (3,5,6-trichloro-2-pyridinol) in agricultural soils amended with drinking water treatment residuals. J. Soils. Sediments 2017, 17, 889–900. [Google Scholar] [CrossRef]
- Sparling, D.W.; Fellers, G. Comparative toxicity of chlorpyrifos, diazinon, malathion and their oxon derivatives to larval Rana boylii. Environ. Pollut. 2007, 147, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Antonious, G.F.; Turley, E.T.; Abubakari, M.; Snyder, J.C. Dissipation, half-lives, and mass spectrometric identification of chlorpyrifos and its two metabolites on field-grown collard and kale. J. Environ. Sci. Health. B 2017, 52, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Briceño, G.; Fuentes, M.S.; Palma, G.; Jorquera, M.A.; Amoroso, M.J.; Diez, M.C. Chlorpyrifos biodegradation and 3,5,6-trichloro-2-pyridinol production by actinobacteria isolated from soil. Int. Biodeterior. Biodegrad. 2012, 73, 1–7. [Google Scholar] [CrossRef]
- Das, S.; Adhya, T.K. Degradation of chlorpyrifos in tropical rice soils. J. Environ. Manag. 2015, 152, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nabil, S.A.; Hussein, H.S.; Salah, E.M.A.A.; Reda, A.B. Isolation, characterization and fingerprinting of some chlorpyrifos- degrading bacterial strains isolated from Egyptian pesticides-polluted soils. Afr. J. Microbiol. Res. 2011, 5, 2855–2862. [Google Scholar] [CrossRef]
- Saito-Shida, S.; Nemoto, S.; Teshima, R.; Akiyama, H. Quantitative analysis of pesticide residues in vegetables and fruits by liquid chromatography quadrupole time-of-flight mass spectrometry. Food. Addit. Contam. Part. A. 2016, 33, 119–127. [Google Scholar] [CrossRef]
- Yang, X.F.; Luo, J.H.; Duan, Y.; Li, S.H.; Liu, C.H. Simultaneous analysis of multiple pesticide residues in minor fruits by ultrahigh-performance liquid chromatography/hybrid quadrupole time-of-fight mass spectrometry. Food Chem. 2018, 241, 188–198. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Y.; Chang, C.P.; Lo, C.K.; Wang, X. High-throughput screening and quantification of pesticides in paprika by UHPLC-Q-TOF/MS. Food. Anal. Methods 2021, 14, 2186–2198. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Codex Pesticide Residues Limits in Food and Feed Database. 2003. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticide-detail/en/?p_id=17 (accessed on 12 June 2021).
- United States Environmental Protection Agency. Office of Pesticide Programs. Index to Pesticide Chemical Names, Part 180 Tolerance Information, and Food and Feed Commodities (by Commodity). 2012. Available online: https://www.epa.gov/sites/default/files/2015-01/documents/tolerances-commodity.pdf (accessed on 20 November 2021).
- Japan Food Chemical Research Foundation. The Japanese Positive List System for Agricultural Chemical Residues in Foods. 2021. Available online: http://db.ffcr.or.jp/front/pesticide_detail?id=21800 (accessed on 25 April 2022).
- National Standard of the People’s Republic of China (NSPRC). GB 2763-2021; Maximum Residue Limits for Pesticides in Food. China Standard Publishing House: Beijing, China, 2016; pp. 95–97.
- López, M.G.; Fussell, R.J.; Stead, S.L.; Roberts, D.; McCullagh, M.; Rao, R. Evaluation and validation of an accurate mass screening method for the analysis of pesticides in fruits and vegetables using liquid chromatography-quadrupole-time of flight-mass spectrometry with automated detection. J. Chromatogr. A. 2014, 1373, 40–50. [Google Scholar] [CrossRef]
- Bauer, A.; Luetjohann, J.; Hanschen, F.S.; Schreiner, M.; Kuballa, J.; Jantzen, E.; Rohn, S. Identification and characterization of pesticide metabolites in brassica species by liquid chromatography travelling wave ion mobility quadrupole time-of-flight mass spectrometry (UPLC-TWIMS-QTOF-MS). Food Chem. 2018, 244, 292–303. [Google Scholar] [CrossRef]
- Sánchez-Hernández, L.; Hernández-Domínguez, D.; Martín, M.T.; Nozal, M.J.; Higes, M.; Bernal Yagüe, J.L. Residues of neonicotinoids and their metabolites in honey and pollen from sunflower and maize seed dressing crops. J. Chromatogr. A. 2016, 1428, 220–227. [Google Scholar] [CrossRef] [PubMed]
- González-Curbelo, M.Á.; Socas-Rodríguez, B.; Herrero, M.; Herrera-Herrera, A.V.; Hernández-Borges, J. Dissipation kinetics of organophosphorus pesticides in milled toasted maize and wheat flour (gofio) during storage. Food Chem. 2017, 229, 854–859. [Google Scholar] [CrossRef]
- Kong, Z.Q.; Li, M.M.; Chen, J.Y.; Gui, Y.J.; Bao, Y.M.; Fan, B.; Jian, Q.; Francis, F.; Dai, X.F. Behavior of field-applied triadimefon, malathion, dichlorvos, and their main metabolites during barley storage and beer processing. Food Chem. 2016, 211, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, Y.; Yu, X.Y.; Liu, X.J. Dissipation of chlorpyrifos and residue analysis in rice, soil and water under paddy field conditions. Ecotoxicol. Environ. Saf. 2012, 78, 276–280. [Google Scholar] [CrossRef]
- Mahdavi, V.; Farimani, M.M.; Fathi, F.; Ghassempour, A. A targeted metabolomics approach toward understanding metabolic variations in rice under pesticide stress. Anal. Biochem. 2015, 478, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Z.; Jiang, M.; Xie, C.F.; Song, S.Y.; Wang, J.; Bai, G.; Luo, G.A. Metabolic fingerprinting of acs7 mutant and wild-type arabidopsis thaliana under salt stress by ultra performance liquid chromatography coupled with quadrupole/time of flight mass spectrometry. Anal. Lett. 2012, 45, 1786–1798. [Google Scholar] [CrossRef]
- Regueiro, J.; López-Fernández, O.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. A review on the fermentation of foods and the residues of pesticides—Biotransformation of pesticides and effects on fermentation and food quality. Crit. Rev. Food. Sci. Nutr. 2015, 55, 839–863. [Google Scholar] [CrossRef]
- Racke, K.D. Environmental fate of chlorpyrifos. Rev. Environ. Contam. Toxicol. 1993, 131, 1–150. [Google Scholar]
- Zhang, Z.Y.; Jiang, W.W.; Jian, Q.; Song, W.C.; Zheng, Z.T.; Wang, D.L.; Liu, X.J. Changes of field incurred chlorpyrifos and its toxic metabolite residues in rice during food processing from-RAC-to-consumption. PLoS ONE 2015, 10, e0116467. [Google Scholar] [CrossRef][Green Version]
- Chai, L.K.; Mohd-Tahir, N.; Bruun Hansen, H.C. Dissipation of acephate, chlorpyrifos, cypermethrin and their metabolites in a humid-tropical vegetable production system. Pest. Manage. Sci. 2009, 65, 189–196. [Google Scholar] [CrossRef]
- Abraham, J.; Silambarasan, S. Biodegradation of chlorpyrifos and its hydrolyzing metabolite 3,5,6-trichloro-2-pyridinol by Sphingobacterium sp. JAS3. Process. Biochem. 2013, 48, 1559–1564. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Li, Q.F.; Liu, H.M.; Feng, Z.Z.; Yan, X.; Hong, Q.; Li, S.P. Biodegradation of chlorpyrifos and 3,5,6-trichloro-2-pyridinol by Cupriavidus sp. DT-1. Bioresour. Technol. 2013, 127, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.C.; Minard, R.D.; Bollag, J.M. Microbial and photolytic degradation of 3,5,6-trichloro-2-pyridinol. Environ. Toxicol. Chem. 2010, 17, 814–819. [Google Scholar] [CrossRef]

| Compound | Retention Time (min) | Formula | Observed Neutral Mass (Da) | Fragments | Adducts |
|---|---|---|---|---|---|
| Chlorpyrifos 3,5,6-TCP | 11.20 6.00 | C9H11Cl3NO3PS C5H2 Cl3NO | 349.9335 197.9268 | 311.2577 197.9273 96.9505 184.0724 116.0693 70.0646 | +H, +Na +H |
| Compound | Formula | Exact Mass (Da) |
|---|---|---|
| 3,5,6-TCP | C5H2Cl3NO | 198.4345 |
| DEP | C4H11O4P | 154.1015 |
| DETP | C4H11O3PS | 170.1671 |
| CPO | C9H11Cl3NO4P | 332.9491 |
| Compound | Matrix | R2 | Average Recovery and Standard Deviations (%) | LOD (µg/kg) | LOQ (µg/kg) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Spiking Level (µg/kg) | |||||||||
| 20 | 50 | 100 | 200 | 500 | |||||
| Chlorpyrifos | Wheatear | 0.9995 | 72.00 ± 2.18 | 71.33 ± 6.43 | 72.67 ± 7.57 | 82.83 ± 1.76 | 102.13 ± 3.35 | 0.38 | 1.30 |
| Leaf | 0.9974 | 76.67 ± 5.77 | 83.33 ± 5.03 | 85.00 ± 2.00 | 85.50 ± 2.78 | 76.73 ± 4.27 | 0.85 | 2.70 | |
| Stem | 0.9983 | 77.00 ± 3.29 | 78.46 ± 5.87 | 79.16 ± 7.22 | 85.45 ± 2.77 | 89.53 ± 4.88 | 0.70 | 2.20 | |
| 3, 5, 6-TCP | Wheatear | 0.9977 | 66.33 ± 6.24 | 70.01 ± 7.35 | 72.37 ± 8.35 | 83.57 ± 7.37 | 83.36 ± 8.99 | 2.90 | 11.00 |
| Leaf | 0.9958 | 65.79 ± 5.34 | 63.38 ± 4.26 | 69.24 ± 6.38 | 72.43 ± 8.46 | 77.84 ± 6.59 | 4.50 | 14.00 | |
| Stem | 0.9983 | 67.86 ± 5.76 | 71.47 ± 7.58 | 73.15 ± 5.37 | 81.38 ± 3.58 | 87.67 ± 5.68 | 4.25 | 13.50 | |
| Matrix | Time | Treatment | First-Order Kinetic Equation | C0 (mg/kg) | R2 | K (1/d) | t1/2 (d) |
|---|---|---|---|---|---|---|---|
| Wheatear | 2017 | 1× | Ct = 2.4668 e−0.2765t | 2.4668 | 0.9201 | 0.2765 | 2.51 |
| 2× | Ct = 4.5245 e−0.2609t | 4.5245 | 0.9638 | 0.2609 | 2.66 | ||
| 5× | Ct = 12.3650 e−0.2510t | 12.3650 | 0.9653 | 0.2510 | 2.76 | ||
| 10× | Ct = 24.3582 e−0.2080t | 24.3582 | 0.9265 | 0.2080 | 3.33 | ||
| 2018 | 1× | Ct = 2.4479 e−0.1921t | 2.4479 | 0.9693 | 0.1921 | 3.61 | |
| 2× | Ct = 2.9560 e−0.1653t | 2.9560 | 0.8646 | 0.1653 | 4.19 | ||
| 5× | Ct = 9.3164 e−0.1775t | 9.3164 | 0.8839 | 0.1775 | 3.90 | ||
| 10× | Ct = 34.7860 e−0.1562t | 34.7860 | 0.9595 | 0.1562 | 4.44 | ||
| Leaf | 2017 | 1× | Ct = 7.6341 e−0.2199t | 7.6341 | 0.9771 | 0.2199 | 3.15 |
| 2× | Ct = 10.8377 e−0.1580t | 10.8377 | 0.9726 | 0.1580 | 4.39 | ||
| 5× | Ct = 32.4195 e−0.1954t | 32.4195 | 0.9381 | 0.1954 | 3.55 | ||
| 10× | Ct = 56.8813 e−0.1494t | 56.8813 | 0.9898 | 0.1494 | 4.64 | ||
| 2018 | 1× | Ct = 8.6738 e−0.2117t | 8.6738 | 0.9863 | 0.2117 | 3.27 | |
| 2× | Ct = 10.5375 e−0.1516t | 10.5375 | 0.9778 | 0.1516 | 4.57 | ||
| 5× | Ct = 25.7491 e−0.1512t | 25.7491 | 0.9909 | 0.1512 | 4.58 | ||
| 10× | Ct = 62.3189 e−0.1372t | 62.3189 | 0.9919 | 0.1372 | 5.05 | ||
| Stem | 2017 | 1× | Ct = 1.0919 e−0.2687t | 1.0919 | 0.8979 | 0.2687 | 2.58 |
| 2× | Ct = 1.8643 e−0.2954t | 1.8643 | 0.9253 | 0.2954 | 2.35 | ||
| 5× | Ct = 6.8317 e−0.3142t | 6.8317 | 0.9685 | 0.3142 | 2.21 | ||
| 10× | Ct = 27.7730 e−0.2979t | 27.7730 | 0.9742 | 0.2979 | 2.33 | ||
| 2018 | 1× | Ct = 1.8867 e−0.2950t | 1.8867 | 0.9893 | 0.2950 | 2.35 | |
| 2× | Ct = 2.3523 e−0.2311t | 2.3523 | 0.9799 | 0.2311 | 3.00 | ||
| 5× | Ct = 8.0059 e−0.2213t | 8.0059 | 0.9843 | 0.2213 | 3.13 | ||
| 10× | Ct = 20.6520 e−0.2002t | 20.6520 | 0.9923 | 0.2002 | 3.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Li, J.; Feng, M.; Tang, Q.; Jiang, Z.; Chen, H.; Shan, T.; Li, J. Identification and Dissipation of Chlorpyrifos and Its Main Metabolite 3,5,6-TCP during Wheat Growth with UPLC-QTOF/MS. Metabolites 2022, 12, 1162. https://doi.org/10.3390/metabo12121162
Yu L, Li J, Feng M, Tang Q, Jiang Z, Chen H, Shan T, Li J. Identification and Dissipation of Chlorpyrifos and Its Main Metabolite 3,5,6-TCP during Wheat Growth with UPLC-QTOF/MS. Metabolites. 2022; 12(12):1162. https://doi.org/10.3390/metabo12121162
Chicago/Turabian StyleYu, Lili, Jia Li, Meiqin Feng, Qian Tang, Zejun Jiang, Hui Chen, Tingting Shan, and Junhui Li. 2022. "Identification and Dissipation of Chlorpyrifos and Its Main Metabolite 3,5,6-TCP during Wheat Growth with UPLC-QTOF/MS" Metabolites 12, no. 12: 1162. https://doi.org/10.3390/metabo12121162
APA StyleYu, L., Li, J., Feng, M., Tang, Q., Jiang, Z., Chen, H., Shan, T., & Li, J. (2022). Identification and Dissipation of Chlorpyrifos and Its Main Metabolite 3,5,6-TCP during Wheat Growth with UPLC-QTOF/MS. Metabolites, 12(12), 1162. https://doi.org/10.3390/metabo12121162






