GC-TOF-MS-Based Non-Targeted Metabolomic Analysis of Differential Metabolites in Chinese Ultra-Long-Term Industrially Fermented Kohlrabi and Their Associated Metabolic Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Ultra-Long-Term Industrially Fermented Kohlrabi
2.2. Physicochemical Analysis
2.3. Extraction of Metabolites from Ultra-Long-Term Industrially Fermented Kohlrabi
2.4. Detection of Metabolites from Ultra-Long-Term Industrially Fermented Kohlrabi by GC-TOF-MS
2.5. GC-TOF-MS Data Preprocessing and Analysis
2.6. Metabolic Pathway Analysis
2.7. E-Nose Analysis
2.8. Statistical Analysis
3. Results
3.1. Physicochemical Analysis
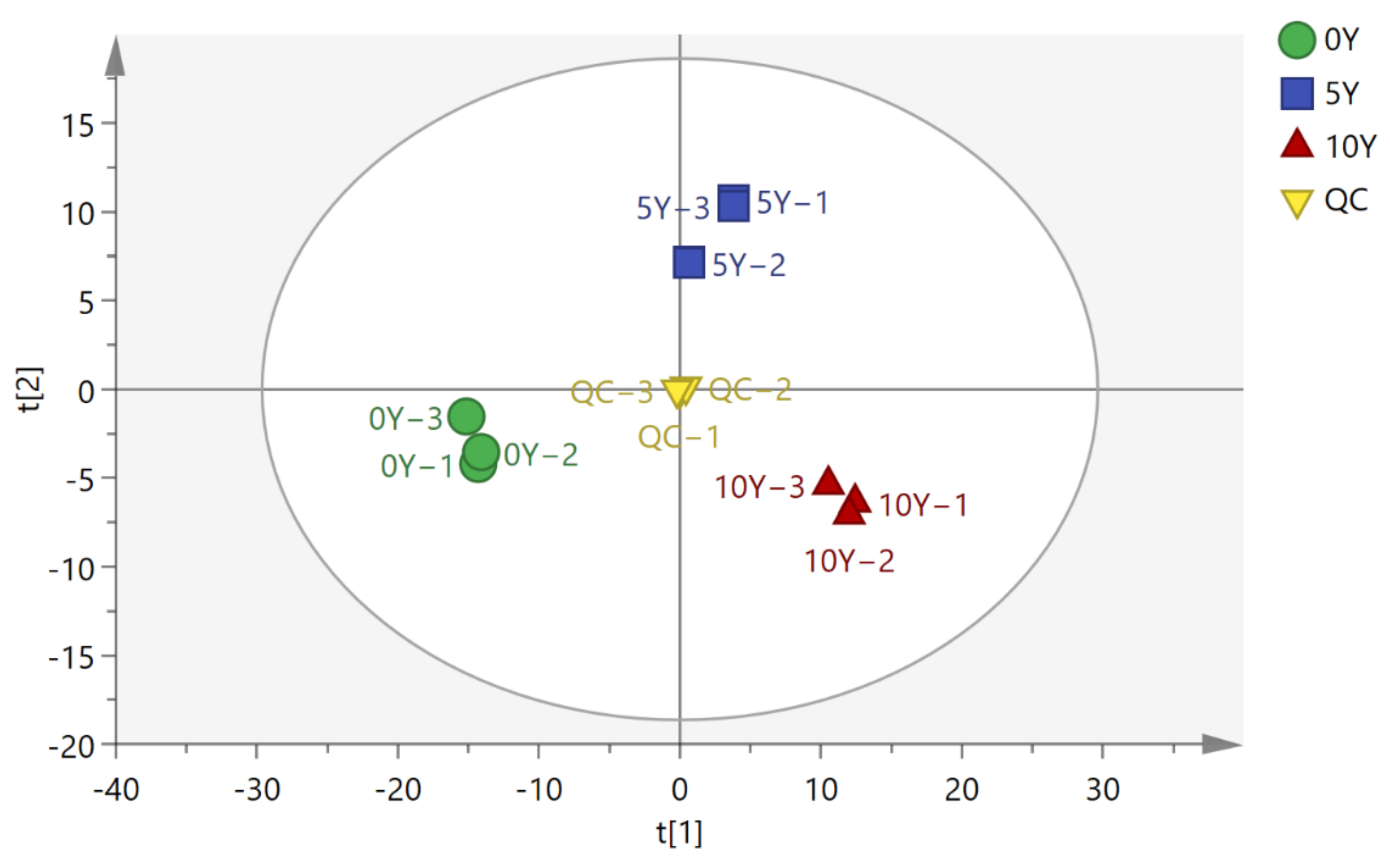
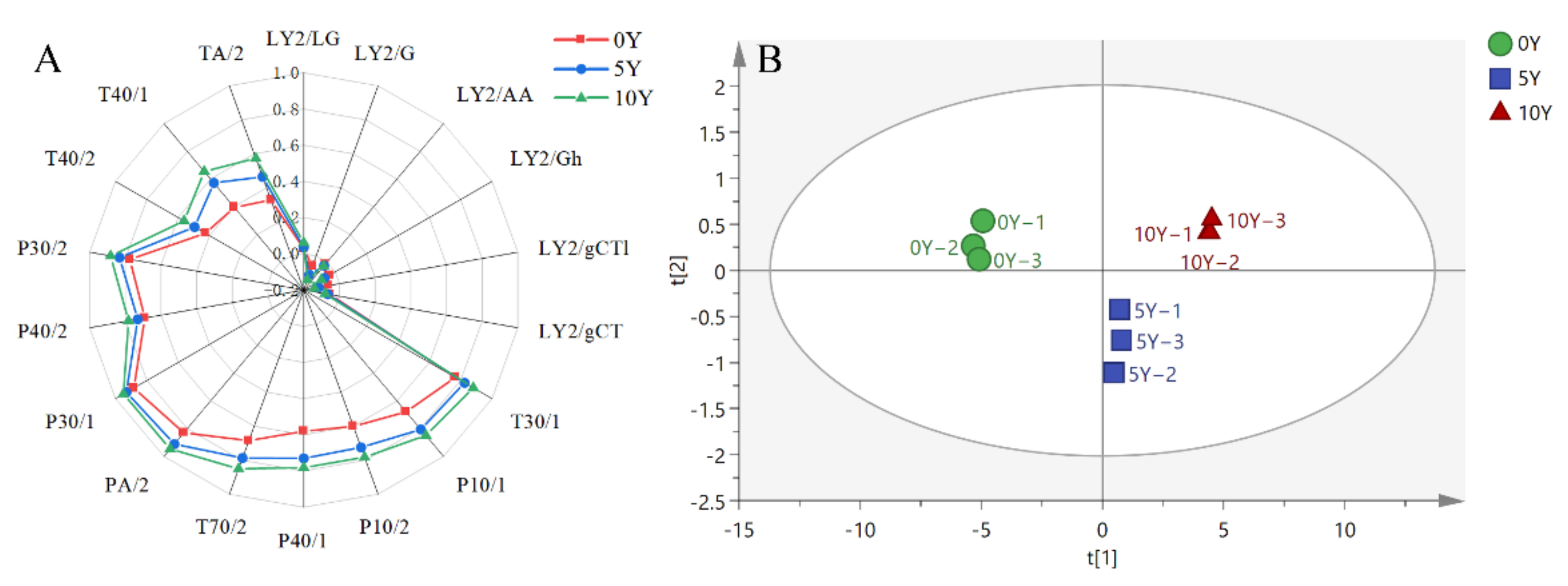
3.2. PCA Analysis for the Metabolomics of Ultra-Long-Term Industrially Fermented Kohlrabies
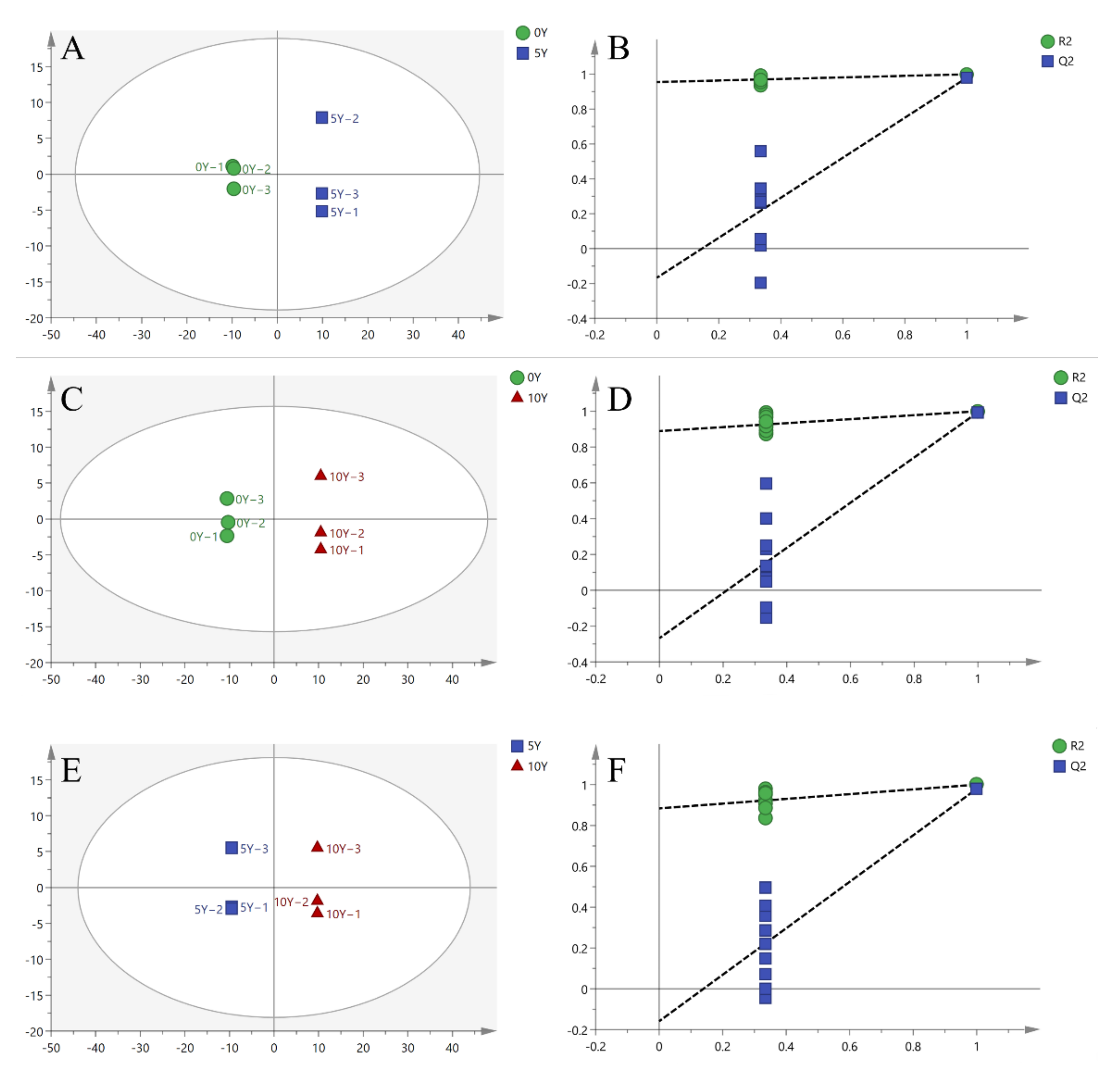
3.3. OPLA-DA Analysis of Metabolites from Three Different Kohlrabies
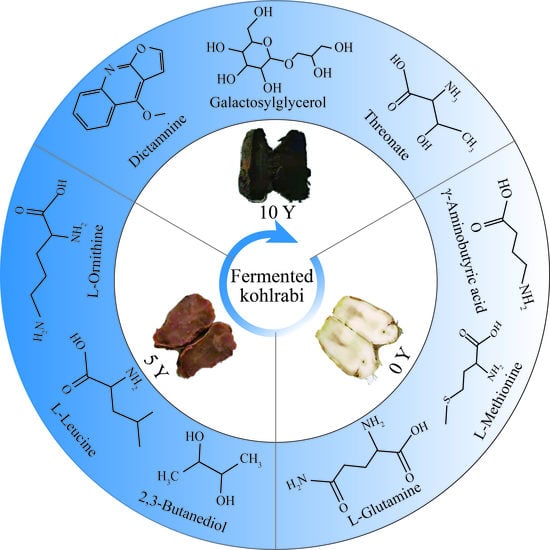
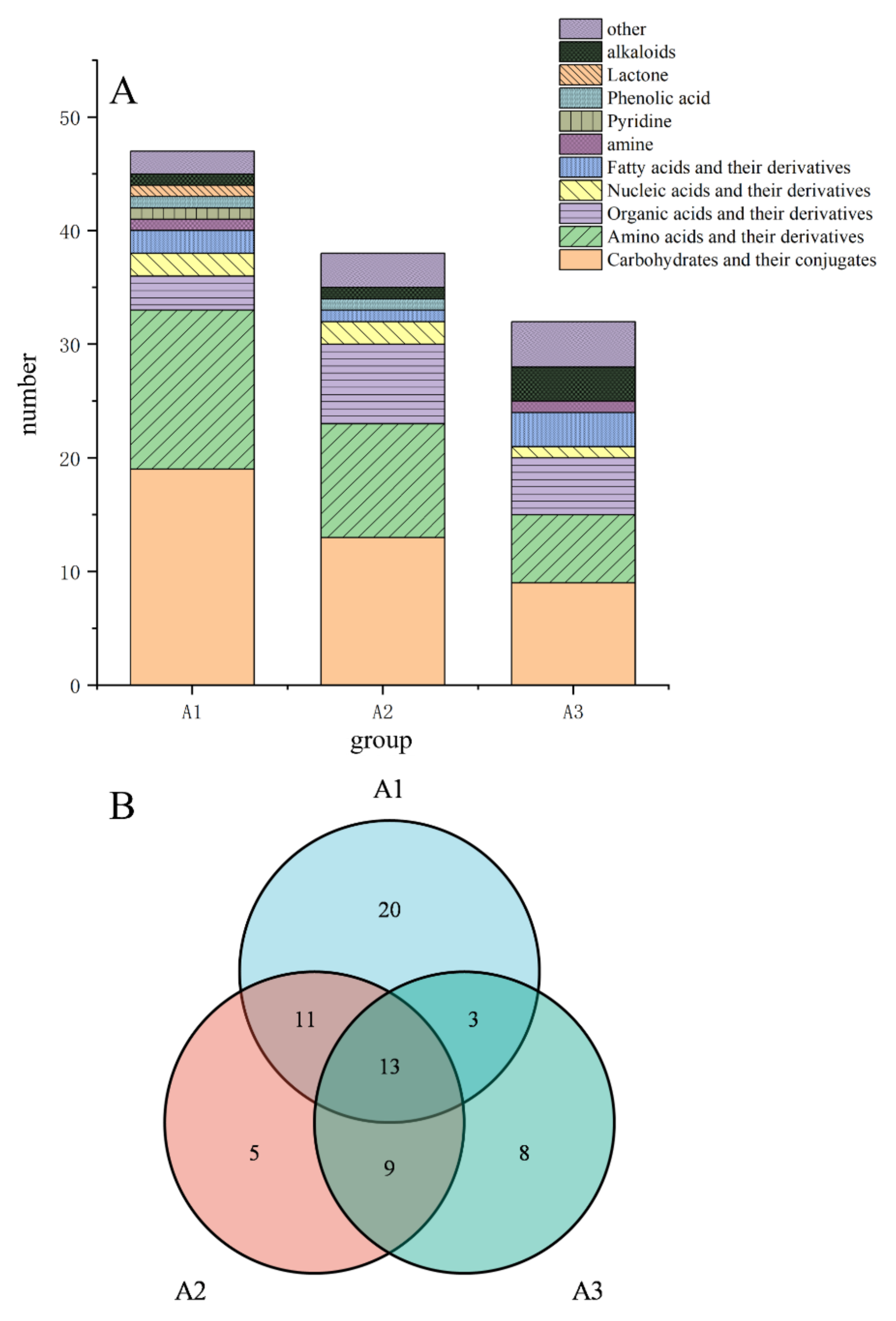
3.4. Screening and Identification of Differential Metabolites from the Three Industrially Fermented Kohlrabies
3.5. Heat Map Analysis for Differential Metabolites
3.6. Metabolic Pathway Analysis for Differential Metabolites in Ultra-Long-Term Industrially Fermented Kohlrabi
3.7. E-Nose Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasko, P.; Galanty, A.; Żmudzki, P.; Gdula-Argasińska, J.; Zagrodzki, P. Influence of Different Light Conditions and Time of Sprouting on Harmful and Beneficial Aspects of Rutabaga Sprouts in Comparison to Their Roots and Seeds. J. Sci. Food Agric 2018, 99, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Kapusta-Duch, J.; Florkiewicz, A.; Leszczyńska, T.; Borczak, B. Directions of Changes in the Content of Selected Macro- and Micronutrients of Kale, Rutabaga, Green and Purple Cauliflower Due to Hydrothermal Treatment. Appl. Sci. 2021, 11, 3452. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, X.; Yang, J.; Wang, H.; Liu, F.; Xu, X.; Pan, S. Effects of high hydrostatic pressure and thermal treatment on texture properties of pickled kohlrabi. LWT 2022, 157, 113078. [Google Scholar] [CrossRef]
- Jiang, L.; Xian, S.; Liu, X.; Shen, G.; Zhang, Z.; Hou, X.; Chen, A. Metagenomic Study on Chinese Homemade Paocai: The Effects of Raw Materials and Fermentation Periods on the Microbial Ecology and Volatile Components. Foods 2021, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C.; Xin, X.; Liu, D.; Zhang, W. Comparative Analysis of Traditional and Modern Fermentation for Xuecai and Correlations Between Volatile Flavor Compounds and Bacterial Community. Front. Microbiol. 2021, 12, 631054. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, Y.; Chan, L. Application of Metabonomics Approach in Food Safety Research-A Review. Food Rev. Int. 2020, 36, 547–558. [Google Scholar] [CrossRef]
- Ding, M.; Cheng, J.; Xiao, W.; Qiao, B.; Yuan, Y. Comparative metabolomic analysis on industrial continuous and batch ethanol fermentation processes by GC-TOF-MS. Metabolomics 2008, 5, 229–238. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261.e212. [Google Scholar] [CrossRef]
- GB/T 12456—2008; Determination of Total Acid in Foods. State Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, China National Standardization Administration Committee. China Standards Press: Beijing, China, 2008.
- GB/T 5009.7—2016; Determination of Reducing Sugar in Foods. State Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, China National Standardization Administration Committee. China Standards Press: Beijing, China, 2016.
- GB/T 5009.5—2016; Determination of Protein in Foods. State Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, China National Standardization Administration Committee. China Standards Press: Beijing, China, 2016.
- Doughty, H.W. Mohr’s method for the determination of silver and halogens in other than neutral solutions. J. Am. Chem. Soc. 1924, 46, 2707–2709. [Google Scholar] [CrossRef]
- Fiehn, O.; Kind, T. Metabolite profiling in blood plasma. Methods Mol. Biol. 2007, 358, 3–17. [Google Scholar] [CrossRef]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tao, L.; Zhang, T.; Zhang, J.; Wu, T.; Luan, D.; Ni, L.; Wang, X.; Zhong, J. Effect of four types of thermal processing methods on the aroma profiles of acidity regulator-treated tilapia muscles using E-nose, HS-SPME-GC-MS, and HS-GC-IMS. LWT 2021, 147, 111585. [Google Scholar] [CrossRef]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.; Guo, B.; Chen, H.; Sun, B.; Zhang, Y. Comparison of Volatile Flavor Compounds in Yu-Shiang Shredded Pork Processed by Two Different Methods by SDE-GC-MS. Food Sci. 2015, 36, 70–75. [Google Scholar] [CrossRef]
- Wu, R.; Yu, M.; Liu, X.; Meng, L.; Wang, Q.; Xue, Y.; Wu, J.; Yue, X. Changes in flavour and microbial diversity during natural fermentation of suan-cai, a traditional food made in Northeast China. Int. J. Food Microbiol. 2015, 211, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Jiang, H. Microbial production of metabolites and associated enzymatic reactions under high pressure. World J. Microbiol. Biotechnol. 2016, 32, 178. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Zhang, J.; Xin, X.; Liao, X. Metagenomics reveals the formation mechanism of flavor metabolites during the spontaneous fermentation of potherb mustard (Brassica juncea var. multiceps). Food Res. Int. 2021, 148, 110622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Z.; Zhang, K.; Liu, X.; Lin, X.; Zhang, Z.; Bao, T.; Feng, Z. Nutrient consumption patterns of Lactobacillus plantarum and their application in suancai. Int. J. Food Microbiol. 2021, 354, 109317. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Ding, P.; Wang, D.; Cheng, Y.; He, J.; Gao, M.; Xu, F.; Li, Y.; Zhu, Z.; et al. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18220–18225. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wu, C.; Wang, M.; Xu, B.; Du, L. Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J. Agric. Food Chem. 2012, 60, 9642–9648. [Google Scholar] [CrossRef] [PubMed]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; Del Río, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell. Environ. 2012, 35, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Eprintsev, A.T. Organic Acids: The Pools of Fixed Carbon Involved in Redox Regulation and Energy Balance in Higher Plants. Front. Plant Sci. 2016, 7, 1042. [Google Scholar] [CrossRef]
- Shang, Z.; Ye, Z.; Li, M.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Dynamics of microbial communities, flavor, and physicochemical properties of pickled chayote during an industrial-scale natural fermentation: Correlation between microorganisms and metabolites. Food Chem. 2022, 377, 132004. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Jung, J.Y.; Lee, S.H.; Jin, H.M.; Jeon, C.O. Microbial succession and metabolite changes during fermentation of dongchimi, traditional Korean watery kimchi. Int. J. Food Microbiol. 2013, 164, 46–53. [Google Scholar] [CrossRef]
- Labanauskas, C.K.; Stolzy, L.H.; Handy, M.F. Protein and free amino acids in wheat grain as affected by soil types and salinity levels in irrigation water. Plant Soil. 1981, 59, 299–316. [Google Scholar] [CrossRef]
- Li, X.; Kim, Y.B.; Uddin, M.R.; Lee, S.; Kim, S.J.; Park, S.U. Influence of light on the free amino acid content and γ-aminobutyric acid synthesis in Brassica juncea seedlings. J. Agric. Food Chem. 2013, 61, 8624–8631. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wen, Z.; Li, H.; Yuan, D.; Li, J.; Zhang, H.; Huang, Z.; Cui, S.; Du, W. Identification of the quantitative trait loci (QTL) underlying water soluble protein content in soybean. Theor. Appl. Genet. 2013, 126, 425–433. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, C.; Yang, Q.; Guo, Z.; Yang, B.; Lu, W.; Li, D.; Tian, F.; Liu, X.; Zhang, H.; et al. Selection of Taste Markers Related to Lactic Acid Bacteria Microflora Metabolism for Chinese Traditional Paocai: A Gas Chromatography-Mass Spectrometry-Based Metabolomics Approach. J. Agric. Food Chem. 2016, 64, 2415–2422. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Leaf Amino Acid Supply Affects Photosynthetic and Plant Nitrogen Use Efficiency under Nitrogen Stress. Plant Physiol. 2018, 178, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Shahid, M.; Babar, M. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Xu, Q.; Mei, X.; Yuan, H.; Jiabu, D.; Sang, Z.; Nyima, T. Metabolite profiling in two contrasting Tibetan hulless barley cultivars revealed the core salt-responsive metabolome and key salt-tolerance biomarkers. AoB Plants 2019, 11, plz021. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Taamalli, M.; Gevi, F.; Timperio, A.M.; Zolla, L.; Ghnaya, T. Cadmium stress responses in Brassica juncea: Hints from proteomics and metabolomics. J. Proteome Res. 2013, 12, 4979–4997. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, O.; Nakayama, Y.; Emori, K.; Takeba, G.; Sugino, M. Flower-Inducing Activity of Lysine in Lemna paucicostata 6746. Plant Cell Physiol 1997, 38, 124–128. [Google Scholar] [CrossRef][Green Version]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd Allah, E.F.; Gucel, S.; Tran, L.-S.P. Nitric Oxide Mitigates Salt Stress by Regulating Levels of Osmolytes and Antioxidant Enzymes in Chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Al-Nuri, M.; Warad, I.; Segura-Carretero, A. Untargeted metabolite profiling and phytochemical analysis of Micromeria fruticosa L. (Lamiaceae) leaves. Food Chem. 2019, 279, 128–143. [Google Scholar] [CrossRef]
- Zhang, J.; Morris-Natschke, S.L.; Ma, D.; Shang, X.; Yang, C.; Liu, Y.; Lee, K.H. Biologically active indolizidine alkaloids. Med. Res. Rev. 2021, 41, 928–960. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Zhu, Y.; Sun, J.; Yerke, A.; Sang, S.; Yu, Z. Metabolism of dictamnine in liver microsomes from mouse, rat, dog, monkey, and human. J. Pharm. Biomed. 2016, 119, 166–174. [Google Scholar] [CrossRef]
- Zhao, D.; Du, R.; Song, G.; Sun, J.; Ping, W.; Ge, J. Chemical Composition and Bacterial Diversity of Lactobacillus casei 11MZ-5-1 Fermented Chinese Cabbage Pickle. Food Sci. 2018, 39, 116–122. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, W.; Peng, H.; Liu, L.; Huang, H.; Ji, X. Metabolic engineering for efficient microbial production of 2,3-butanediol. CIESC J. 2016, 67, 2656–2671. [Google Scholar] [CrossRef]
- Xiong, T.; Li, J.; Liang, F.; Wang, Y.; Guan, Q.; Xie, M. Effects of salt concentration on Chinese sauerkraut fermentation. LWT 2016, 69, 169–174. [Google Scholar] [CrossRef]
- Dátilo, M.N.; Sant’Ana, M.R.; Formigari, G.P.; Rodrigues, P.B.; de Moura, L.P.; da Silva, A.S.R.; Ropelle, E.R.; Pauli, J.R.; Cintra, D.E. Omega-3 from Flaxseed Oil Protects Obese Mice Against Diabetic Retinopathy Through GPR120 Receptor. Sci. Rep. 2018, 8, 14318. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, G.; Zhang, J. The suitable condition for Lactobacillus parafarraginis ZH1 producing hexadecanoic acid and inhibiting pathogenic and spoilage yeasts. Biol. Control 2020, 149, 104318. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Enteshari, M.; Metzger, L. Lactitol: Production, properties, and applications. Trends Food Sci. Technol. 2019, 83, 181–191. [Google Scholar] [CrossRef]
- Gart, E.; van Duyvenvoorde, W.; Caspers, M.P.M.; van Trigt, N.; Snabel, J.; Menke, A.; Keijer, J.; Salic, K.; Morrison, M.C.; Kleemann, R. Intervention with isoleucine or valine corrects hyperinsulinemia and reduces intrahepatic diacylglycerols, liver steatosis, and inflammation in Ldlr-/-.Leiden mice with manifest obesity-associated NASH. FASEB J. 2022, 36, e22435. [Google Scholar] [CrossRef]
- Sun, Q.; Weinger, J.G.; Mao, F.; Liu, G. Regulation of structural and functional synapse density by L-threonate through modulation of intraneuronal magnesium concentration. Neuropharmacology 2016, 108, 426–439. [Google Scholar] [CrossRef]
- Jayamuthunagai, J.; Gautam, P.; Srisowmeya, G.; Chakravarthy, M. Biocatalytic production of D-tagatose: A potential rare sugar with versatile applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3430–3437. [Google Scholar] [CrossRef]
- Ma, C.; Teng, L.; Lin, G.; Guo, B.; Zhuo, R.; Qian, X.; Guan, T.; Wu, R.; Liu, Y.; Liu, M. L-leucine promotes axonal outgrowth and regeneration via mTOR activation. FASEB J. 2021, 35, e21526. [Google Scholar] [CrossRef]
- Hunt, B.J. The current place of tranexamic acid in the management of bleeding. Anaesthesia 2015, 70, 50–53.e18. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jung, H.; Kim, K.; Lim, K.M.; Kim, J.Y.; Jho, E.H.; Oh, E.S. D-tyrosine negatively regulates melanin synthesis by competitively inhibiting tyrosinase activity. Pigment. Cell. Melanoma Res. 2018, 31, 374–383. [Google Scholar] [CrossRef] [PubMed]


| 0Y | 5Y | 10Y | |
|---|---|---|---|
| pH | 6.26 ± 0.01 a | 3.59 ± 0.04 b | 3.47 ± 0.02 c |
| Total acid (%) | 0.26 ± 0.02 a | 0.88 ± 0.06 b | 0.91 ± 0.01 b |
| Reducing sugar (g/100 g) | 5.47 ± 0.1 a | 2.47 ± 0.01 b | 2.05 ± 0.04 c |
| Protein (g/100 g) | 2.68 ± 0.15 a | 1.87 ± 0.13 b | 1.24 ± 0.13 c |
| Salt content (%) | 12.06 ± 0.2 a | 12.64 ± 0.14 b | 12.66 ± 0.16 b |
| Brightness (L*) | 56.31 ± 1.2 a | 31.54 ± 1.08 b | 22.03 ± 0.52 b |
| Redness (a*) | 3.47 ± 0.17 a | 10.4 ± 0.19 b | 5.75 ± 0.29 c |
| Yellowness (b*) | 20.78 ± 0.65 a | 14.34 ± 0.4 b | 9.64 ± 0.16 c |
| Group | R2X | R2Y | Q2 |
|---|---|---|---|
| A1 (0Y–5Y) | 0.719 | 0.999 | 0.980 |
| A2 (0Y–10Y) | 0.775 | 1.000 | 0.994 |
| A3 (5Y–10Y) | 0.688 | 1.000 | 0.979 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Chen, H.; Xiang, L.; Zhang, Y.; Liu, D.; Zhao, Z. GC-TOF-MS-Based Non-Targeted Metabolomic Analysis of Differential Metabolites in Chinese Ultra-Long-Term Industrially Fermented Kohlrabi and Their Associated Metabolic Pathways. Metabolites 2022, 12, 991. https://doi.org/10.3390/metabo12100991
Nie X, Chen H, Xiang L, Zhang Y, Liu D, Zhao Z. GC-TOF-MS-Based Non-Targeted Metabolomic Analysis of Differential Metabolites in Chinese Ultra-Long-Term Industrially Fermented Kohlrabi and Their Associated Metabolic Pathways. Metabolites. 2022; 12(10):991. https://doi.org/10.3390/metabo12100991
Chicago/Turabian StyleNie, Xin, Hongfan Chen, Lu Xiang, Yulin Zhang, Dayu Liu, and Zhiping Zhao. 2022. "GC-TOF-MS-Based Non-Targeted Metabolomic Analysis of Differential Metabolites in Chinese Ultra-Long-Term Industrially Fermented Kohlrabi and Their Associated Metabolic Pathways" Metabolites 12, no. 10: 991. https://doi.org/10.3390/metabo12100991
APA StyleNie, X., Chen, H., Xiang, L., Zhang, Y., Liu, D., & Zhao, Z. (2022). GC-TOF-MS-Based Non-Targeted Metabolomic Analysis of Differential Metabolites in Chinese Ultra-Long-Term Industrially Fermented Kohlrabi and Their Associated Metabolic Pathways. Metabolites, 12(10), 991. https://doi.org/10.3390/metabo12100991