Influence of 24 h Simulated Altitude on Red Blood Cell Deformability and Hematological Parameters in Patients with Fontan Circulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Procedure, Blood Sampling, and Processing
2.3. Density Gradient Centrifugation
2.4. Red Blood Cell Deformability
2.5. RBC Nitrite/RSNO/Fe-NO
2.6. Immunostaining of RBC NOS Serine 1177 Residue and Nitrotyrosine
2.7. Oxidative Status
2.8. ATP and EPO Concentrations
2.9. Statistical Analysis
3. Results
3.1. Oxygen Saturation and RBC Parameters
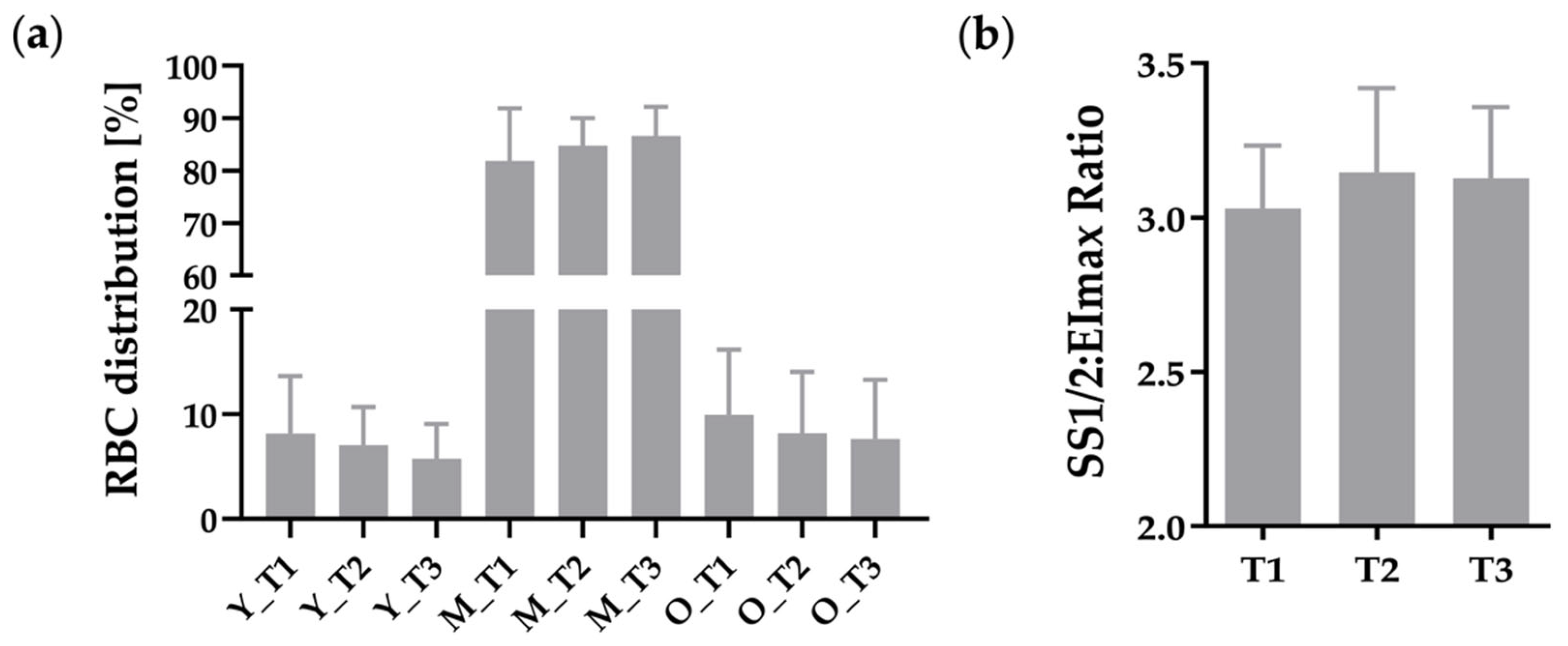
3.2. RBC Distribution of RBC Sub-Fractions and RBC Deformability
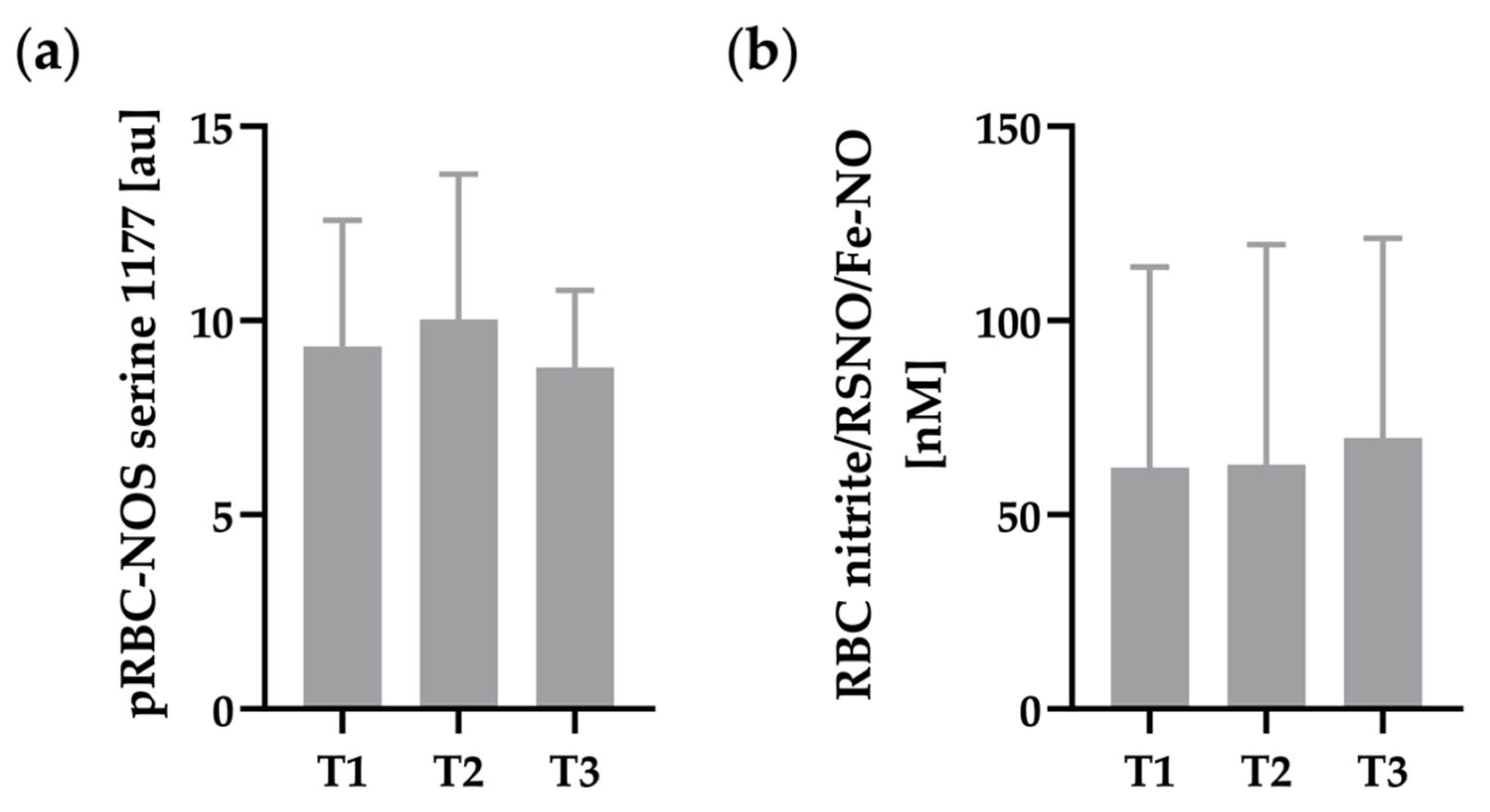
3.3. RBC-NOS Serine 1177 and RBC Nitrite/RSNO/Fe-NO
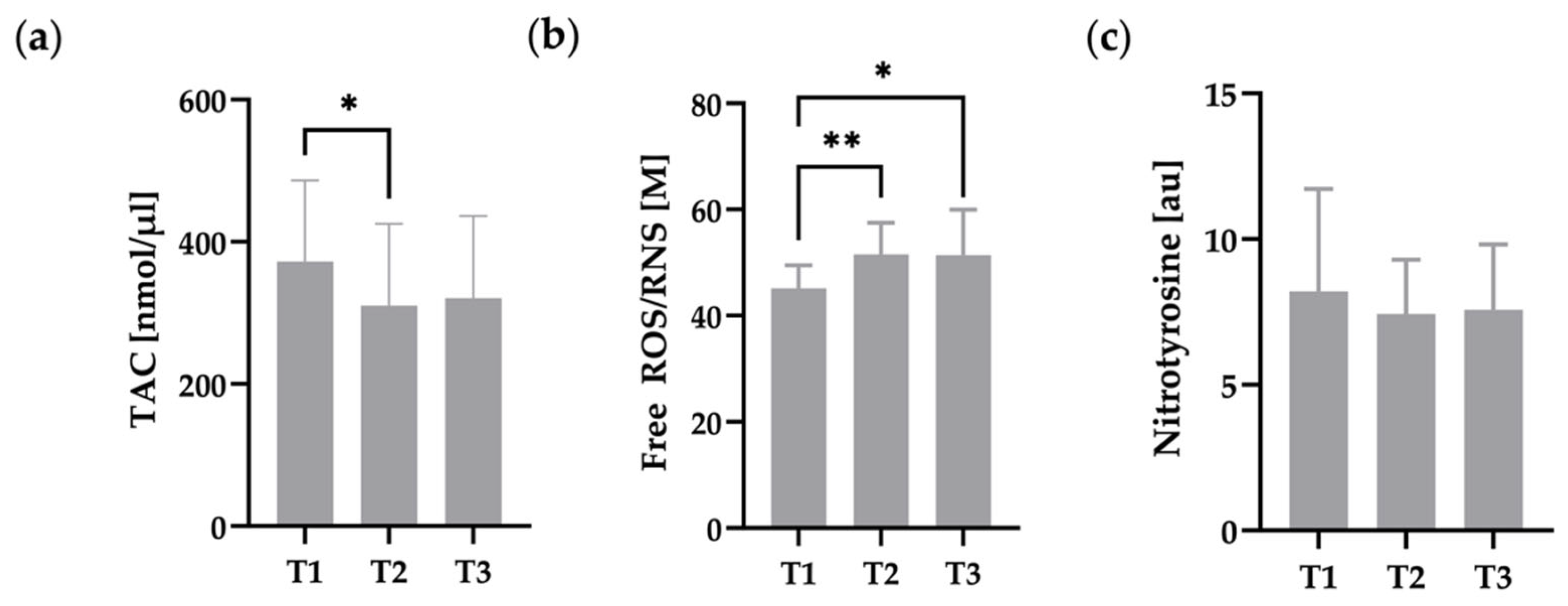
3.4. Oxidative Status of RBC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontan, F.; Baudet, E. Surgical repair of tricuspid atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Gewillig, M. The Fontan circulation. Heart 2005, 91, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Binotto, M.A.; Maeda, N.Y.; Lopes, A.A. Evidence of endothelial dysfunction in patients with functionally univentricular physiology before completion of the Fontan operation. Cardiol. Young 2005, 15, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.; d’Udekem, Y.; Cheung, M.; Sari, C.I.; Inman, J.; Ahimastos, A.; Eikelis, N.; Pathak, A.; King, I.; Grigg, L.; et al. Sympathetic and vascular dysfunction in adult patients with Fontan circulation. Int. J. Cardiol. 2013, 167, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, J.J. Altitude exposures during aircraft flight. Flying higher. Chest 1988, 93, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Bärtsch, P.; Gibbs, J.S. Effect of altitude on the heart and the lungs. Circulation 2007, 116, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Staempfli, R.; Schmid, J.P.; Schenker, S.; Eser, P.; Trachsel, L.D.; Deluigi, C.; Wustmann, K.; Thomet, C.; Greutmann, M.; Tobler, D.; et al. Cardiopulmonary adaptation to short-term high altitude exposure in adult Fontan patients. Heart 2016, 102, 1296–1301. [Google Scholar] [CrossRef]
- Takken, T.; Evertse, A.; de Waard, F.; Spoorenburg, M.; Kuijpers, M.; Schroer, C.; Hulzebos, E.H. Exercise responses in children and adults with a Fontan circulation at simulated altitude. Congenit. Heart Dis. 2019, 14, 1005–1012. [Google Scholar] [CrossRef]
- Garcia, J.A.; McMinn, S.B.; Zuckerman, J.H.; Fixler, D.E.; Levine, B.D. The role of the right ventricle during hypobaric hypoxic exercise: Insights from patients after the Fontan operation. Med. Sci. Sports Exerc. 1999, 31, 269–276. [Google Scholar] [CrossRef]
- Müller, N.; Herberg, U.; Jung, T.; Breuer, J.; Härtel, J.A. Adequate exercise response at artificial altitude in Fontan patients. Front. Pediatr. 2022, 10, 947433. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Blood rheology and hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, N.; Gallagher, P.G. Red cell membrane: Past, present, and future. Blood 2008, 112, 3939–3948. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.K.; Secomb, T.W. Theoretical analysis of the determinants of lung oxygen diffusing capacity. J. Theor. Biol. 2014, 351, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Viallat, A.; Abkarian, M. Red blood cell: From its mechanics to its motion in shear flow. Int. J. Lab. Hematol. 2014, 36, 237–243. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Shin, S. Advances in the measurement of red blood cell deformability: A brief review. J. Cell. Biotechnol. 2015, 1, 63–79. [Google Scholar] [CrossRef]
- Grau, M.; Lauten, A.; Hoeppener, S.; Goebel, B.; Brenig, J.; Jung, C.; Bloch, W.; Suhr, F. Regulation of red blood cell deformability is independent of red blood cell-nitric oxide synthase under hypoxia. Clin. Hemorheol. Microcirc. 2016, 63, 199–215. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Bor-Kucukatay, M.; Wenby, R.B.; Meiselman, H.J.; Baskurt, O.K. Effects of nitric oxide on red blood cell deformability. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1577–H1584. [Google Scholar] [CrossRef]
- Connes, P.; Simmonds, M.J.; Brun, J.F.; Baskurt, O.K. Exercise hemorheology: Classical data, recent findings and unresolved issues. Clin. Hemorheol. Microcirc. 2013, 53, 187–199. [Google Scholar] [CrossRef]
- Suhr, F.; Brenig, J.; Muller, R.; Behrens, H.; Bloch, W.; Grau, M. Moderate exercise promotes human RBC-NOS activity, NO production and deformability through Akt kinase pathway. PLoS ONE 2012, 7, e45982. [Google Scholar] [CrossRef]
- Płoszczyca, K.; Langfort, J.; Czuba, M. The Effects of Altitude Training on Erythropoietic Response and Hematological Variables in Adult Athletes: A Narrative Review. Front. Physiol. 2018, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Stray-Gundersen, J.; Chapman, R.F.; Levine, B.D. “Living high-training low” altitude training improves sea level performance in male and female elite runners. J. Appl. Physiol. 2001, 91, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hou, C.W.; Bernard, J.R.; Chen, C.C.; Hung, T.C.; Cheng, L.L.; Liao, Y.H.; Kuo, C.H. Rhodiola crenulata- and Cordyceps sinensis-based supplement boosts aerobic exercise performance after short-term high altitude training. High Alt. Med. Biol. 2014, 15, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Czuba, M.; Maszczyk, A.; Gerasimuk, D.; Roczniok, R.; Fidos-Czuba, O.; Zajac, A.; Golas, A.; Mostowik, A.; Langfort, J. The effects of hypobaric hypoxia on erythropoiesis, maximal oxygen uptake and energy cost of exercise under normoxia in elite biathletes. J. Sports Sci. Med. 2014, 13, 912–920. [Google Scholar] [PubMed]
- Friedmann, B.; Jost, J.; Rating, T.; Weller, E.; Werle, E.; Eckardt, K.U.; Bartsch, P.; Mairbaurl, H. Effects of iron supplementation on total body hemoglobin during endurance training at moderate altitude. Int. J. Sports Med. 1999, 20, 78–85. [Google Scholar] [CrossRef]
- Dehnert, C.; Hütler, M.; Liu, Y.; Menold, E.; Netzer, C.; Schick, R.; Kubanek, B.; Lehmann, M.; Böning, D.; Steinacker, J.M. Erythropoiesis and performance after two weeks of living high and training low in well trained triathletes. Int. J. Sports Med. 2002, 23, 561–566. [Google Scholar] [CrossRef]
- Härtel, J.A.; Müller, N.; Herberg, U.; Breuer, J.; Bizjak, D.A.; Bloch, W.; Grau, M. Altered Hemorheology in Fontan Patients in Normoxia and After Acute Hypoxic Exercise. Front. Physiol. 2019, 10, 1443. [Google Scholar] [CrossRef]
- Treff, G.; Sareban, M.; Schmidt, W. Hypoxic training in natural and artificial altitude. Dtsch. Z. Sportmed. 2022, 73, 112–117. [Google Scholar] [CrossRef]
- Tuvia, S.; Levin, S.; Korenstein, R. Oxygenation-deoxygenation cycle of erythrocytes modulates submicron cell membrane fluctuations. Biophys. J. 1992, 63, 599–602. [Google Scholar] [CrossRef]
- Kaniewski, W.S.; Hakim, T.S.; Freedman, J.C. Cellular deformability of normoxic and hypoxic mammalian red blood cells. Biorheology 1994, 31, 91–101. [Google Scholar] [CrossRef]
- Moon, H.W.; Shin, S.H.; Lee, C.H.; Park, H.Y.; Sunoo, S.; Nam, S.S. Effects of various acute hypoxic conditions on the hemorheological response during exercise and recovery1. Clin. Hemorheol. Microcirc. 2016, 63, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.S.; Kang, H.; Rasheed, I.D.; Zhou, S.; Lou, N.; Gershteyn, A.; McConnell, E.D.; Wang, Y.; Richardson, K.E.; Palmer, A.F.; et al. Erythrocytes Are Oxygen-Sensing Regulators of the Cerebral Microcirculation. Neuron 2016, 91, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Takao, C.M.; Wenby, R.B.; Meiselman, H.J.; Wood, J.C.; Detterich, J.A. Elevated Low-Shear Blood Viscosity is Associated with Decreased Pulmonary Blood Flow in Children with Univentricular Heart Defects. Pediatr. Cardiol. 2016, 37, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Breda, F.L.; Manchado-Gobatto, F.B.; de Barros Sousa, F.A.; Beck, W.R.; Pinto, A.; Papoti, M.; Scariot, P.P.M.; Gobatto, C.A. Complex networks analysis reinforces centrality hematological role on aerobic-anaerobic performances of the Brazilian Paralympic endurance team after altitude training. Sci. Rep. 2022, 12, 1148. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, D.A.; Brinkmann, C.; Bloch, W.; Grau, M. Increase in Red Blood Cell-Nitric Oxide Synthase Dependent Nitric Oxide Production during Red Blood Cell Aging in Health and Disease: A Study on Age Dependent Changes of Rheologic and Enzymatic Properties in Red Blood Cells. PLoS ONE 2015, 10, e0125206. [Google Scholar] [CrossRef]
- Grau, M.; Kuck, L.; Dietz, T.; Bloch, W.; Simmonds, M.J. Sub-Fractions of Red Blood Cells Respond Differently to Shear Exposure Following Superoxide Treatment. Biology 2021, 10, 47. [Google Scholar] [CrossRef]
- Tomschi, F.; Bizjak, D.; Bloch, W.; Latsch, J.; Predel, H.G.; Grau, M. Deformability of different red blood cell populations and viscosity of differently trained young men in response to intensive and moderate running. Clin. Hemorheol. Microcirc. 2018, 69, 503–514. [Google Scholar] [CrossRef]
- Nemeth, N.; Kiss, F.; Miszti-Blasius, K. Interpretation of osmotic gradient ektacytometry (osmoscan) data: A comparative study for methodological standards. Scand. J. Clin. Lab. Invest. 2015, 75, 213–222. [Google Scholar] [CrossRef]
- Grau, M.; Zollmann, E.; Bros, J.; Seeger, B.; Dietz, T.; Noriega Urena, J.A.; Grolle, A.; Zacher, J.; Notbohm, H.L.; Suck, G.; et al. Autologous Blood Doping Induced Changes in Red Blood Cell Rheologic Parameters, RBC Age Distribution, and Performance. Biology 2022, 11, 647. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Comparison of three commercially available ektacytometers with different shearing geometries. Biorheology 2009, 46, 251–264. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Data reduction methods for ektacytometry in clinical hemorheology. Clin. Hemorheol. Microcirc. 2013, 54, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.M.; Kleinbongard, P.; Ringwood, L.; Hito, R.; Hunter, C.J.; Schechter, A.N.; Gladwin, M.T.; Dejam, A. The measurement of blood and plasma nitrite by chemiluminescence: Pitfalls and solutions. Free Radic. Biol. Med. 2006, 41, 541–548. [Google Scholar] [CrossRef]
- Hendgen-Cotta, U.; Grau, M.; Rassaf, T.; Gharini, P.; Kelm, M.; Kleinbongard, P. Reductive Gas-Phase Chemiluminescence and Flow Injection Analysis for Measurement of the Nitric Oxide Pool in Biological Matrices. Methods Enzymol. 2008, 441, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Hendgen-Cotta, U.B.; Brouzos, P.; Drexhage, C.; Rassaf, T.; Lauer, T.; Dejam, A.; Kelm, M.; Kleinbongard, P. Recent methodological advances in the analysis of nitrite in the human circulation: Nitrite as a biochemical parameter of the L-arginine/NO pathway. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 851, 106–123. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Jungen, P.; Bloch, W.; Grau, M. Cryopreservation of red blood cells: Effect on rheologic properties and associated metabolic and nitric oxide related parameters. Cryobiology 2018, 84, 59–68. [Google Scholar] [CrossRef]
- Grau, M.; Pauly, S.; Ali, J.; Walpurgis, K.; Thevis, M.; Bloch, W.; Suhr, F. RBC-NOS-dependent S-nitrosylation of cytoskeletal proteins improves RBC deformability. PLoS ONE 2013, 8, e56759. [Google Scholar] [CrossRef] [PubMed]
- d’Udekem, Y.; Iyengar, A.J.; Galati, J.C.; Forsdick, V.; Weintraub, R.G.; Wheaton, G.R.; Bullock, A.; Justo, R.N.; Grigg, L.E.; Sholler, G.F.; et al. Redefining expectations of long-term survival after the Fontan procedure: Twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 2014, 130, S32–S38. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, E.R.; Lundell, B.; Soderstrom, L.; Sjoberg, G. Can endurance training improve physical capacity and quality of life in young Fontan patients? Cardiol. Young 2018, 28, 438–446. [Google Scholar] [CrossRef]
- Härtel, J.A.; Herberg, U.; Jung, T.; Winkler, C.; Breuer, J.; Müller, N. Physical activity and heart rate monitoring in Fontan patients—Should we recommend activities in higher intensities? PLoS ONE 2020, 15, e0228255. [Google Scholar] [CrossRef]
- Takken, T.; Hulzebos, H.J.; Blank, A.C.; Tacken, M.H.; Helders, P.J.; Strengers, J.L. Exercise prescription for patients with a Fontan circulation: Current evidence and future directions. Neth. Heart J. 2007, 15, 142–147. [Google Scholar] [CrossRef]
- Tomkiewicz-Pajak, L.; Plazak, W.; Kolcz, J.; Pajak, J.; Kopec, G.; Dluzniewska, N.; Olszowska, M.; Moryl-Bujakowska, A.; Podolec, P. Iron deficiency and hematological changes in adult patients after Fontan operation. J. Cardiol. 2014, 64, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Takken, T.; Tacken, M.H.; Blank, A.C.; Hulzebos, E.H.; Strengers, J.L.; Helders, P.J. Exercise limitation in patients with Fontan circulation: A review. J. Cardiovasc. Med. 2007, 8, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Koistinen, P.O.; Rusko, H.; Irjala, K.; Rajamäki, A.; Penttinen, K.; Sarparanta, V.P.; Karpakka, J.; Leppäluoto, J. EPO, red cells, and serum transferrin receptor in continuous and intermittent hypoxia. Med. Sci. Sports Exerc. 2000, 32, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Rennie, D. Water intake at high altitude. J. Wilderness Med. 1993, 4, 224–227. [Google Scholar]
- Bärtsch, P. Höhenanpassung. Ger. J. Sports Med. 2000, 51, 139–140. [Google Scholar]
- Mairbäurl, H. Höhenakklimatisation. Dtsch. Z. Für Sportmed. 2000, 12, 390–395. [Google Scholar]
- Simmonds, M.J.; Meiselman, H.J.; Baskurt, O.K. Blood rheology and aging. J. Geriatr. Cardiol. 2013, 10, 291–301. [Google Scholar] [CrossRef]
- Smith, J.A.; Martin, D.T.; Telford, R.D.; Ballas, S.K. Greater erythrocyte deformability in world-class endurance athletes. Am. J. Physiol. 1999, 276, H2188–H2193. [Google Scholar] [CrossRef]
- Klipp, M.; Holzwarth, A.-U.; Poeschl, J.M.; Nelle, M.; Linderkamp, O. Effects of erythropoietin on erythrocyte deformability in non-transfused preterm infants. Acta Paediatr. 2007, 96, 253–256. [Google Scholar] [CrossRef]
- Chou, S.L.; Huang, Y.C.; Fu, T.C.; Hsu, C.C.; Wang, J.S. Cycling Exercise Training Alleviates Hypoxia-Impaired Erythrocyte Rheology. Med. Sci. Sports Exerc. 2016, 48, 57–65. [Google Scholar] [CrossRef]
- Suhr, F.; Porten, S.; Hertrich, T.; Brixius, K.; Schmidt, A.; Platen, P.; Bloch, W. Intensive exercise induces changes of endothelial nitric oxide synthase pattern in human erythrocytes. Nitric Oxide 2009, 20, 95–103. [Google Scholar] [CrossRef] [PubMed]
- McMahon, T.J.; Darrow, C.C.; Hoehn, B.A.; Zhu, H. Generation and Export of Red Blood Cell ATP in Health and Disease. Front. Physiol. 2021, 12, 754638. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Adhikary, G.; McCormick, A.A.; Holcroft, J.J.; Kumar, G.K.; Prabhakar, N.R. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J. Physiol. 2004, 557, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Debevec, T.; Pialoux, V.; Mekjavic, I.B.; Eiken, O.; Mury, P.; Millet, G.P. Moderate exercise blunts oxidative stress induced by normobaric hypoxic confinement. Med. Sci. Sports Exerc. 2014, 46, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Barodka, V.M.; Nagababu, E.; Mohanty, J.G.; Nyhan, D.; Berkowitz, D.E.; Rifkind, J.M.; Strouse, J.J. New insights provided by a comparison of impaired deformability with erythrocyte oxidative stress for sickle cell disease. Blood Cells Mol. Dis. 2014, 52, 230–235. [Google Scholar] [CrossRef]
- Samaja, M.; Rubinacci, A.; Motterlini, R.; De Ponti, A.; Portinaro, N. Red cell aging and active calcium transport. Exp. Gerontol. 1990, 25, 279–286. [Google Scholar] [CrossRef][Green Version]
- Kiefer, C.R.; Snyder, L.M. Oxidation and erythrocyte senescence. Curr. Opin. Hematol. 2000, 7, 113–116. [Google Scholar] [CrossRef]
- Kehrer, J.P.; Lund, L.G. Cellular reducing equivalents and oxidative stress. Free Radic. Biol. Med. 1994, 17, 65–75. [Google Scholar] [CrossRef]
- Mazzeo, R.S.; Child, A.; Butterfield, G.E.; Mawson, J.T.; Zamudio, S.; Moore, L.G. Catecholamine response during 12 days of high-altitude exposure (4300 m) in women. J. Appl. Physiol. 1998, 84, 1151–1157. [Google Scholar] [CrossRef]
- Knight, A.R.; Taylor, E.L.; Lukaszewski, R.; Jensen, K.T.; Jones, H.E.; Carre, J.E.; Isupov, M.N.; Littlechild, J.A.; Bailey, S.J.; Brewer, E.; et al. A high-sensitivity electrochemiluminescence-based ELISA for the measurement of the oxidative stress biomarker, 3-nitrotyrosine, in human blood serum and cells. Free Radic. Biol. Med. 2018, 120, 246–254. [Google Scholar] [CrossRef]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Lima, D.J.; Mendes-Ribeiro, A.C.; Brunini, T.M.; Martins, M.A.; Mury, W.V.; Freire, R.A.; Monteiro, W.D.; Farinatti, P.T.; Matsuura, C. Erythrocyte nitric oxide availability and oxidative stress following exercise. Clin. Hemorheol. Microcirc. 2017, 65, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Kiernan, W.; Merker, H.J.; Wenzel, M.; Beneke, R. Red cell membrane skeletal changes in marathon runners. Int. J. Sports Med. 1998, 19, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Coppel, J.; Hennis, P.; Gilbert-Kawai, E.; Grocott, M.P. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: A systematic review of crossover trials. Extrem Physiol. Med. 2015, 4, 2. [Google Scholar] [CrossRef] [PubMed]

| Age | Years | 24.5 [16.3, 38.8] * |
| Gender | f/m | 9/9 |
| Years since Fontan Completion | 20.3 ± 5.98 | |
| Congenital Heart Malformation | ||
| Tricuspid Atresia (TA) | 4 | |
| Pulmonary Atresia (PA) | 1 | |
| Pulmonary Atresia with ccTGA | 2 | |
| Hypoplastic Left Heart Syndrome (HLHS) | 2 | |
| Double Inlet Left ventricle (DILV) | 5 | |
| Double Outlet Right Ventricle (DORV) | 2 | |
| Double Inlet Right Ventricle (DIRV) | 1 | |
| Single Ventricle with ccTGA (SV) | 1 | |
| Functional Ventricle | ||
| Left | 10 | |
| Right | 8 | |
| Anticoagulation | ||
| Aspirin | 6 | |
| Phenprocoumon | 8 | |
| Warfarin | 1 | |
| New Oral Anticoagulants | 2 | |
| No Anticoagulation | 1 | |
| T1 | T2 | T3 | Significance | |||
|---|---|---|---|---|---|---|
| (p-Value) | ||||||
| SpO2 | % | 94 ± 3 | 87 ± 4 | 95 ± 4 | <0.0001 ****,†††† | |
| pH (n = 15) | 7.39 ± 0.03 | 7.4 ± 0.02 | 7.38 ± 0.03 | 0.008 **, 0.019 † | ||
| Red blood cells | T/L | 5.41 ± 0.63 | 5.57 ±0.77 | 5.5 ± 0.64 | ns | |
| Reticulocytes (n = 11) | G/L | 74.86 ± 30.35 | 82.85 ± 34.07 | 0.005 ** | ||
| Hemoglobin | g/dL | 16.84 ± 2.01 | 17.66 ± 2.58 | 17.03 ± 2.33 | ns | |
| Hematocrit | % | 47.89 ± 4.94 | 49.33 ± 6.55 | 48.77 ± 5.62 | ns | |
| MCV | fl | 88.75 ± 3.33 | 88.63 ± 3.56 | 88.66 ± 3.27 | ns | |
| MCH | pg | 30.91 ± 2.11 | 31.75 ± 2.67 | 30.91 ± 1.83 | ns | |
| MCHC | g/dL | 34.81 ± 1.78 | 35.76 ± 2.23 | 34.86 ± 1.35 | ns | |
| RDW | % | 13.54 ± 0.82 | 13.75 ± 0.89 | 13.69 ± 0.82 | 0.018 * | |
| EPOplasma | mIU/mL | 13.06 ± 2.82 | 30.95 ± 15.4 | 31.68 ± 14.19 | <0.0001 ****,‡‡‡‡ | |
| RBC ATP | ng/µL | 215.19 ± 29.89 | 203.53 ± 42.69 | 235.67 ± 47.5 | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Härtel, J.A.; Müller, N.; Breuer, J.; Jordan, J.; Tank, J.; Bros, J.; Seeger, B.; Zollmann, E.; Bloch, W.; Grau, M. Influence of 24 h Simulated Altitude on Red Blood Cell Deformability and Hematological Parameters in Patients with Fontan Circulation. Metabolites 2022, 12, 1025. https://doi.org/10.3390/metabo12111025
Härtel JA, Müller N, Breuer J, Jordan J, Tank J, Bros J, Seeger B, Zollmann E, Bloch W, Grau M. Influence of 24 h Simulated Altitude on Red Blood Cell Deformability and Hematological Parameters in Patients with Fontan Circulation. Metabolites. 2022; 12(11):1025. https://doi.org/10.3390/metabo12111025
Chicago/Turabian StyleHärtel, Julian Alexander, Nicole Müller, Johannes Breuer, Jens Jordan, Jens Tank, Janina Bros, Benedikt Seeger, Emily Zollmann, Wilhelm Bloch, and Marijke Grau. 2022. "Influence of 24 h Simulated Altitude on Red Blood Cell Deformability and Hematological Parameters in Patients with Fontan Circulation" Metabolites 12, no. 11: 1025. https://doi.org/10.3390/metabo12111025
APA StyleHärtel, J. A., Müller, N., Breuer, J., Jordan, J., Tank, J., Bros, J., Seeger, B., Zollmann, E., Bloch, W., & Grau, M. (2022). Influence of 24 h Simulated Altitude on Red Blood Cell Deformability and Hematological Parameters in Patients with Fontan Circulation. Metabolites, 12(11), 1025. https://doi.org/10.3390/metabo12111025








