Untargeted Metabolomic Approach to Study the Impact of Aging on Salivary Metabolome in Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Selection Criteria for Participants
2.3. Collection and Storage of Salivary Samples
2.4. Instrumentation and Analytical Conditions
2.5. Quality Control for Untargeted Metabolomics
2.6. Data Analysis
2.7. Metabolic Pathways
3. Results and Discussion
3.1. Comparison of the LC-MS Profiles of Young and Elderly Women
3.2. Identification of the over = or Under-Expressed Compounds
3.3. Evolution of Saliva Metabolome with Age and Potential Affection of Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nassar, M.; Hiraishi, N.; Islam, M.S.; Otsuki, M.; Tagami, J. Age-Related Changes in Salivary Biomarkers. J. Dent. Sci. 2014, 9, 85–90. [Google Scholar] [CrossRef]
- Khalil, N.A. Saliva Can Be an Indicator for Aging. A Review. Curr. Sci. Int. 2022, 11, 84–98. [Google Scholar]
- Kudryashova, K.S.; Burka, K.; Kulaga, A.Y.; Vorobyeva, N.S.; Kennedy, B.K. Aging Biomarkers: From Functional Tests to Multi-Omics Approaches. Proteomics 2020, 20, 1900408. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A Review of Saliva: Normal Composition, Flow, and Function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Carpenter, G.H. The Secretion, Components, and Properties of Saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T.W. Salivary Biomarkers: Toward Future Clinical and Diagnostic Utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef]
- Roblegg, E.; Coughran, A.; Sirjani, D. Saliva: An All-Rounder of Our Body. Eur. J. Pharm. Biopharm. 2019, 142, 133–141. [Google Scholar] [CrossRef]
- Nagler, R.M.; Hershkovich, O. Age-Related Changes in Unstimulated Salivary Function and Composition and Its Relations to Medications and Oral Sensorial Complaints. Aging Clin. Exp. Res. 2005, 17, 358–366. [Google Scholar] [CrossRef]
- Vissink, A.; Spijkervet, F.K.L.; Amerongen, A.V.N. Aging and Saliva: A Review of the Literature. Spec. Care Dent. 1996, 16, 95–103. [Google Scholar] [CrossRef]
- Xu, F.; Laguna, L.; Sarkar, A. Aging-Related Changes in Quantity and Quality of Saliva: Where Do We Stand in Our Understanding? J. Texture Stud. 2019, 50, 27–35. [Google Scholar] [CrossRef]
- Toan, N.K.; Ahn, S.-G. Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature. IJMS 2021, 22, 5835. [Google Scholar] [CrossRef] [PubMed]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-Analysis of Salivary Flow Rates in Young and Older Adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Teruya, T.; Goga, H.; Yanagida, M. Human Age-Declined Saliva Metabolic Markers Determined by LC–MS. Sci. Rep. 2021, 11, 18135. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Cesari, M.; Landi, F.; Bernabei, R.; Coelho-Júnior, H.J.; Marzetti, E. Biomarkers of Physical Frailty and Sarcopenia: Coming up to the Place? Int. J. Mol. Sci. 2020, 21, 5635. [Google Scholar] [CrossRef]
- Miyamoto, K.; Hirayama, A.; Sato, Y.; Ikeda, S.; Maruyama, M.; Soga, T.; Tomita, M.; Nakamura, M.; Matsumoto, M.; Yoshimura, N.; et al. A Metabolomic Profile Predictive of New Osteoporosis or Sarcopenia Development. Metabolites 2021, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Bosman, P.; Pichon, V.; Acevedo, A.C.; Chardin, H.; Combes, A. Development of Analytical Methods to Study the Salivary Metabolome: Impact of the Sampling. Anal. Bioanal. Chem. 2022, 414, 6899–6909. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A Web-Based Platform to Process Untargeted Metabolomic Data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef]
- Montenegro-Burke, J.R.; Guijas, C.; Siuzdak, G. METLIN: A Tandem Mass Spectral Library of Standards. Methods Mol. Biol. 2020, 2104, 149–163. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Tanaka, S.; Machino, M.; Akita, S.; Yokote, Y.; Sakagami, H. Changes in Salivary Amino Acid Composition during Aging. In Vivo 2010, 24, 853–856. [Google Scholar]
- Antonelli, T.; Carlà, V.; Lambertini, L.; Moroni, F.; Bianchi, C. Pyroglutamic Acid Administration Modifies the Electrocorticogram and Increases the Release of Acetylcholine and GABA from the Guinea-Pig Cerebral Cortex. Pharmacol. Res. Commun. 1984, 16, 189–197. [Google Scholar] [CrossRef]
- Kaduce, T.L.; Spector, A.A.; Bar, R.S. Linoleic Acid Metabolism and Prostaglandin Production by Cultured Bovine Pulmonary Artery Endothelial Cells. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1982, 2, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, R.; Viola, A.U.; Tarokh, L.; Cajochen, C.; Brown, S.A. The Human Circadian Metabolome. Proc. Natl. Acad. Sci. USA 2012, 109, 2625–2629. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Yamamoto, K.; Yokota, H.; Hakozaki-Usui, K.; Hino, F.; Kato, I. Rapid, Simple Enzymatic Assay of Free L-Fucose in Serum and Urine, and Its Use as a Marker for Cancer, Cirrhosis, and Gastric Ulcers. Clin. Chem. 1990, 36, 474–476. [Google Scholar] [CrossRef]
- Yamauchi, M.; Kimura, K.; Maezawa, Y.; Ohata, M.; Mizuhara, Y.; Hirakawa, J.; Nakajima, H.; Toda, G. Urinary Level of L-Fucose as a Marker of Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 1993, 17, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, C.; Pitchika, V.; Pink, C.; Samietz, S.; Kastenmüller, G.; Artati, A.; Suhre, K.; Adamski, J.; Nauck, M.; Völzke, H.; et al. The Saliva Metabolome in Association to Oral Health Status. J. Dent. Res. 2019, 98, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Losman, J.-A.; Koivunen, P.; Kaelin, W.G. 2-Oxoglutarate-Dependent Dioxygenases in Cancer. Nat. Rev. Cancer 2020, 20, 710–726. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary Electrophoresis Mass Spectrometry-Based Saliva Metabolomics Identified Oral, Breast and Pancreatic Cancer-Specific Profiles. Metabolomics 2009, 6, 78–95. [Google Scholar] [CrossRef]
- Tsuruoka, M.; Hara, J.; Hirayama, A.; Sugimoto, M.; Soga, T.; Shankle, W.R.; Tomita, M. Capillary Electrophoresis-Mass Spectrometry-Based Metabolome Analysis of Serum and Saliva from Neurodegenerative Dementia Patients. Electrophoresis 2013, 34, 2865–2872. [Google Scholar] [CrossRef]
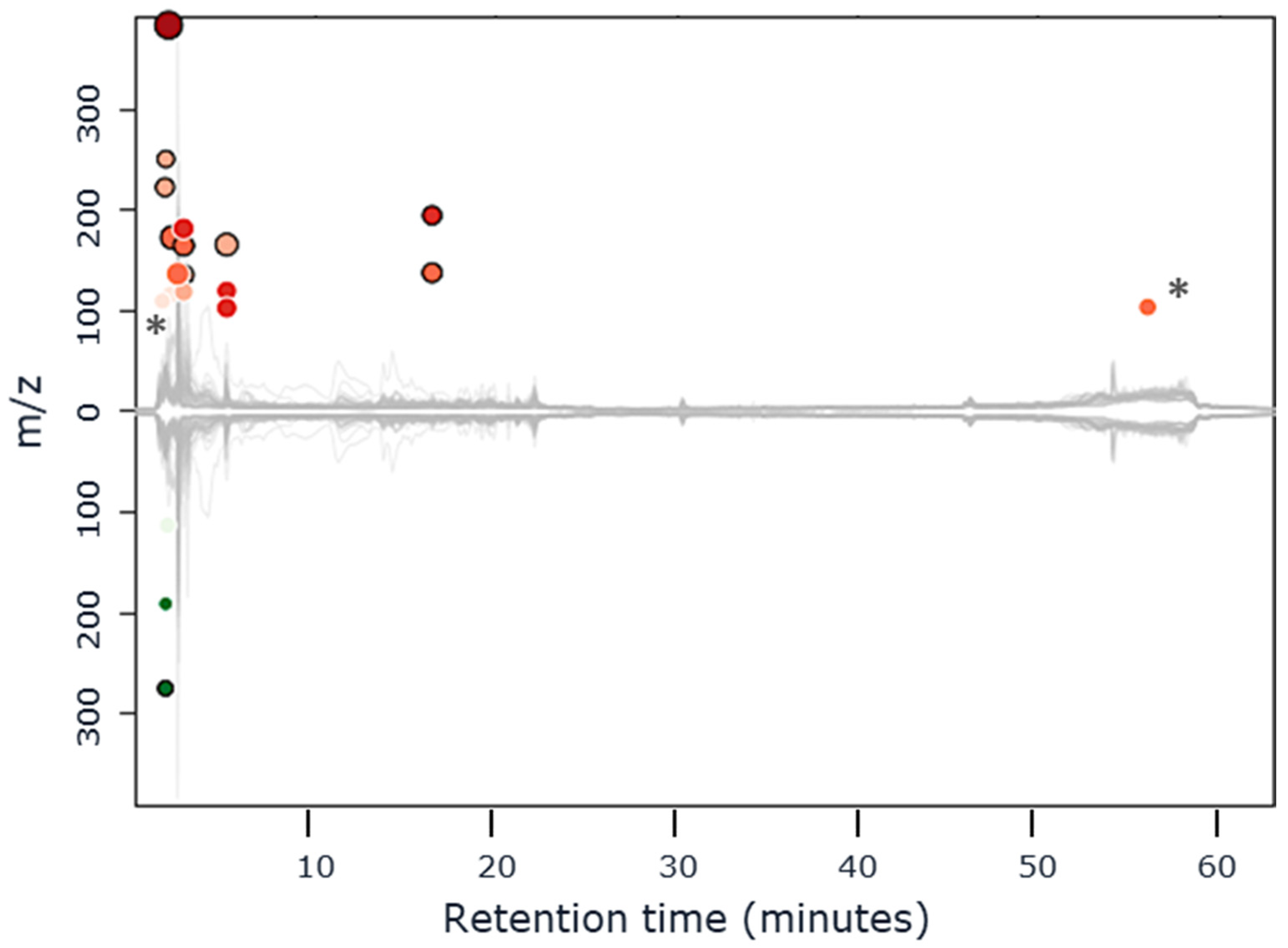

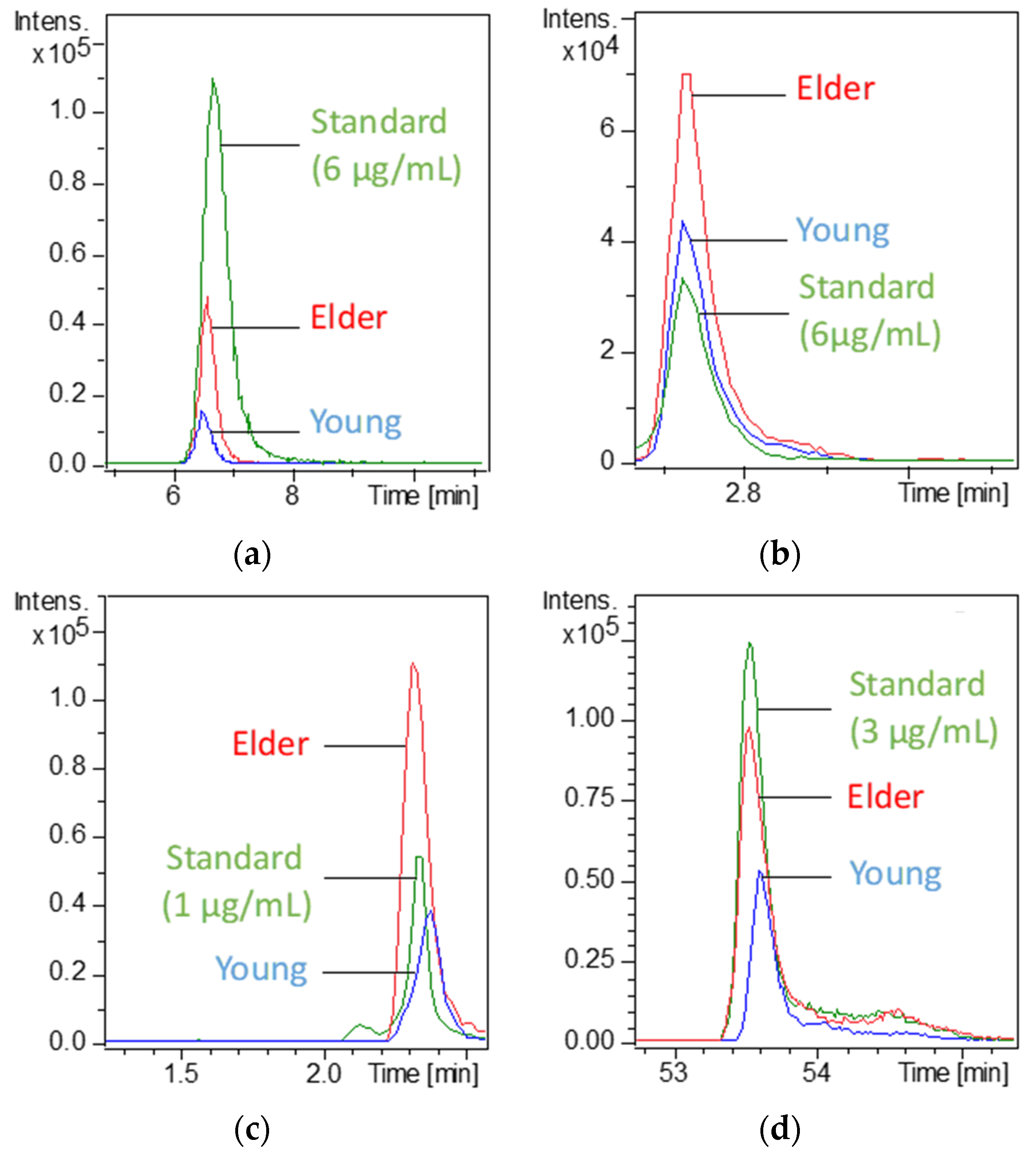
| Measured m/z | Fold Change (over Expressed) | Raw Formula | Confirmed Identification * | Log P | Analytical Method | Retention Time (min) |
|---|---|---|---|---|---|---|
| 88.041 | 1.8 | C3H7NO2 | Alanine | −3.2 | RPLC− | 1.73 |
| 102.058 | 2.7 | C4H9NO2 | γ-aminobutyric acid (GABA) | −2.3 | RPLC− | 1.75 |
| 103.056 | 2.4 | C5H10O2 | Isovaleric acid | 1.21 | RPLC+ | 5.04 |
| 104.037 | 2.6 | C3H7NO3 | Serine | −3.9 | RPLC− | 1.69 |
| 119.051 | 1.9 | C4H6O4 | Succinic acid ** | −0.4 | RPLC+ HILIC− | 2.66 6.61 |
| 117.020 | 3.0 | |||||
| 120.083 | 2.3 | C4H9NO3 | Threonine | −3.5 | RPLC+ | 5.04 |
| 123.046 | 2.0 | C4H10O4 | Threitol | −2.5 | RPLC+ | 2.65 |
| 128.037 | 4.6 | C5H7NO3 | Pyroglutamic acid | −0.89 | RPLC− | 2.54 |
| 130.089 | 3.1 | C6H13NO2 | Leucine/Isoleucine | −1.6 | RPLC− | 3.12 |
| 131.083 | 2.3 | C5H12N2O2 | Ornithine | −3.7 | RPLC− | 1.68 |
| 137.047 | 3.0 | C5H4N4O | Hypoxanthine | −0.05 | RPLC+ | 2.32 |
| 145.101 | 2.8 | C6H14N2O2 | Lysine | −3.2 | RPLC− | 1.56 |
| 146.050 | 2.5 | C5H9NO4 | Glutamic acid | −3.2 | RPLC− | 1.75 |
| 165.056 | 2.2 | C6H12O5 | Fucose | −1.9 | RPLC+ | 2.65 |
| 166.087 | 2.5 | C9H11NO2 | Phenylalanine | −1.2 | RPLC+ | 5.03 |
| 182.083 | 2.6 | C9H11NO3 | Tyrosine | −1.5 | RPLC+ | 2.65 |
| 279.236 | 1.9 | C18H32O2 | Linoleic acid | 6.42 | RPLC− | 53.75 |
| Measured m/z | Fold Change (over or under Expressed) | Raw Formula | Putative Identification (Error in ppm) | Analytical Method | Retention Time (min) |
|---|---|---|---|---|---|
| 136.076 | 2.1 (Over) | C8H9NO | Phenylacetamide (5) | RPLC+ | 2.65 |
| 138.068 | 2.0 (Over) | C6H13N | Cyclohexylammonium (4) | 16.41 | |
| 173.094 | 2.7 (Over) | C10H14O | Thymol (4) | 2.03 | |
| 195.088 | 1.9 (Over) | C8H10N4O2 | Caffein (4) | 16.41 | |
| 223.026 | 1.9 (Over) | C11H8N2O | Fuberidazole (3) | 1.62 | |
| 384.150 | 4.1 (Over) | C14H25NO11 | N-acetyllactosamine or Β-mannose-N-acetylglucosamine (1) | 1.82 | |
| 98.984 | 2.5 (Over) | H3PO4 | Phosphoric acid (4) | HILIC+ | 2.27 |
| 256.103 | 2.6 (Over) | C9H15N5O4 | Tetrahydroneoptin (10) | RPLC− | 2.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosman, P.; Pichon, V.; Acevedo, A.C.; Le Pottier, L.; Pers, J.O.; Chardin, H.; Combès, A. Untargeted Metabolomic Approach to Study the Impact of Aging on Salivary Metabolome in Women. Metabolites 2022, 12, 986. https://doi.org/10.3390/metabo12100986
Bosman P, Pichon V, Acevedo AC, Le Pottier L, Pers JO, Chardin H, Combès A. Untargeted Metabolomic Approach to Study the Impact of Aging on Salivary Metabolome in Women. Metabolites. 2022; 12(10):986. https://doi.org/10.3390/metabo12100986
Chicago/Turabian StyleBosman, Pauline, Valérie Pichon, Ana Carolina Acevedo, Laëtitia Le Pottier, Jacques Olivier Pers, Hélène Chardin, and Audrey Combès. 2022. "Untargeted Metabolomic Approach to Study the Impact of Aging on Salivary Metabolome in Women" Metabolites 12, no. 10: 986. https://doi.org/10.3390/metabo12100986
APA StyleBosman, P., Pichon, V., Acevedo, A. C., Le Pottier, L., Pers, J. O., Chardin, H., & Combès, A. (2022). Untargeted Metabolomic Approach to Study the Impact of Aging on Salivary Metabolome in Women. Metabolites, 12(10), 986. https://doi.org/10.3390/metabo12100986








