Normal Thermostability of p.Ser113Leu and p.Arg631Cys Variants of Mitochondrial Carnitine Palmitoyltransferase II (CPT II) in Human Muscle Homogenate
Abstract
1. Introduction
2. Patients and Controls
3. Materials and Methods
3.1. CPT Assay
- (i)
- For 6 min at different temperatures (30 °C, 37 °C, 40 °C, 45 °C, 49 °C);
- (ii)
- At 40 °C and 46 °C for up to 15 min (0, 5, 7, 10, 15 min).
3.2. Protein Determination
4. Results
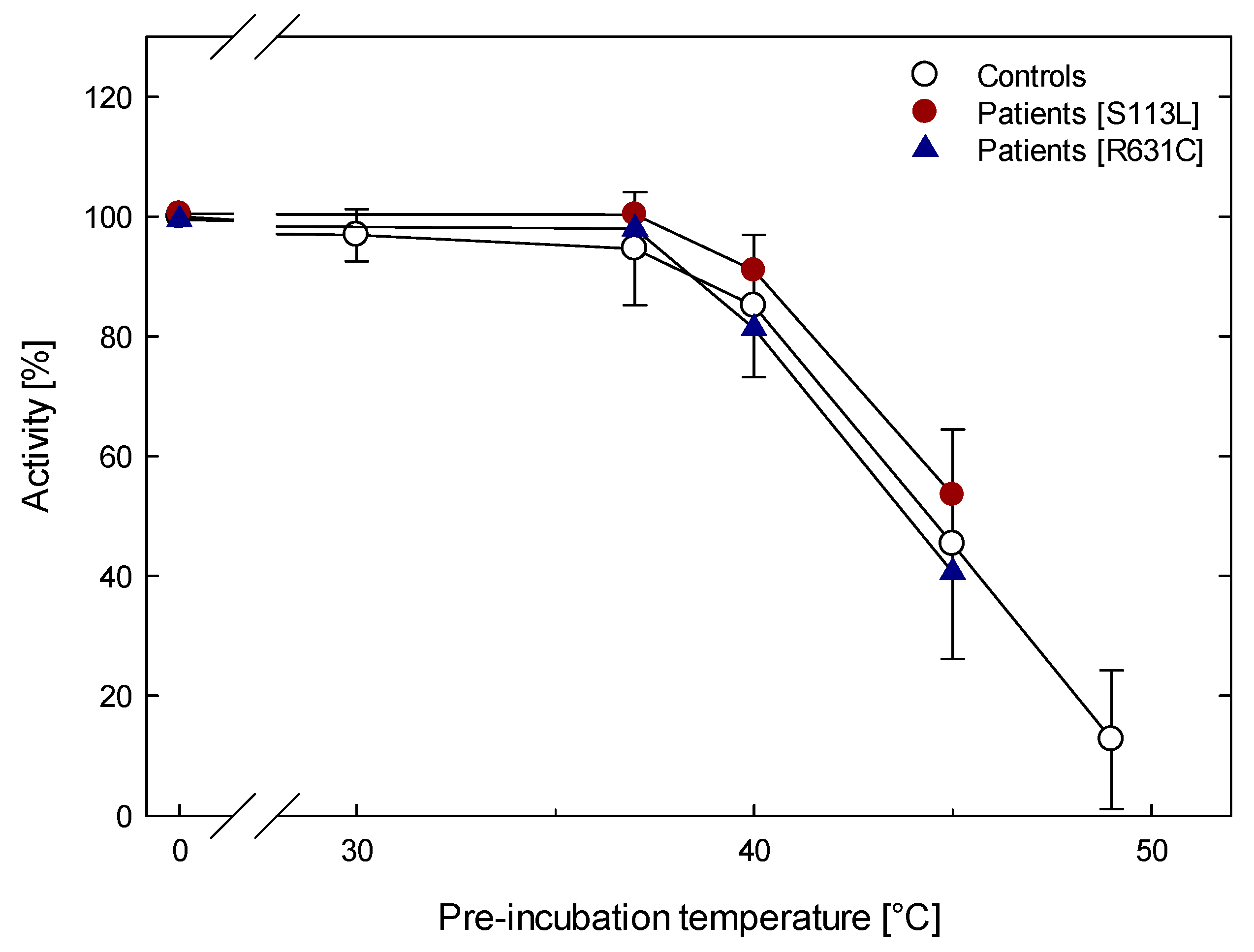
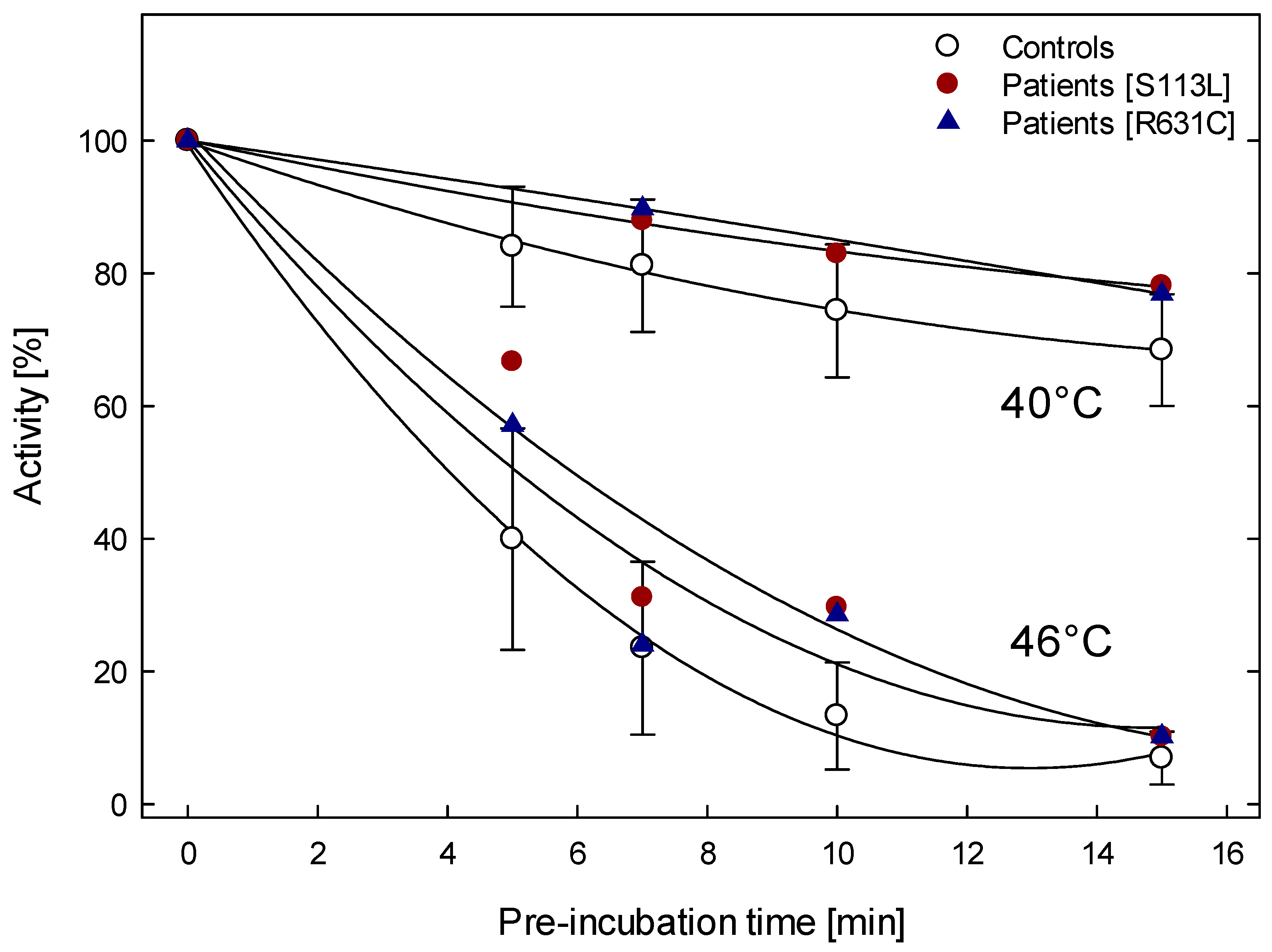
4.1. Thermolability at Different Temperatures and Times
4.2. Preincubation with Cardiolipin (CLP)
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finocchiaro, G.; Taroni, F.; Rocchi, M.; Martin, A.L.; Colombo, I.; Tarelli, G.T.; DiDonato, S. cDNA cloning, sequence analysis, and chromosomal localization of the gene for human carnitine palmitoyltransferase. Proc. Natl. Acad. Sci. 1991, 88, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; di San Filippo, C.A.; Pasquali, M. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. Part C Semin. Med. Genet. 2006, 142C, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Vladutiu, G.D.; Quackenbush, E.J.; Hainline, B.E.; Albers, S.; Smail, D.S.; Bennett, M.J. Lethal neonatal and severe late infantile forms of carnitine palmitoyltransferase II deficiency associated with compound heterozygosity for different protein truncation mutations. J. Pediatr. 2002, 141, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Sigauke, E.; Rakheja, D.; Kitson, K.; Bennett, M.J. Carnitine Palmitoyltransferase II Deficiency: A Clinical, Biochemical, and Molecular Review. Lab. Investig. 2003, 83, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.R.; Pridjian, G.; Morrison, S.; Pickoff, A.S. Fatal carnitine palmitoyltransferase II deficiency in a newborn: New phenotypic features. Clin. Pediatr. 1999, 38, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Boemer, F.; DeBerg, M.; Schoos, R.; Caberg, J.-H.; Gaillez, S.; Dugauquier, C.; Delbecque, K.; François, A.; Maton, P.; Demonceau, N.; et al. Diagnostic pitfall in antenatal manifestations of CPT II deficiency. Clin. Genet. 2016, 89, 193–197. [Google Scholar] [CrossRef]
- Demaugre, F.; Bonnefont, J.-P.; Colonna, M.; Cepanec, C.; Leroux, J.P.; Saudubray, J.M. Infantile form of carnitine palmitoyltransferase II deficiency with hepatomuscular symptoms and sudden death. Physiopathological approach to carnitine palmitoyltransferase II deficiencies. J. Clin. Investig. 1991, 87, 859–864. [Google Scholar] [CrossRef][Green Version]
- Yahyaoui, R.; Espinosa, M.G.; Gómez, C.; Dayaldasani, A.; Rueda, I.; Roldán, A.; Ugarte, M.; Lastra, G.; Pérez, V. Neonatal carnitine palmitoyltransferase II deficiency associated with Dandy-Walker syndrome and sudden death. Mol. Genet. Metab. 2011, 104, 414–416. [Google Scholar] [CrossRef]
- Bouchireb, K.; Teychene, A.; Rigal, O.; de Lonlay, P.; Valayannopoulos, V.; Gaudelus, J.; Sellier, N.; Bonnefont, J.P.; Brivet, M.; de Pontual, L. Post-mortem MRI reveals CPT2 deficiency after sudden infant death. Eur. J. Pediatr. 2010, 169, 1561–1563. [Google Scholar] [CrossRef]
- Joshi, P.R.; Zierz, S. Muscle Carnitine Palmitoyltransferase II (CPT II) Deficiency: A Conceptual Approach. Molecules 2020, 25, 1784. [Google Scholar] [CrossRef]
- Joshi, P.R.; Deschauer, M.; Zierz, S. Phenotype of carnitine palmitoyltransferase II (CPT II) deficiency: A questionnaire-based survey. J. Clin. Neurosci. 2018, 59, 32–36. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0967586818312232 (accessed on 20 November 2018). [CrossRef] [PubMed]
- Corti, S.; Bordoni, A.; Ronchi, D.; Musumeci, O.; Aguennouz, M.; Toscano, A.; Lamperti, C.; Bresolin, N.; Comi, G. Clinical features and new molecular findings in Carnitine Palmitoyltransferase II (CPT II) deficiency. J. Neurol. Sci. 2008, 266, 97–103. [Google Scholar] [CrossRef]
- DiMauro, S.; DiMauro, P.M.M. Muscle Carnitine Palmityltransferase Deficiency and Myoglobinuria. Science 1973, 182, 929–931. [Google Scholar] [CrossRef]
- Joshi, P.R.; Deschauer, M.; Zierz, S. Carnitine palmitoyltransferase II (CPT II) deficiency: Genotype-phenotype analysis of 50 patients. J. Neurol. Sci. 2014, 338, 107–111. [Google Scholar] [CrossRef]
- Zierz, S. Limited trypsin proteolysis renders carnitine palmitoyltransferase insensitive to inhibition by malonyl-CoA in patients with muscle carnitine palmitoyltransferase deficiency. Clin. Investig. 1994, 72, 957–960. [Google Scholar] [CrossRef]
- Lehmann, D.; Zierz, S. Normal protein content but abnormally inhibited enzyme activity in muscle carnitine palmitoyltransferase II deficiency. J. Neurol. Sci. 2014, 339, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, L.; Golbik, R.; Sippl, W.; Zierz, S. Malony-CoA inhibits the S113L variant of carnitine-palmitoyltransferase II. Biochim. Biophys Acta. 2016, 1861, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Olpin, S.E.; Afifi, A.; Clark, S.; Manning, N.J.; Bonham, J.R.; Dalton, A.; Leonard, J.V.; Land, J.M.; Andresen, B.S.; Morris, A.A.; et al. Mutation and biochemical analysis in carnitine palmitoyltransferase type II (CPT II) deficiency. J. Inherit. Metab. Dis. 2003, 26, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, L.; Golbik, R.; Sippl, W.; Zierz, S. Stabilization of the thermolabile variant S113L of carnitine palmitoyltransferase II. Neurol. Genet. 2016, 2, e53. [Google Scholar] [CrossRef]
- Kashfi, K.; Mynatt, R.L.; Park, E.A.; Cook, G.A. Membrane microenvironment regulation of carnitine palmitoyltranferases I and II. Biochem. Soc. Trans. 2011, 39, 833–837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meinhardt, B.; Scholle, L.M.; Seifert, F.; Anwand, M.; Pietzsch, M.; Zierz, S. Cardiolipin Stabilizes and Increases Catalytic Efficiency of Carnitine Palmitoyltransferase II and Its Variants S113L, P50H, and Y479F. Int. J. Mol. Sci. 2021, 22, 4831. [Google Scholar] [CrossRef] [PubMed]
- Zierz, S.; Engel, A.G. Regulatory properties of a mutant carnitine palmitoyltransferase in human skeletal muscle. JBIC J. Biol. Inorg. Chem. 1985, 149, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- van den Berg, S.A.A.; van Marken Lichtenbelt, W.; Willems van Dijk, K.; Schrauwen, P. Skeletal muscle mitochondrial uncoupling, adaptive thermogenesis and energy expenditure. Curr. Opin.Clin. Nutr. Metab. Care 2011, 14, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Stier, A.; Massemin, S.; Criscuolo, F. Chronic mitochondrial uncoupling treatment prevents acute cold-induced oxidative stress in birds. J. Comp. Physiol. B 2014, 184, 1021–1029. [Google Scholar] [CrossRef]
- Motlagh Scholle, L.; Thaele, A.; Beckers, M.; Meinhardt, B.; Zierz, S. Lack of activation of the S113L variant of carnitine palmitoyltransfersase II by cardiolipin. J. Bioenerg. Biomembr. 2018, 50, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Woldegiorgis, G.; Bremer, J.; Shrago, E. Substrate inhibition of carnitine palmitoyltransferase by palmitoyl-CoA and activation by phospholipids and proteins. Biochim. Biophys. Acta. 1985, 837, 135–140. [Google Scholar] [CrossRef]
| Patients (m/f) | Age at Biopsy (Years) | Genotype |
|---|---|---|
| 1 (f) | 19 | p.Ser113Leu/p.Ser113Leu |
| 2 (m) | 21 | p.Ser113Leu/p.Ser113Leu |
| 3 (m) | 36 | p.Ser113Leu/p.Ser113Leu |
| 4 (m) | 24 | p.Arg631Cys/p.Arg631Cys |
| 5 (m) | 44 | p.Arg631Cys/p.Arg631Cys |
| Pre-Incubation Temperature [°C] | Controls | Patients | |
|---|---|---|---|
| p.Ser113Leu | p.Arg631Cys | ||
| 0 °C | 1.39 ± 0.13 (n = 5) | 1.14 (n = 3) | 1.12 (n = 2) |
| 40 °C | |||
| 7 min | 1.16 ± 0.07 (n = 3) | 0.64 (n = 2) | 0.61 (n = 1) |
| 15 min | 0.94 ± 0.06 (n = 3) | 0.57 (n = 2) | 0.59 (n = 1) |
| 46 °C | |||
| 7 min | 4.79 ± 1.66 (n = 3) | 3.92 (n = 1) | 5.75 (n = 1) |
| 15 min | 9.22 ± 4.37 (n = 3) | 20.47 (n = 1) | 9.02 (n = 1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, P.R.; Gräfin zu Stolberg-Stolberg, M.; Scholle, L.M.; Meinhardt, B.; Pegoraro, E.; Zierz, S. Normal Thermostability of p.Ser113Leu and p.Arg631Cys Variants of Mitochondrial Carnitine Palmitoyltransferase II (CPT II) in Human Muscle Homogenate. Metabolites 2022, 12, 1141. https://doi.org/10.3390/metabo12111141
Joshi PR, Gräfin zu Stolberg-Stolberg M, Scholle LM, Meinhardt B, Pegoraro E, Zierz S. Normal Thermostability of p.Ser113Leu and p.Arg631Cys Variants of Mitochondrial Carnitine Palmitoyltransferase II (CPT II) in Human Muscle Homogenate. Metabolites. 2022; 12(11):1141. https://doi.org/10.3390/metabo12111141
Chicago/Turabian StyleJoshi, Pushpa Raj, Maria Gräfin zu Stolberg-Stolberg, Leila Motlagh Scholle, Beate Meinhardt, Elena Pegoraro, and Stephan Zierz. 2022. "Normal Thermostability of p.Ser113Leu and p.Arg631Cys Variants of Mitochondrial Carnitine Palmitoyltransferase II (CPT II) in Human Muscle Homogenate" Metabolites 12, no. 11: 1141. https://doi.org/10.3390/metabo12111141
APA StyleJoshi, P. R., Gräfin zu Stolberg-Stolberg, M., Scholle, L. M., Meinhardt, B., Pegoraro, E., & Zierz, S. (2022). Normal Thermostability of p.Ser113Leu and p.Arg631Cys Variants of Mitochondrial Carnitine Palmitoyltransferase II (CPT II) in Human Muscle Homogenate. Metabolites, 12(11), 1141. https://doi.org/10.3390/metabo12111141






