Study on the Regulation of Exogenous Hormones on the Absorption of Elements and the Accumulation of Secondary Metabolites in the Medicinal Plant Artemisia argyi Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Treatment Methods
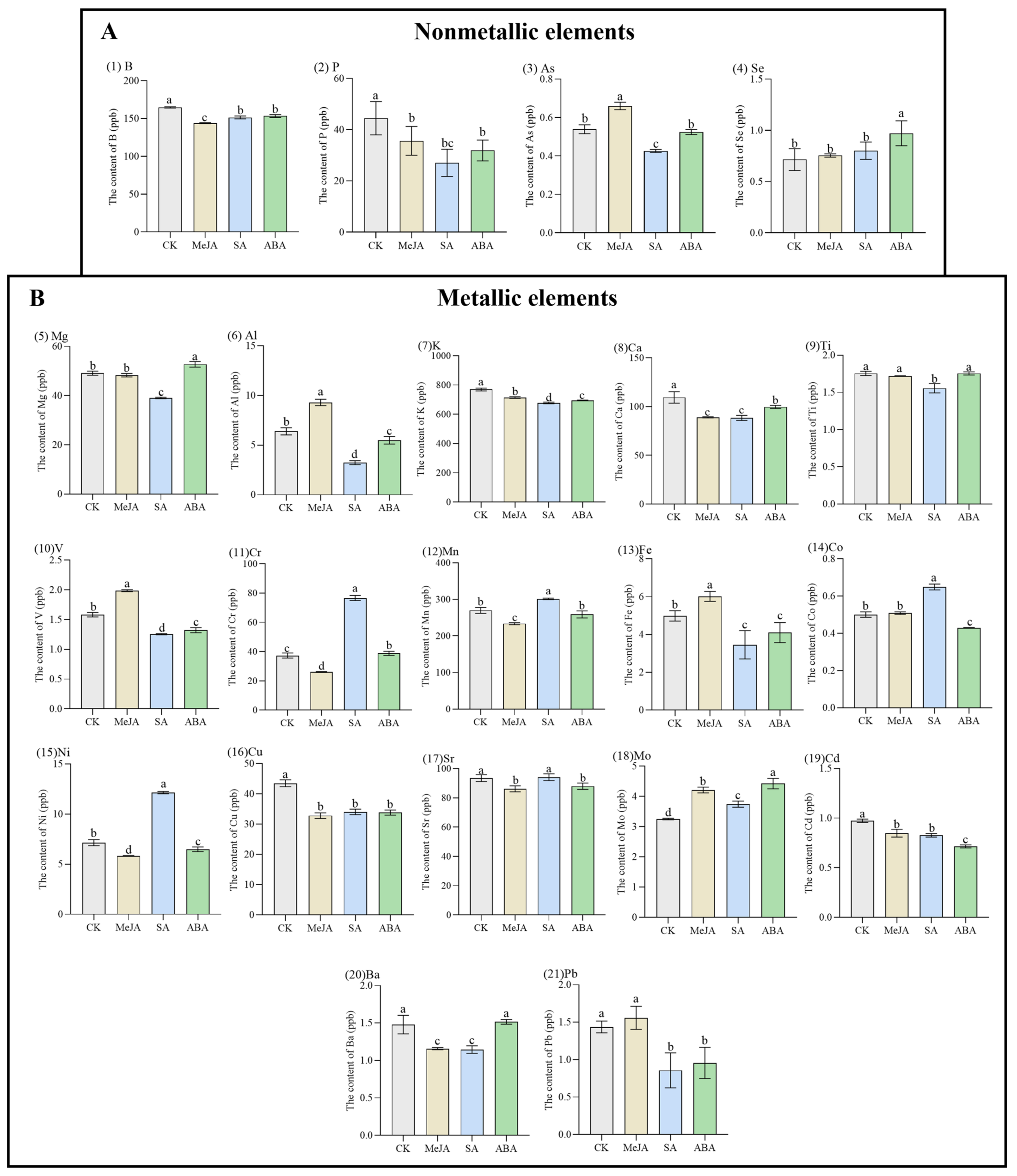
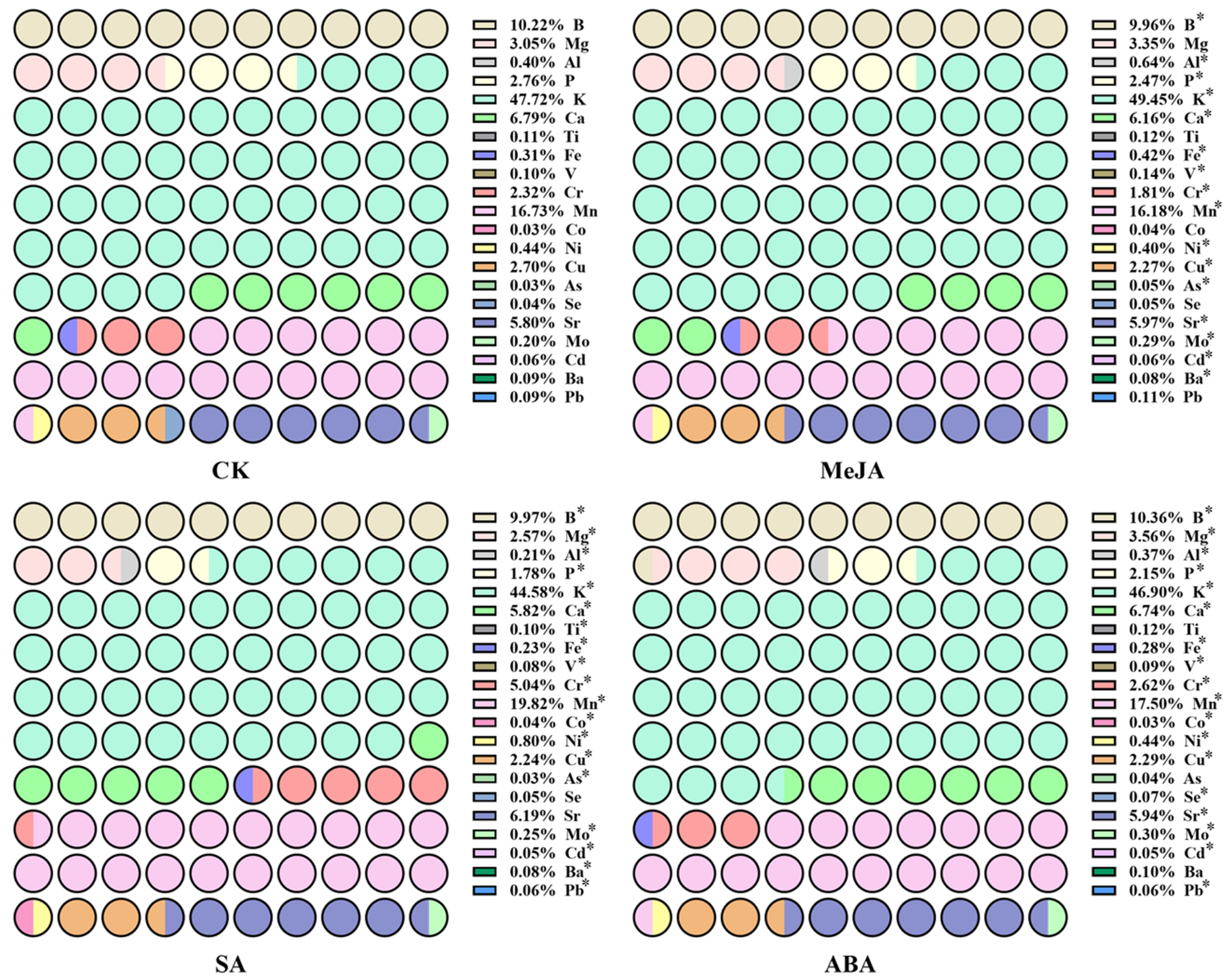
2.2. Determination of 21 Element Contents in A. argyi Leaves by ICP-MS
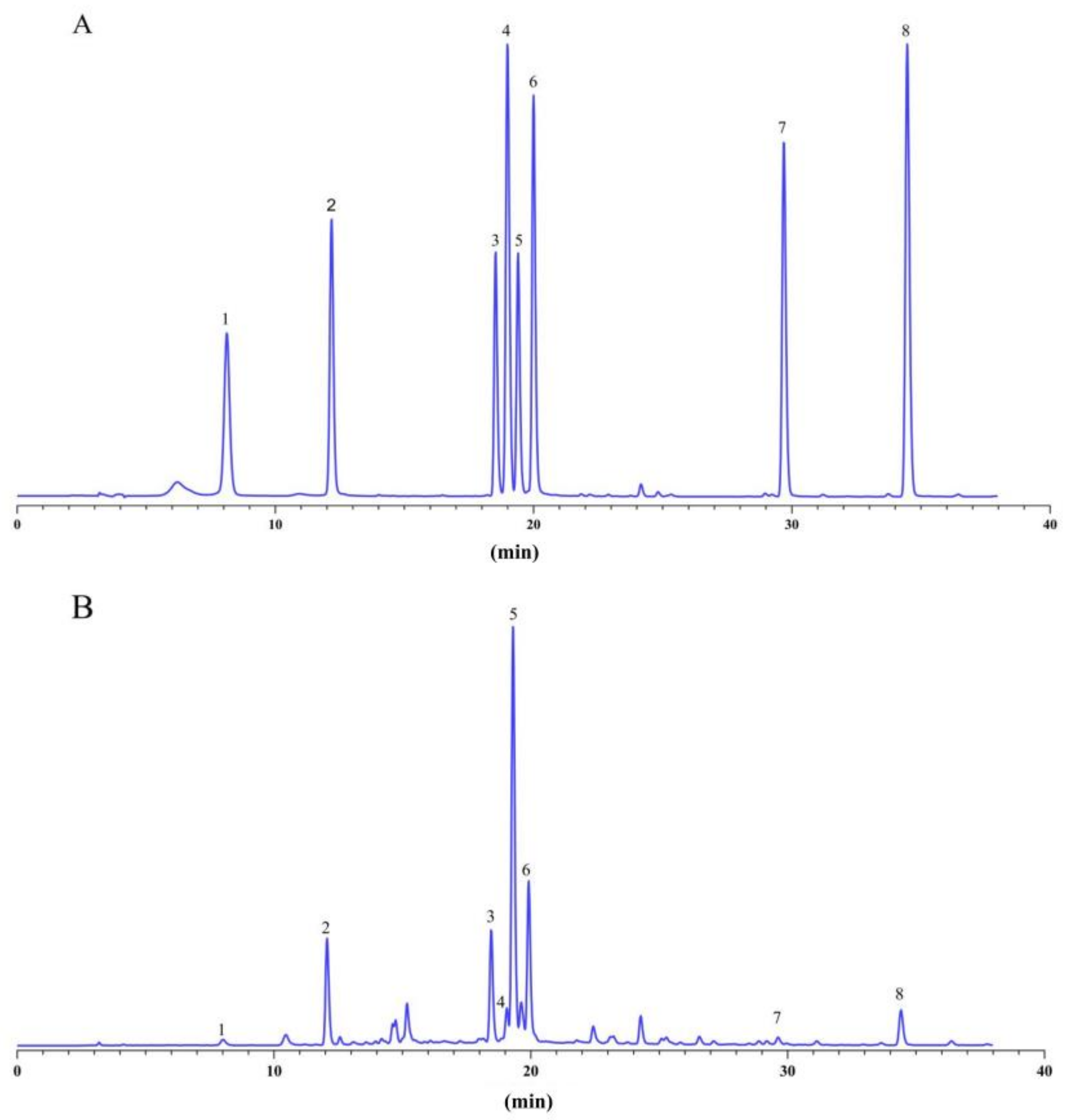
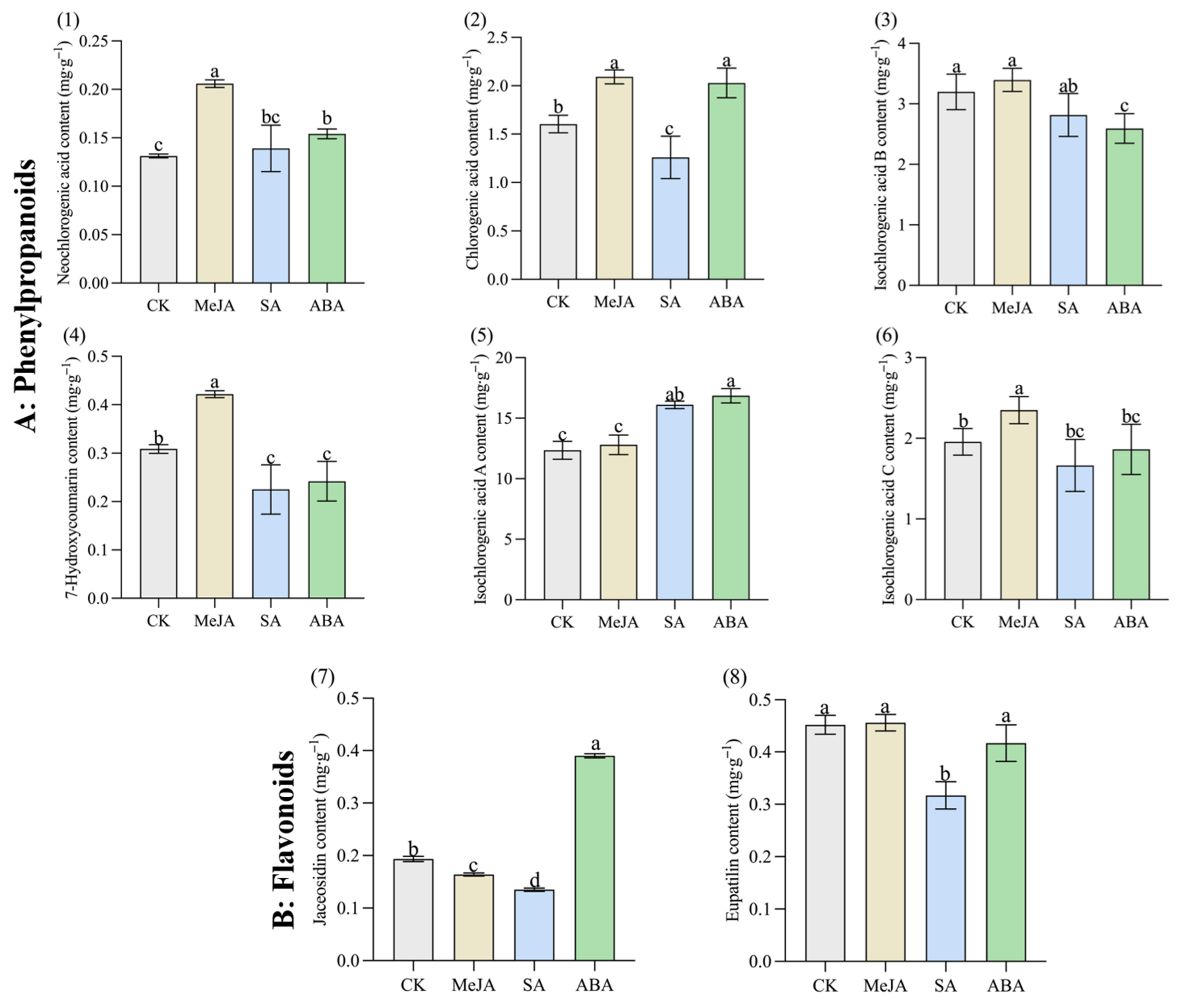
2.3. Determination of Eight Bioactive Components in A. argyi Leaves by HPLC
2.4. Widely Untargeted Metabolomic Analysis of A. argyi Leaves
2.5. Statistical Analysis
3. Results
3.1. The Content of 21 Element Contents in A. argyi Leaves
3.2. The Content of Bioactive Components in A. argyi Leaves
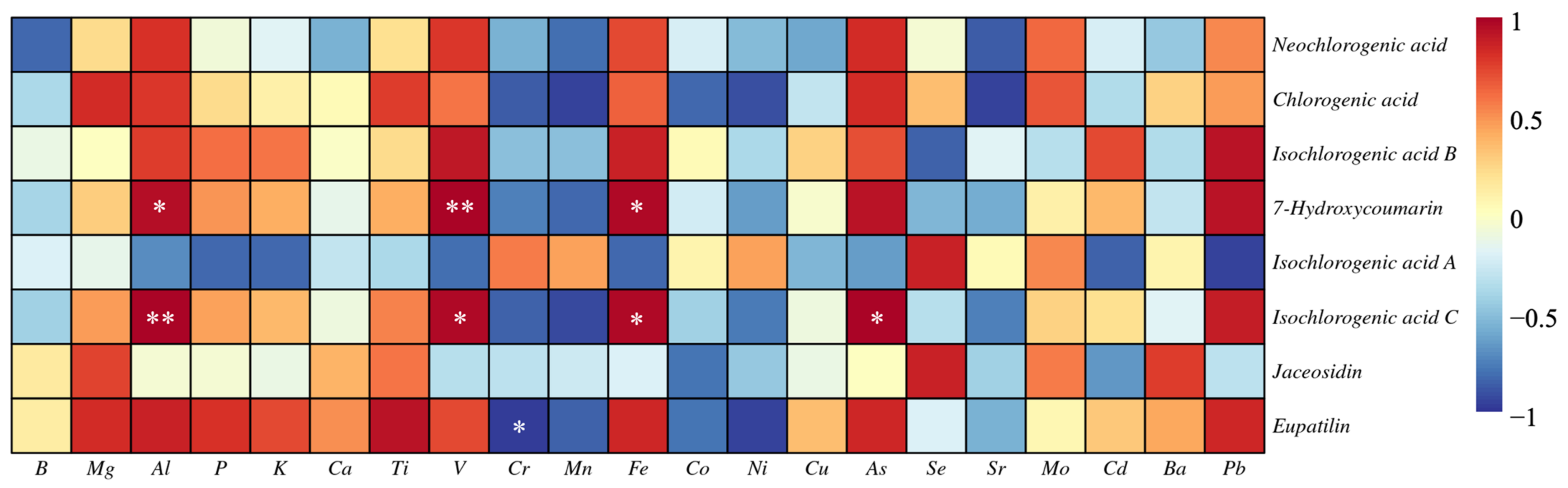
3.3. The Correlation between the Elements and the Bioactive Components
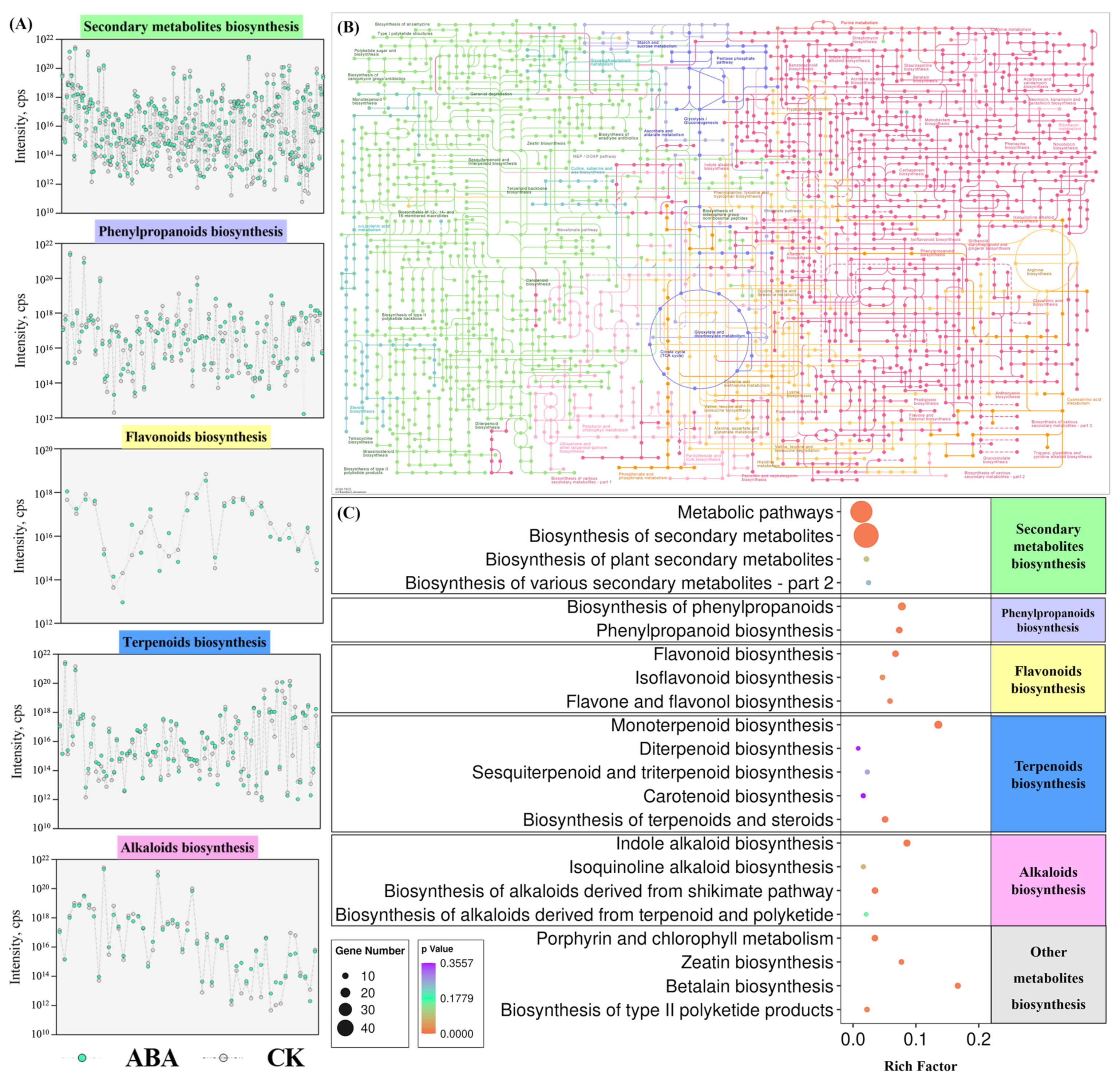
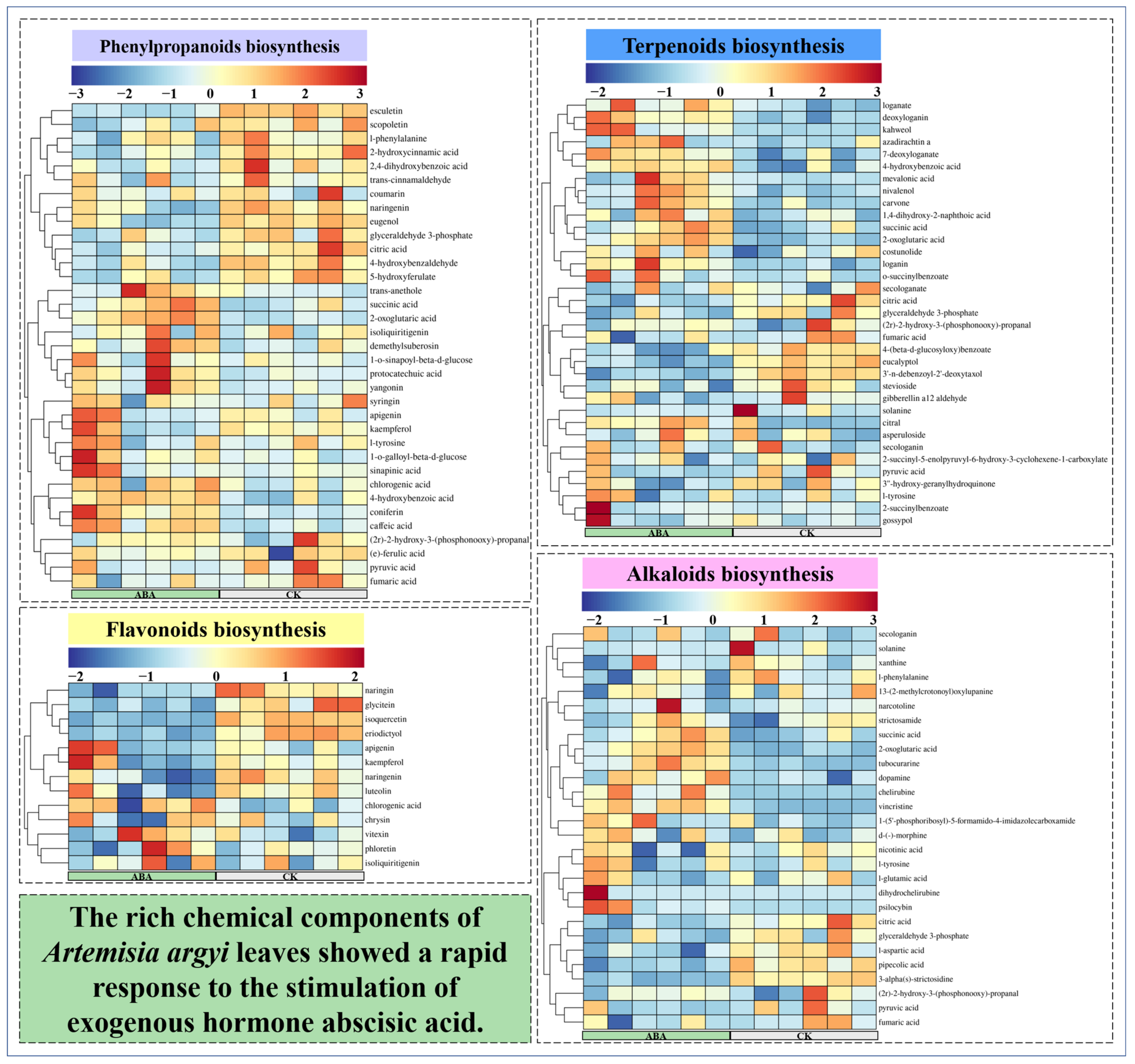
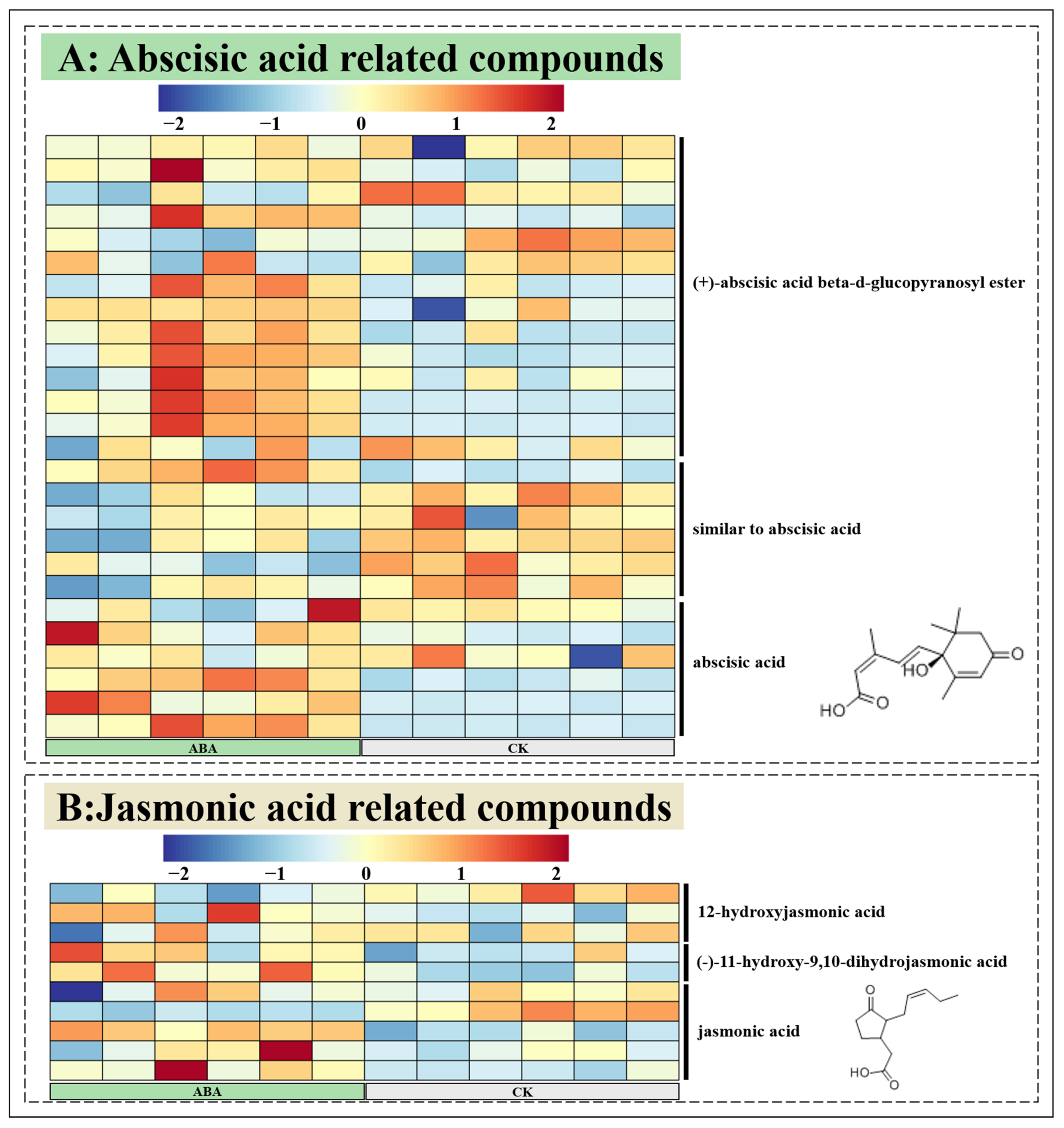
3.4. Widely Untargeted Metabolomic Analysis of A. argyi Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Li, X.; Jiang, Q.; Sun, H.; Jiang, J.; Chen, S.; Guan, Z.; Fang, W.; Chen, F. GC-MS analysis of the volatile constituents in the leaves of 14 Compositae plants. Molecules 2018, 23, 166. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, Z.; Guo, Y.; Lu, S.; Du, H.; Cao, Y. Antioxidant capacity of flavonoids from Folium Artemisiae Argyi and the molecular mechanism in Caenorhabditis elegans. J. Ethnopharmacol. 2021, 279, 114398. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Luo, D.; Zhao, T.; Du, H.; Liu, Z.; Xu, Z.; Guo, L.; Chen, C.; Peng, S.; Li, J.X.; et al. Genome sequencing reveals chromosome fusion and extensive expansion of genes related to secondary metabolism in Artemisia argyi. Plant Biotechnol. J. 2022, 20, 1902–1915. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, L.; Chen, Q.; Miao, Y.; Peng, Z.; Huang, B.; Guo, L.; Liu, D.; Du, H. Allelopathic effect of Artemisia argyi on the germination and growth of various weeds. Sci. Rep. 2021, 11, 4303. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-B.; Wang, Z.-G.; Xiong, Y.; Huang, X.-J.; Mei, Z.-N.; Hong, Z.-G. Anti-inflammatory and blood stasis activities of essential oil extracted from Artemisia argyi leaf in animals. J. Nat. Med. 2016, 70, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Y.; Wan, X.; Shi, N.; Huang, L.; Wan, D. Artemisiae Argyi Folium and its geo-authentic crude drug qi ai. J. Tradit. Chin. Med. Sci. 2017, 4, 20–23. [Google Scholar] [CrossRef]
- Xiao-Yan, L.; Yuan, Z.; Long-Bo, Z.; Da-Hui, L.; Xian-Zhang, H.; Li, Z.; Li-Ping, K. Research progress on chemical constituents from Artemisiae Argyi Folium and their pharmacological activities and quality control. China J. Chin. Mater. Med. 2020, 45, 4017–4030. (In Chinese) [Google Scholar]
- Liu, Y.; He, Y.; Wang, F.; Xu, R.; Yang, M.; Ci, Z.; Wu, Z.; Zhang, D.; Lin, J. From longevity grass to contemporary soft gold: Explore the chemical constituents, pharmacology, and toxicology of Artemisia argyi H.Lév. & vaniot essential oil. J. Ethnopharmacol. 2021, 279, 114404. [Google Scholar]
- Hou, M.-Z.; Chen, L.-L.; Chang, C.; Zan, J.-F.; Du, S.-M. Pharmacokinetic and tissue distribution study of eight volatile constituents in rats orally administrated with the essential oil of Artemisiae argyi Folium by GC–MS/MS. J. Chromatogr. B 2021, 1181, 122904. [Google Scholar] [CrossRef]
- Chen, L.-L.; Zhang, H.-J.; Chao, J.; Liu, J.-F. Essential oil of Artemisia argyi suppresses inflammatory responses by inhibiting JAK/STATs activation. J. Ethnopharmacol. 2017, 204, 107–117. [Google Scholar] [CrossRef]
- Luo, D.Y.; Yan, Z.T.; Che, L.R.; Zhu, J.J.; Chen, B. Repellency and insecticidal activity of seven Mugwort (Artemisia argyi) essential oils against the malaria vector Anopheles sinensis. Sci. Rep. 2022, 12, 5337. [Google Scholar] [CrossRef]
- Song, X.; Wen, X.; He, J.; Zhao, H.; Li, S.; Wang, M. Phytochemical components and biological activities of Artemisia argyi. J. Funct. Foods 2019, 52, 648–662. [Google Scholar] [CrossRef]
- Bouis, H.E. Micronutrient fortification of plants through plant breeding: Can it improve nutrition in man at low cost? Proc. Nutr. Soc. 2003, 62, 403–411. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Zhan, L.; Li, L. Mineral elements and active ingredients in root of wild Paeonia lactiflora growing at Duolun County, Inner Mongolia. Biol. Trace Elem. Res. 2020, 193, 548–554. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, F.; Yang, L.; Xiao, Y.; Zhou, G. A seasonal change of active ingredients and mineral elements in root of Astragalus membranaceus in the Qinghai-Tibet Plateau. Biol. Trace Elem. Res. 2021, 199, 3950–3959. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Yan, Z.Y.; Shen, X.; Yang, X. Prediction of active ingredients in Salvia miltiorrhiza Bunge. based on soil elements and artificial neural network. PeerJ 2022, 10, e12726. [Google Scholar] [CrossRef]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1934578X19850354. [Google Scholar]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of phytohormone signaling: A primary function of flavonoids in plant–environment interactions. Front. Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Spoel, S.H. Fine-tuning plant defence signalling: Salicylate versus jasmonate. Plant Biol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Qiao, J.; Luo, Z.; Li, Y.; Ren, G.; Liu, C.; Ma, X. Effect of abscisic acid on accumulation of five active components in root of Glycyrrhiza uralensis. Molecules 2017, 22, 1982. [Google Scholar] [CrossRef]
- Yang, L.-L.; Yang, L.; Lan, Y.-M.; Zhao, Y.; Han, M.; Yang, L.-M. Exogenous abscisic acid reduces saikosaponin accumulation by inhibiting saikosaponin synthesis pathway gene expression under drought stress in Bupleurum chinense DC. Ind. Crops Prod. 2020, 154, 112686. [Google Scholar] [CrossRef]
- Jirakiattikul, Y.; Rithichai, P.; Kwanthong, P.; Itharat, A. Effect of jasmonic acid elicitation period on enhancement of bioactive compounds and antioxidant activity in callus cultures of Hibicus sabdariffa Linn. Hortic. Environ. Biotechnol. 2021, 62, 629–636. [Google Scholar] [CrossRef]
- Sharifi, Y.; Nematzadeh, G.A.; Ghasemi Omran, V.; Tavabe Ghavami, T.S.; Ebrahimzadeh, M.A. Effect of salicylic acid on phenols and flavonoids content in callus culture of Iranian sodab (Ruta graveolens): A threatened medicinal plant of north of Iran. Tabari Biomed. Stud. Res. J. 2019, 1, 32–36. [Google Scholar]
- Yang, L.; Zhou, S.; Hou, Y.; Ji, B.; Pei, L.; Su, X.; Zhong, H.; Dong, C. Blue light induces biosynthesis of flavonoids in Epimedium sagittatum (Sieb.et Zucc.) Maxim. leaves, a study on a light-demanding medicinal shade herb. Ind. Crops Prod. 2022, 187, 115512. [Google Scholar] [CrossRef]
- Li, J.; Li, C. Seventy-year major research progress in plant hormones by Chinese scholars. Sci. Sin. Vitae 2019, 49, 1227. [Google Scholar]
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front. Environ. Sci. 2016, 4, 69. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–218. [Google Scholar]
- Watters, R.L.; Eberhardt, K.R.; Beary, E.S.; Fassett, J.D. Protocol for isotope dilution using inductively coupled plasma-mass spectrometry (ICP-MS) for the determination of inorganic elements. Metrologia 1997, 34, 87–96. [Google Scholar] [CrossRef]
- Fu, L.; Shi, S. A novel strategy to determine the compositions of inorganic elements in fruit wines using ICP-MS/MS. Food Chem. 2019, 299, 125172. [Google Scholar] [CrossRef] [PubMed]
- Grassin-Delyle, S.; Martin, M.; Hamzaoui, O.; Lamy, E.; Jayle, C.; Sage, E.; Etting, I.; Devillier, P.; Alvarez, J.-C. A high-resolution ICP-MS method for the determination of 38 inorganic elements in human whole blood, urine, hair and tissues after microwave digestion. Talanta 2019, 199, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular-physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol 2021, 229, 2446–2469. [Google Scholar] [CrossRef]
- Passioura, J.B. ‘Soil conditions and plant growth’. Plant Cell Environ. 2002, 25, 311–318. [Google Scholar] [CrossRef]
- Azaizeh, H. Effects of mineral nutrients on physiological and biochemical processes related to secondary metabolites production in medicinal herbs. Chapter Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 105–110. [Google Scholar]
- Konieczynski, P.; Viapiana, A.; Wesolowski, M. Comparison of infusions from Black and Green Teas (Camellia sinensis L. Kuntze) and Erva-mate (Ilex paraguariensis A. St.-Hil.) based on the content of essential elements, secondary metabolites, and antioxidant activity. Food Anal. Methods 2017, 10, 3063–3070. [Google Scholar] [CrossRef]
- Guo, L.; Wang, S.; Zhang, J.; Yang, G.; Zhao, M.; Ma, W.; Zhang, X.; Li, X.; Han, B.; Chen, N.; et al. Effects of ecological factors on secondary metabolites and inorganic elements of Scutellaria baicalensis and analysis of geoherblism. Sci. China Life Sci. 2013, 56, 1047. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, L.; Zhang, Y.; Han, M.; Yang, L. Effects of soil factors on saikosaponin content of Bupleurum chinense in different habitats. Chin. Tradit. Herb. Drugs 2020, 51, 5328–5336. (In Chinese) [Google Scholar]
- Aliyu, A.; Musa, A.; Oshanimi, J.; Ibrahim, H.; Oyewale, A.O. Phytochemical analyses and mineral elements composition of some medicinal plants of Northern Nigeria. Niger. J. Pharm. Sci. 2008, 7, 119–125. [Google Scholar]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant metabolomics: An indispensable system biology tool for plant science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef]
- Scossa, F.; Benina, M.; Alseekh, S.; Zhang, Y.; Fernie, A.R. The integration of metabolomics and next-generation sequencing data to elucidate the pathways of natural product metabolism in medicinal plants. Planta Med. 2018, 84, 855–873. [Google Scholar] [CrossRef]
- Dunn, W.B.; Bailey, N.J.C.; Johnson, H.E. Measuring the metabolome: Current analytical technologies. Analyst 2005, 130, 606–625. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Yan, Y.; Zhao, B.; Xu, H.; Su, X.; Dong, C. Study on the Regulation of Exogenous Hormones on the Absorption of Elements and the Accumulation of Secondary Metabolites in the Medicinal Plant Artemisia argyi Leaves. Metabolites 2022, 12, 984. https://doi.org/10.3390/metabo12100984
Yang L, Yan Y, Zhao B, Xu H, Su X, Dong C. Study on the Regulation of Exogenous Hormones on the Absorption of Elements and the Accumulation of Secondary Metabolites in the Medicinal Plant Artemisia argyi Leaves. Metabolites. 2022; 12(10):984. https://doi.org/10.3390/metabo12100984
Chicago/Turabian StyleYang, Linlin, Yueci Yan, Boyu Zhao, Huaming Xu, Xiuhong Su, and Chengming Dong. 2022. "Study on the Regulation of Exogenous Hormones on the Absorption of Elements and the Accumulation of Secondary Metabolites in the Medicinal Plant Artemisia argyi Leaves" Metabolites 12, no. 10: 984. https://doi.org/10.3390/metabo12100984
APA StyleYang, L., Yan, Y., Zhao, B., Xu, H., Su, X., & Dong, C. (2022). Study on the Regulation of Exogenous Hormones on the Absorption of Elements and the Accumulation of Secondary Metabolites in the Medicinal Plant Artemisia argyi Leaves. Metabolites, 12(10), 984. https://doi.org/10.3390/metabo12100984







