Simultaneous Determination of Methylated Nucleosides by HILIC–MS/MS Revealed Their Alterations in Urine from Breast Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Standard Preparation
2.3. Sample Collection
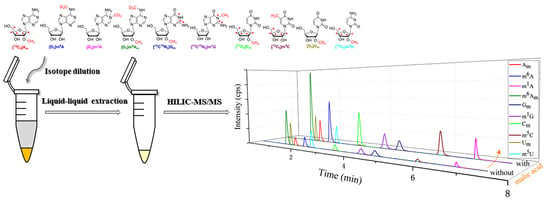
2.4. Sample Pretreatment
2.5. HILIC–MS/MS Analysis
2.6. Method Validation
2.7. Statistical Analysis
3. Results and Discussion
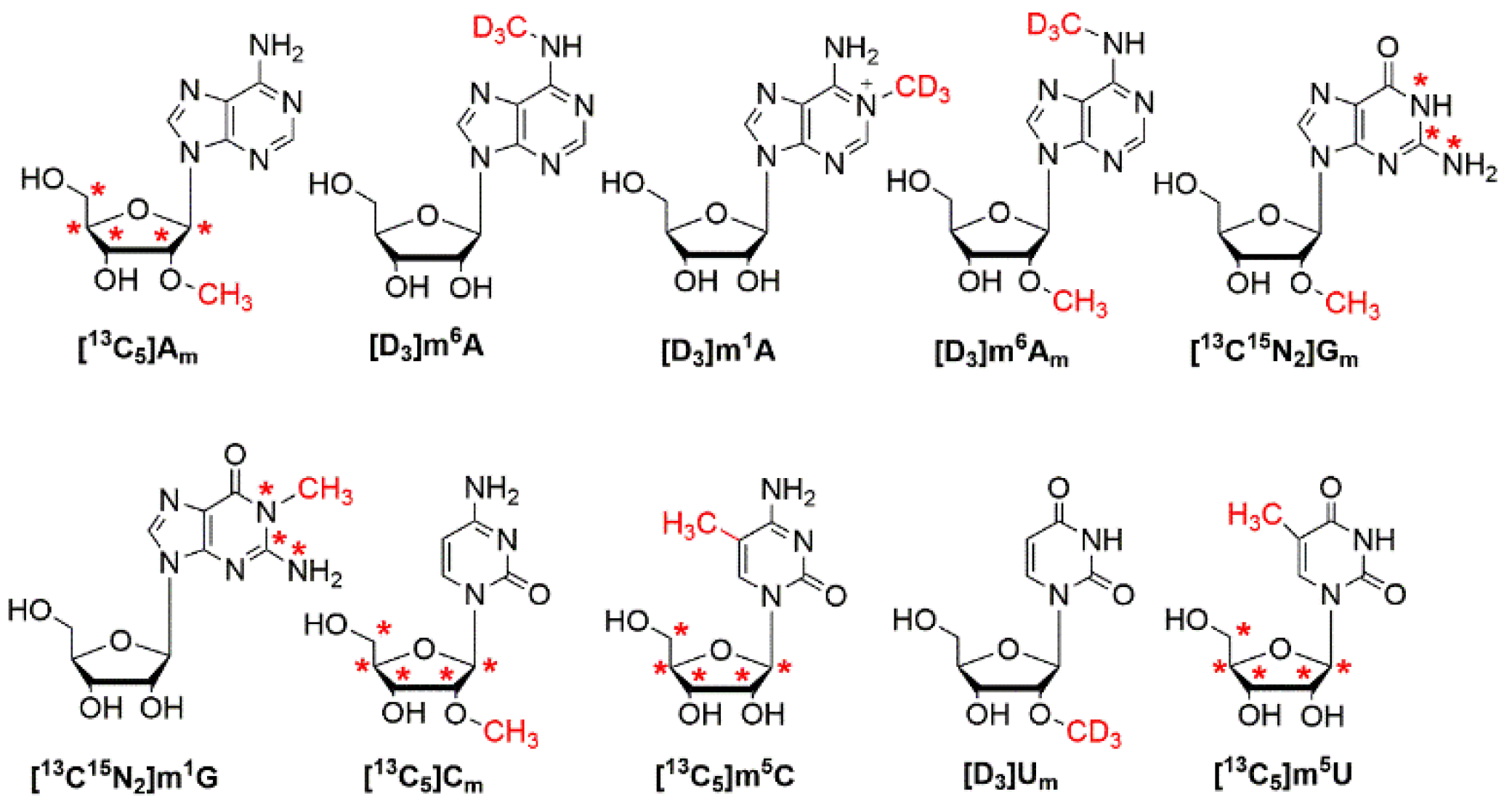
3.1. Synthesis and Characterization of Stable Isotope-Labeled Gm, m1G and Cm
3.2. Establishment of HILIC–MS/MS Method for the Simultaneous Determination of Am, m6A, m1A, m6Am, Gm, m1G, Cm, m5C, Um and m5U
3.3. Urine Samples Pretreatment
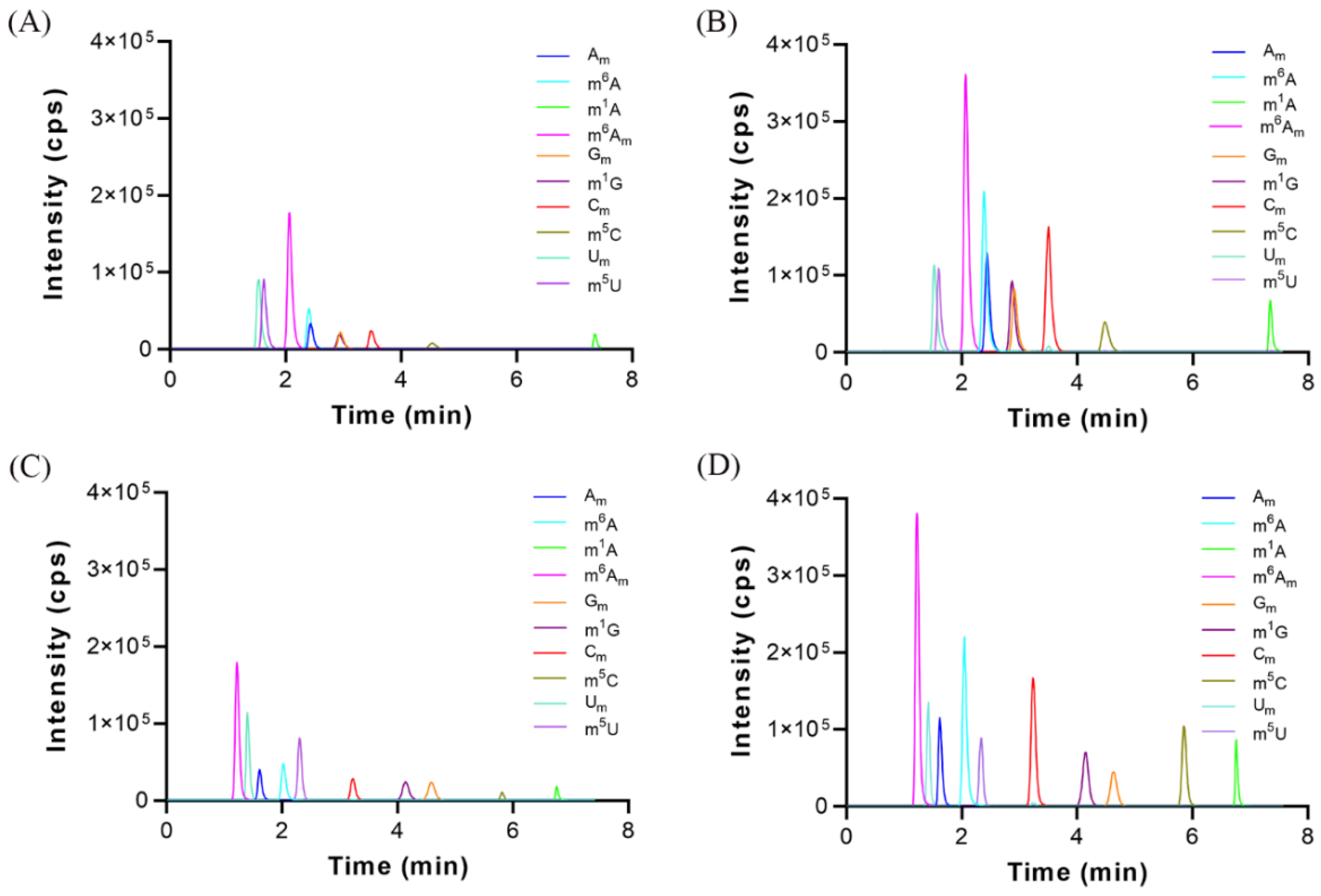
3.4. Validation of the Analytical Method
3.5. Identification of Methylated Nucleoside Modifications in Human Urine
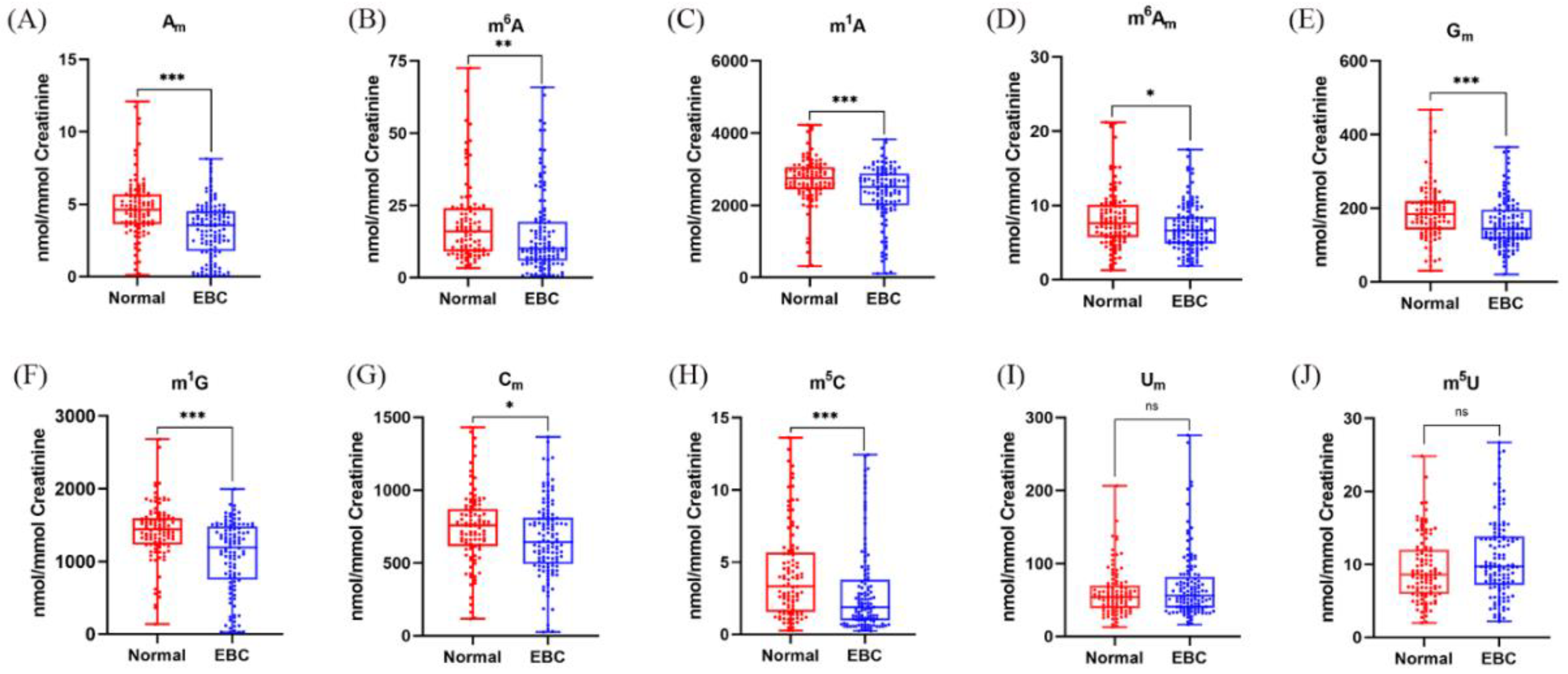
3.6. Quantification of Methylated Nucleoside Modifications in Human Urine
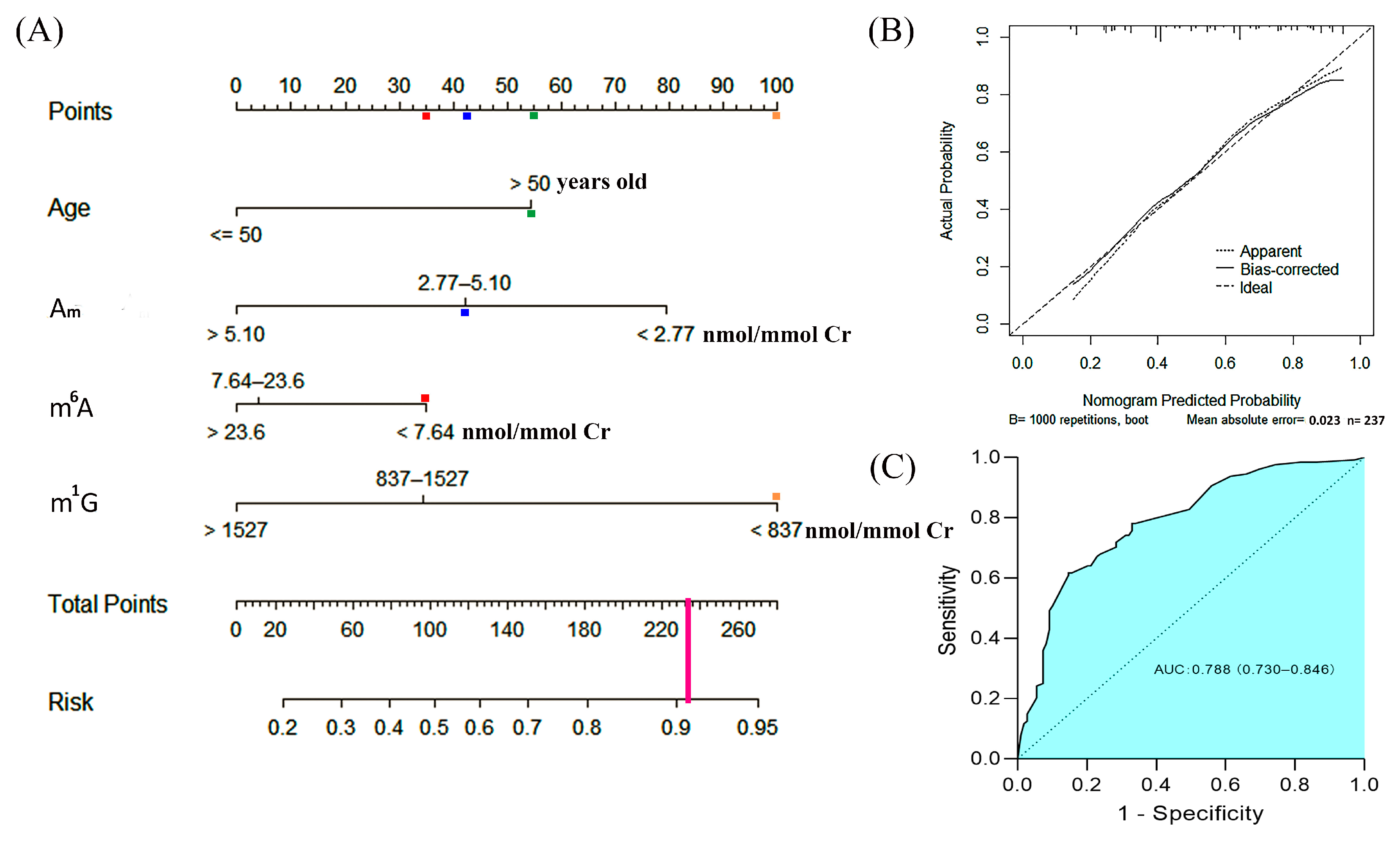
3.7. A Risk Nomogram for Prediction of the Occurrence of Early-Stage Breast Cancer
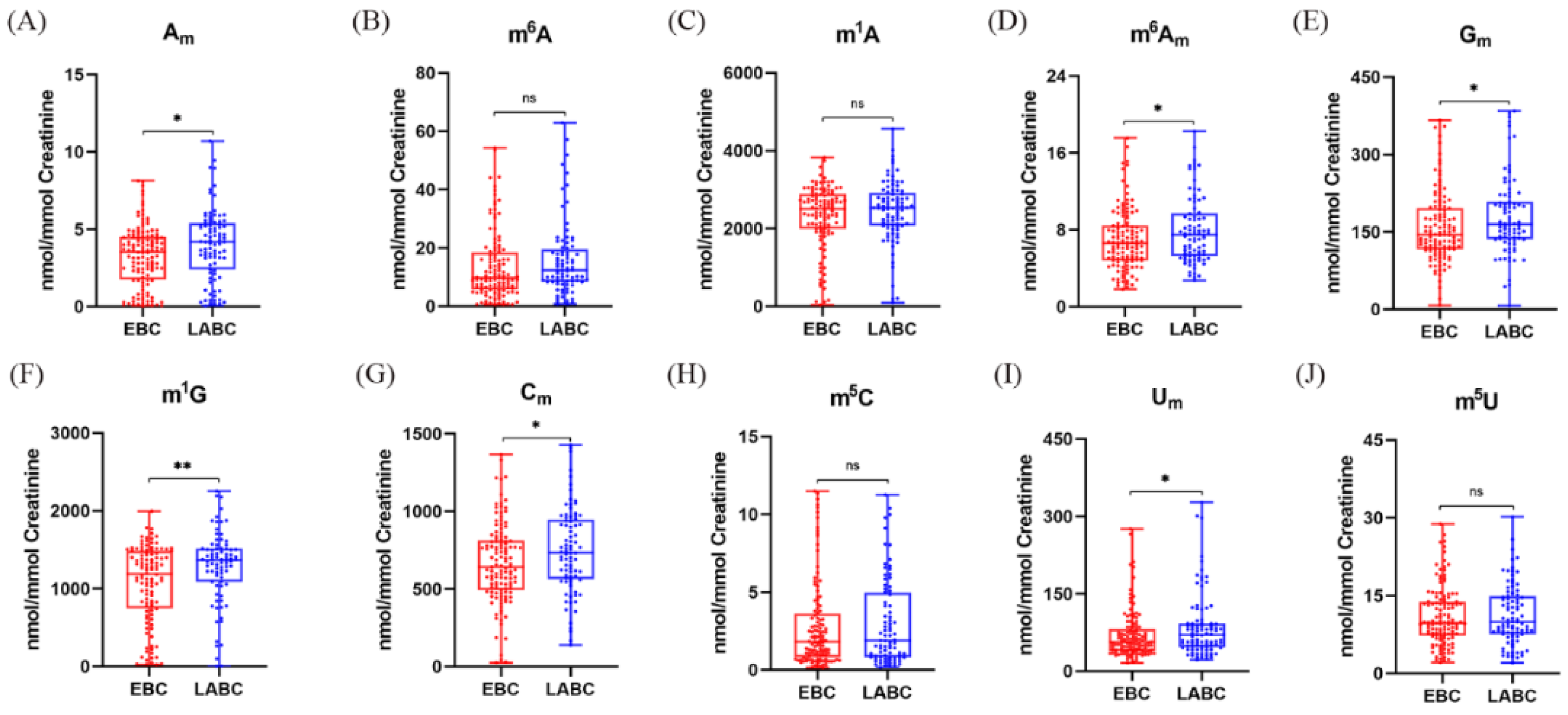
3.8. Elevated Levels of 2′-O-Methylated Nucleoside Modifications in Urine Suggest the Progression of Early-Stage Breast Cancer
3.9. Patients with Triple-Negative Breast Cancer Have Higher Levels of m1G and m5C in Urine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Adderley, H.; Alameddine, M.; Armstrong, A.; Arundell, D.; Fox, R.; Harries, M.; Lim, J.; Salih, Z.; Tetlow, C.; et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: A retrospective survey at two tertiary UK cancer centres. Eur. J. Cancer Care 2021, 30, e13395. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xie, C.; Chen, Q.; Cao, X.; Guo, M.; Zheng, S.; Wang, Y. A novel malic acid-enhanced method for the analysis of 5-methyl-2′-deoxycytidine, 5-hydroxymethyl-2′-deoxycytidine, 5-methylcytidine and 5-hydroxymethylcytidine in human urine using hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2018, 1034, 110–118. [Google Scholar] [CrossRef]
- Guo, C.; Hu, Y.; Cao, X.; Wang, Y. HILIC-MS/MS for the Determination of Methylated Adenine Nucleosides in Human Urine. Anal. Chem. 2021, 93, 17060–17068. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Y.; Fang, Z.; Ye, M.; Li, J.; Zhang, S.; Yuan, Y.; Guo, C. Elevated Levels of Oxidative Nucleic Acid Modification Markers in Urine From Gastric Cancer Patients: Quantitative Analysis by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry. Front. Chem. 2020, 8, 606495. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Qi, C.B.; Yuan, B.F.; Feng, Y.Q. Determination of cytidine modifications in human urine by liquid chromatography—Mass spectrometry analysis. Anal. Chim. Acta 2019, 1081, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Outeiro-Pinho, G.; Barros-Silva, D.; Aznar, E.; Sousa, A.I.; Vieira-Coimbra, M.; Oliveira, J.; Gonçalves, C.S.; Costa, B.M.; Junker, K.; Henrique, R.; et al. MicroRNA-30a-5p(me): A novel diagnostic and prognostic biomarker for clear cell renal cell carcinoma in tissue and urine samples. J. Exp. Clin. Cancer Res. 2020, 39, 98. [Google Scholar] [CrossRef]
- Jobu, K.; Sun, C.; Yoshioka, S.; Yokota, J.; Onogawa, M.; Kawada, C.; Inoue, K.; Shuin, T.; Sendo, T.; Miyamura, M. Metabolomics study on the biochemical profiles of odor elements in urine of human with bladder cancer. Biol. Pharm. Bull. 2012, 35, 639–642. [Google Scholar] [CrossRef]
- Oerlemans, F.; Lange, F. Major and modified nucleosides as markers in ovarian cancer: A pilot study. Gynecol. Obstet. Investig. 1986, 22, 212–217. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Kong, H.W.; Xiong, J.H.; Lv, S.; Xu, G.W. Clinical significance and prognostic value of urinary nucleosides in breast cancer patients. Clin. Biochem. 2005, 38, 24–30. [Google Scholar] [CrossRef]
- Omran, M.M.; Rashed, R.E.; Darwish, H.; Belal, A.A.; Mohamed, F.Z. Development of a gas chromatography-mass spectrometry method for breast cancer diagnosis based on nucleoside metabolomes 1-methyl adenosine, 1-methylguanosine and 8-hydroxy-2′-deoxyguanosine. Biomed. Chromatogr. 2020, 34, e4713. [Google Scholar] [CrossRef] [PubMed]
- Zahran, F.; Rashed, R.; Omran, M.; Darwish, H.; Belal, A. Study on Urinary Candidate Metabolome for the Early Detection of Breast Cancer. Indian J. Clin. Biochem. 2021, 36, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Schmid, H.R.; Lu, X.; Liebich, H.M.; Lu, P. Excretion pattern investigation of urinary normal and modified nucleosides of breast cancer patients by RP-HPLC and factor analysis method. Biomed. Chromatogr. 2000, 14, 459–463. [Google Scholar] [CrossRef]
- Jiang, M.; Prokhorova, A.F.; Rozhmanova, N.B.; Shpigun, O.A. Electrophoretic separation of some nucleosides for the diagnosis of mastopathy and fibroadenoma. J. Anal. Chem. 2016, 71, 1198–1203. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Lin, W.D.; Tsai, Y.; Lin, C.T.; Wang, H.C.; Jeng, L.B.; Lee, C.C.; Lin, Y.C.; Lai, C.C.; Tsai, F.J. Analysis of urinary nucleosides as potential tumor markers in human breast cancer by high performance liquid chromatography/electrospray ionization tandem mass spectrometry. Clin. Chim. Acta 2011, 412, 1861–1866. [Google Scholar] [CrossRef]
- Hu, C.W.; Liu, H.H.; Li, Y.J.; Chao, M.R. Direct analysis of 5-methyl-2’-deoxycytidine in human urine by isotope dilution LC-MS/MS: Correlations with N-methylated purines and oxidized DNA lessions. Chem. Res. Toxicol. 2012, 25, 462–470. [Google Scholar] [CrossRef]
- Li, X.S.; Li, S.; Kellermann, G. Simultaneous extraction and determination of monoamine neurotransmitters in human urine for clinical routine testing based on a dual functional solid phase extraction assisted by phenylboronic acid coupled with liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 2859–2871. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, X.; Li, X.; Zhai, C.; Shi, Y.; Gao, X. BH+/MH+-matching method for discovery of cis-diol-containing modified nucleosides in urine by ribose-targeted solid phase extraction followed by dual-mass spectrometry platform identification. J. Pharm. Biomed. Anal. 2022, 210, 114555. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Singh, V.; Rickert, D.; Khaled, A.; Pawliszyn, J. High throughput determination of free biogenic monoamines and their metabolites in urine using thin-film solid phase microextraction. Talanta 2021, 232, 122438. [Google Scholar] [CrossRef]
- Deng, F.; Yu, H.; Pan, X.; Hu, G.; Wang, Q.; Peng, R.; Tan, L.; Yang, Z. Ultra-high performance liquid chromatography tandem mass spectrometry for the determination of five glycopeptide antibiotics in food and biological samples using solid-phase extraction. J. Chromatogr. A 2018, 1538, 54–59. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, A.; Zhang, Y.; Bie, Z. Recent advances of boronate affinity materials in sample preparation. Anal. Chim. Acta 2019, 1076, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chen, Q.; Chen, J.; Yu, J.; Hu, Y.; Zhang, S.; Zheng, S. 8-Hydroxyguanosine as a possible RNA oxidative modification marker in urine from colorectal cancer patients: Evaluation by ultra performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2020, 1136, 121931. [Google Scholar] [CrossRef]
- Cao, G.; Song, Z.; Yang, Z.; Chen, Z.; Hong, Y.; Cai, Z. Database-assisted global metabolomics profiling of pleural effusion induced by tuberculosis and malignancy. Chin. Chem. Lett. 2021, 32, 3207–3210. [Google Scholar] [CrossRef]
- Xiang, L.; Nie, J.; Wang, L.; Wang, Y.; Shi, J.; Wei, J.; Lau, C.; Cai, Z.; Huang, Y. Integrated metabolomics analysis of the effect of PPARδ agonist GW501516 on catabolism of BCAAs and carboxylic acids in diabetic mice. Chin. Chem. Lett. 2020, 32, 2197–2202. [Google Scholar] [CrossRef]
- Yu, Q.W.; Liu, S.J.; Zheng, F.; Xiao, H.M.; Guan, H.Y.; Feng, Y.Q. Identification and quantification of benzimidazole metabolites of thiophonate-methyl sprayed on celery cabbage SiO2@NiO solid-phase extraction in combination with HIPLC-MS/MS. Chin. Chem. Lett. 2020, 31, 482–486. [Google Scholar] [CrossRef]
- An, Y.P.; Liu, S.; Hao, F.H.; Wang, Y.L.; Tang, H.R. Develpopment and validation of an improved probabilistic quotient normalization method for LC/MS and NMR-based matabonomic analysis. Chin. Chem. Lett. 2020, 31, 1827–1830. [Google Scholar] [CrossRef]
- Xiao, H.M.; Liu, P.; Zheng, S.J.; Wang, X.; Ding, J.; Feng, Y.Q. Screening of amino acids in dried blood spots by stable isotope derivatization-liquid chromatography-electrospray ionization mass spectrometry. Chin. Chem. Lett. 2020, 31, 2423–2427. [Google Scholar] [CrossRef]
- Domenick, T.M.; Gill, E.L.; Vedam-Mai, V.; Yost, R.A. Mass spectrometry-based cellular metabolomics: Current approaches, applications and future directions. Anal. Chem. 2021, 93, 546–566. [Google Scholar] [CrossRef]
- Deda, O.; Virgiliou, C.; Orfanidis, A.; Gika, H.G. Study of Fecal and Urinary Metabolite Perturbations Induced by Chronic Ethanol Treatment in Mice by UHPLC-MS/MS Targeted Profiling. Metabolites 2019, 9, 232. [Google Scholar] [CrossRef]
- Qi, C.B.; Jiang, H.P.; Xiong, J.; Yuan, B.F.; Feng, Y.Q. On-line trapping/capillary hydrophilic-interaction liquid chromatography/mass spectrometry for sensitive determination of RNA modifications from human blood. Chin. Chem. Lett. 2019, 30, 553–557. [Google Scholar] [CrossRef]
- Yamauchi, K.; Nakagima, T.; Kinoshita, M. Methylation of nucleosides with trimethylsulfonium hydroxide. Effects of transition metal ions. J. Org. Chem. 1980, 45, 3865–3868. [Google Scholar] [CrossRef]
- Gątarek, P.; Kałużna-Czaplińska, J.; Pawełczyk, M.; Jastrzębski, K.; Giebułtowicz, J.; Głąbiński, A.; Bobrowska-Korczak, B. LC-MS/MS Determination of Modified Nucleosides in The Urine of Parkinson’s Disease and Parkinsonian Syndromes Patients. Molecules 2020, 25, 4959. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Shi, C.; Liu, L.; Li, P.; Sun, Y.; An, Z. A single-injection targeted metabolomics profiling method for determination of biomarkers to reflect tripterygium glycosides efficacy and toxicity. Toxicol. Appl. Pharmacol. 2020, 389, 114880. [Google Scholar] [CrossRef] [PubMed]
- Raćkowska, E.; Bobrowska-Korczak, B.; Giebułtowicz, J. Development and validation of a rapid LC-MS/MS method for determination of methylated nucleosides and nucleobases in urine. J. Chromatogr. B 2019, 1128, 121775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, Y.; Hong, X.; Wang, M.; Fang, Z.; Cao, X.; Jiang, K.; Guo, C. Determination of adenosine and its modifications in urine and plasma from breast cancer patients by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2022, 1209, 123428. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, J.; Wang, H.; Du, X.; Xu, Y.; Wang, Z.; Chen, D. Prognostic Significance and Tumor Immune Microenvironment Heterogenicity of m5C RNA Methylation Regulators in Triple-Negative Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 657547. [Google Scholar] [CrossRef]

| Linear Equation | R2 Value | Linear Range (nM) | Matrix Factor (%) | |
|---|---|---|---|---|
| Am | y = 1.8251x + 0.0196 | 0.9993 | 1–50 | 109.22 |
| m6A | y = 0.6465x + 0.0034 | 0.9998 | 1–250 | 101.78 |
| m1A | y = 0.9332x + 0.0329 | 0.9996 | 1–10,000 | 94.42 |
| m6Am | y = 1.0074x + 0.0011 | 0.9999 | 1–250 | 103.15 |
| Gm | y = 0.7109x + 0.0077 | 1.0000 | 1–1000 | 95.47 |
| m1G | y = 0.1189x + 0.0173 | 0.9999 | 1–5000 | 100.91 |
| Cm | y = 0.1409x + 0.0316 | 0.9999 | 1–5000 | 102.11 |
| m5C | y = 0.7475x + 0.0023 | 0.9999 | 1–100 | 101.46 |
| Um | y = 3.8871x – 0.0331 | 0.9998 | 1–500 | 109.75 |
| m5U | y = 1.9261x – 0.0852 | 0.9995 | 1–250 | 94.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Hu, Y.; Hong, X.; Zhang, X.; Pan, T.; Pan, C.; Zheng, S.; Guo, C. Simultaneous Determination of Methylated Nucleosides by HILIC–MS/MS Revealed Their Alterations in Urine from Breast Cancer Patients. Metabolites 2022, 12, 973. https://doi.org/10.3390/metabo12100973
Fang Z, Hu Y, Hong X, Zhang X, Pan T, Pan C, Zheng S, Guo C. Simultaneous Determination of Methylated Nucleosides by HILIC–MS/MS Revealed Their Alterations in Urine from Breast Cancer Patients. Metabolites. 2022; 12(10):973. https://doi.org/10.3390/metabo12100973
Chicago/Turabian StyleFang, Zhihao, Yiqiu Hu, Xiujuan Hong, Xiaoxiao Zhang, Tao Pan, Chi Pan, Shu Zheng, and Cheng Guo. 2022. "Simultaneous Determination of Methylated Nucleosides by HILIC–MS/MS Revealed Their Alterations in Urine from Breast Cancer Patients" Metabolites 12, no. 10: 973. https://doi.org/10.3390/metabo12100973
APA StyleFang, Z., Hu, Y., Hong, X., Zhang, X., Pan, T., Pan, C., Zheng, S., & Guo, C. (2022). Simultaneous Determination of Methylated Nucleosides by HILIC–MS/MS Revealed Their Alterations in Urine from Breast Cancer Patients. Metabolites, 12(10), 973. https://doi.org/10.3390/metabo12100973