Energetic Contributions Including Gender Differences and Metabolic Flexibility in the General Population and Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Graded Incremental Exercise Testing
2.3. Calculations of Fat and Carbohydrate Oxidation Rate during GIET
2.4. Calculations of Energetic Contributions during GIET
2.5. Statistical Analyses
3. Results
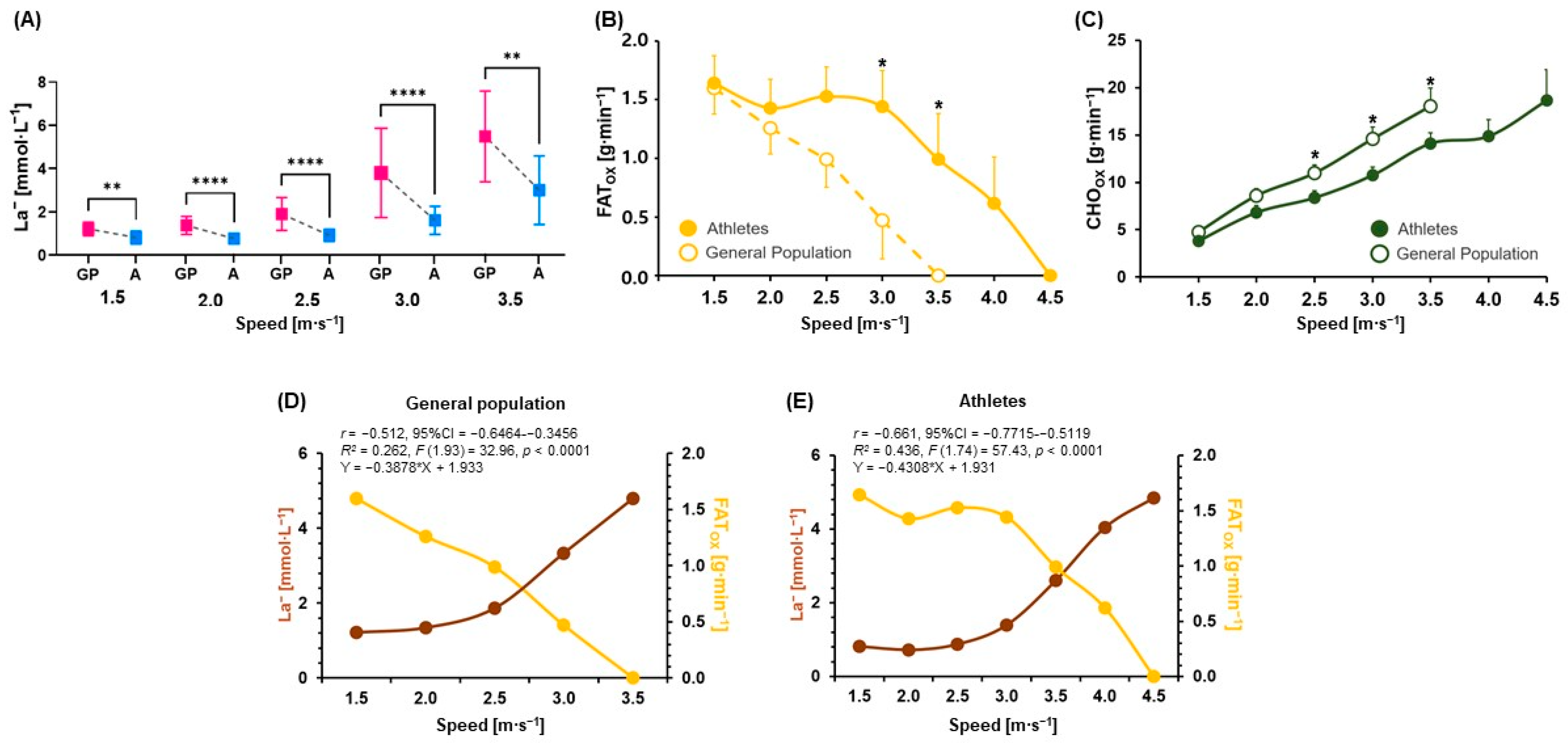
3.1. Comparisons of Physiological Parameters, Metabolic Flexibility (FATox and CHOox), and Correlation and Regression Analyses between La− and FATox
3.2. Jogging/Running Speeds and HR at Certain La−
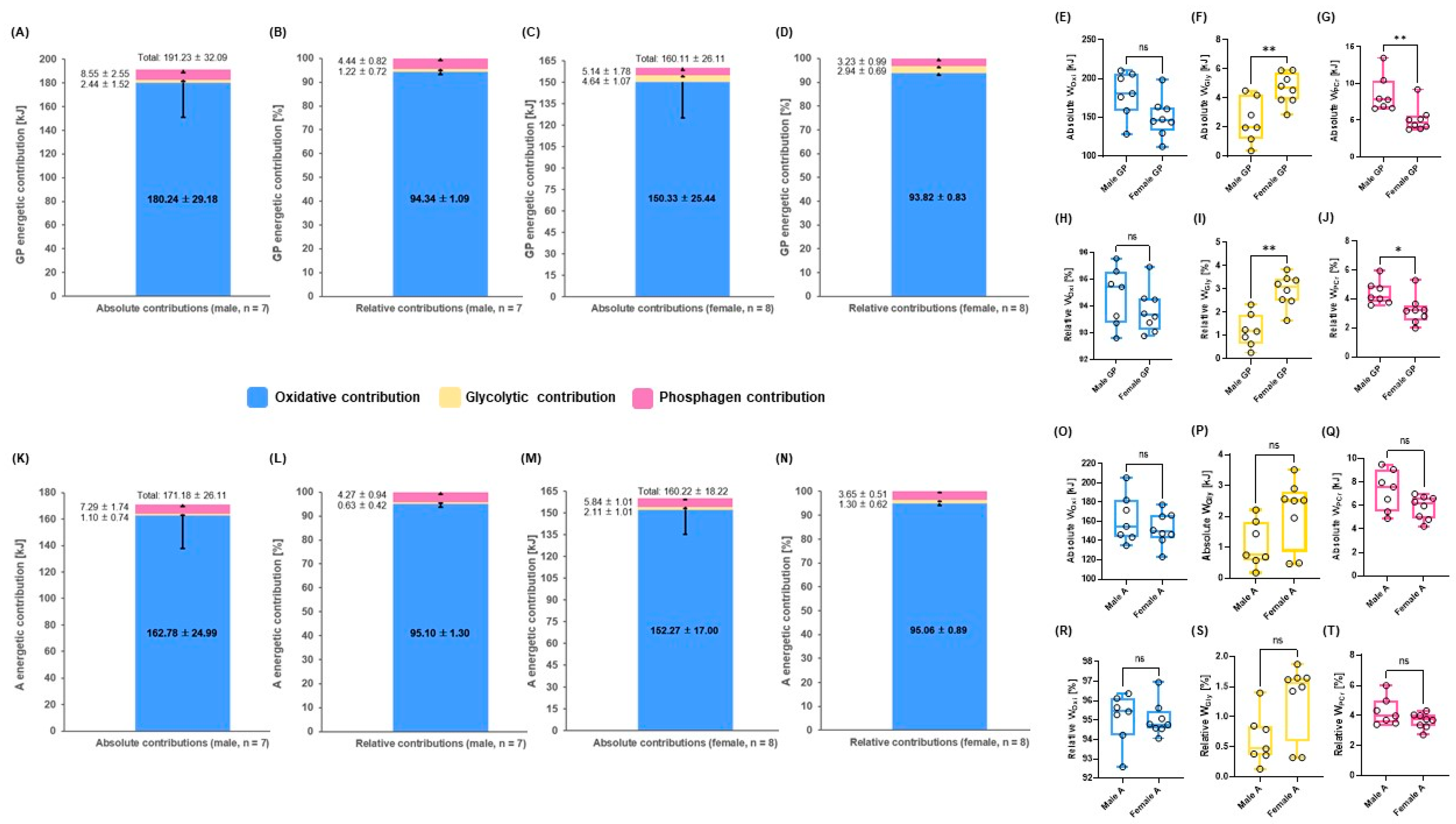
3.3. Mean energetic contributions until 3.5 m∙s−1 Steps during GIET between Males and Females in GP and A
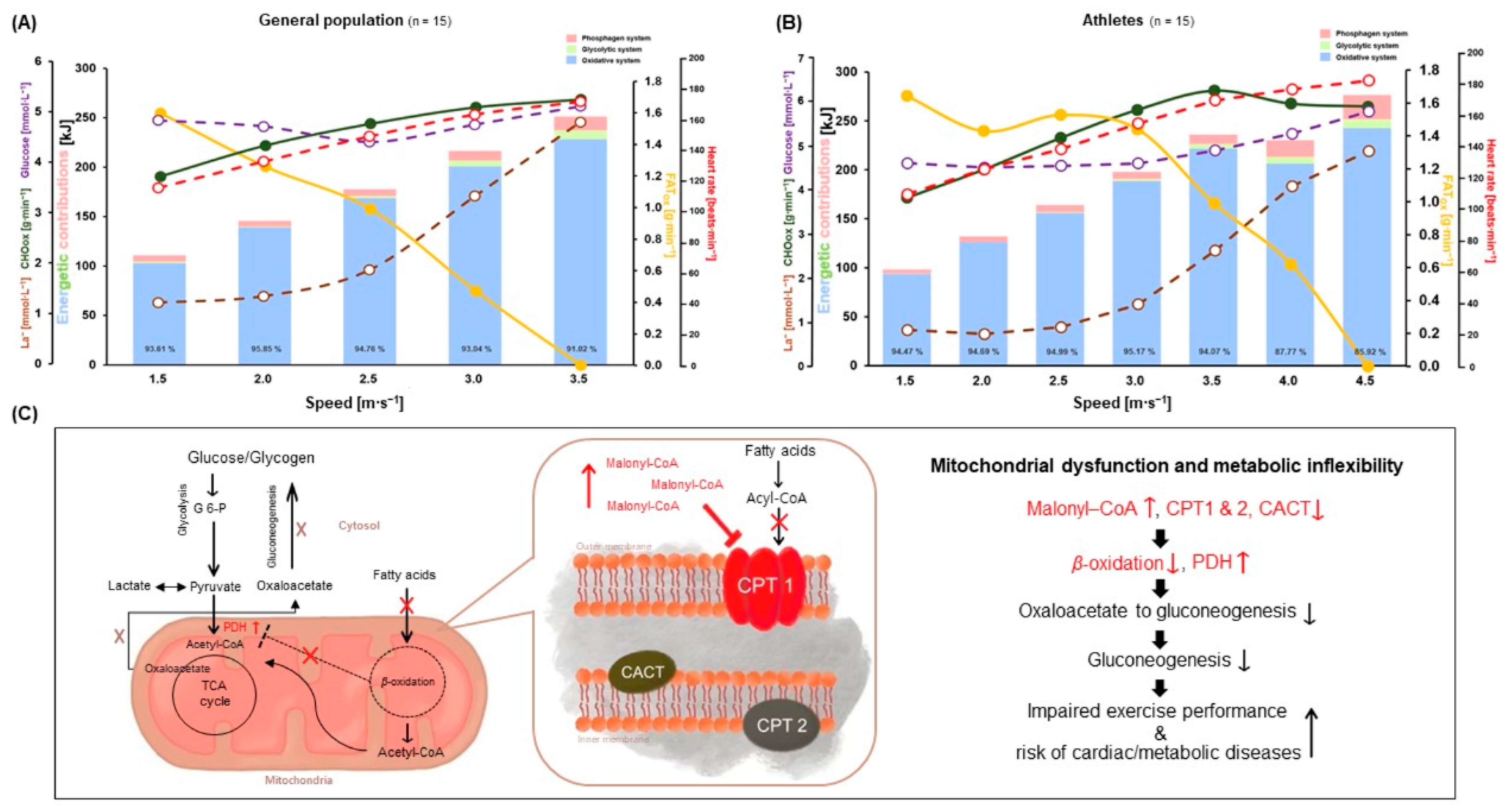
3.4. Energetic Contributions between GP and A during GIET
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Booth, F.W.; Gordon, S.E.; Carlson, C.J.; Hamilton, M.T. Waging war on modern chronic diseases: Primary prevention through exercise biology. J. Appl. Physiol. 2000, 88, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ju, H.M.; Yang, W.H. Metabolic Energy Contributions during High-Intensity Hatha Yoga and Physiological Comparisons between Active and Passive (Savasana) Recovery. Front. Physiol. 2021, 12, 743859. [Google Scholar] [CrossRef] [PubMed]
- Trost, S.G.; Blair, S.N.; Khan, K.M. Physical inactivity remains the greatest public health problem of the 21st century: Evidence, improved methods and solutions using the ‘7 investments that work’ as a framework. Br. J. Sports Med. 2014, 48, 169–170. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Storlien, L.; Oakes, N.D.; Kelley, D.E. Metabolic flexibility. Proc. Nutr. Soc. 2004, 63, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E. Skeletal muscle fat oxidation: Timing and flexibility are everything. J. Clin. Investig. 2005, 115, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Ritov, V.B.; Menshikova, E.V.; He, J.; Ferrell, R.E.; Goodpaster, B.H.; Kelley, D.E. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 2005, 54, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Mercier, J. Balance of carbohydrate and lipid utilization during exercise: The “crossover” concept. J. Appl. Physiol. 1994, 76, 2253–2261. [Google Scholar] [CrossRef]
- Yang, W.-H.; Park, H.; Grau, M.; Heine, O. Decreased blood glucose and lactate: Is a useful indicator of recovery ability in athletes? Int. J. Environ. Res. Public Health 2020, 17, 5470. [Google Scholar] [CrossRef] [PubMed]
- Emhoff, C.A.; Messonnier, L.A.; Horning, M.A.; Fattor, J.A.; Carlson, T.J.; Brooks, G.A. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J. Appl. Physiol. 2013, 114, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Wolfel, E.E.; Butterfield, G.E.; Lopaschuk, G.D.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. 1999, 87, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Role of the Heart in Lactate Shuttling. Front. Nutr. 2021, 8, 663560. [Google Scholar] [CrossRef] [PubMed]
- Rasica, L.; Inglis, E.C.; Iannetta, D.; Soares, R.N.; Murias, J.M. Fitness Level- and Sex-Related Differences in Macrovascular and Microvascular Responses during Reactive Hyperemia. Med. Sci. Sports Exerc. 2022, 54, 497–506. [Google Scholar] [CrossRef]
- Glenn, T.C.; Martin, N.A.; Horning, M.A.; McArthur, D.L.; Hovda, D.A.; Vespa, P.; Brooks, G.A. Lactate: Brain fuel in human traumatic brain injury: A comparison with normal healthy control subjects. J. Neurotrauma 2015, 32, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Son, J.Y.; Ju, H.M.; Won, J.H.; Park, S.B.; Yang, W.H. Effects of Individualized Low-Intensity Exercise and Its Duration on Recovery Ability in Adults. Healthcare 2021, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Moon, N.R.; Heine, O.; Yang, W.H. The ability of energy recovery in professional soccer players is increased by individualized low-intensity exercise. PLoS ONE 2022, 17, e0270484. [Google Scholar] [CrossRef]
- Beneke, R.; Beyer, T.; Jachner, C.; Erasmus, J.; Hütler, M. Energetics of karate kumite. Eur. J. Appl. Physiol. 2004, 92, 518–523. [Google Scholar] [CrossRef]
- di Prampero, P.E.; Ferretti, G. The energetics of anaerobic muscle metabolism: A reappraisal of older and recent concepts. Respir. Physiol. 1999, 118, 103–115. [Google Scholar] [CrossRef]
- Yang, W.-H.; Heine, O.; Grau, M. Rapid weight reduction does not impair athletic performance of Taekwondo athletes–A pilot study. PLoS ONE 2018, 13, e0196568. [Google Scholar] [CrossRef]
- Yang, W.H.; Park, J.H.; Shin, Y.C.; Kim, J. Physiological Profiling and Energy System Contributions during Simulated Epée Matches in Elite Fencers. Int. J. Sports Physiol. Perform. 2022, 17, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Julio, U.F.; Panissa, V.L.G.; Esteves, J.V.; Cury, R.L.; Agostinho, M.F.; Franchini, E. Energy-System Contributions to Simulated Judo Matches. Int. J. Sports Physiol. Perform. 2017, 12, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Gastin, P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26 (Suppl. 1), S28–S37. [Google Scholar] [CrossRef]
- Mader, A. Zur beurteilung der sportartspezifischen ausdauerleistungsfahigkeit im labor. Sportarzt Sport. 1976, 27, 80–88. [Google Scholar]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Quittmann, O.J.; Abel, T.; Zeller, S.; Foitschik, T.; Strüder, H.K. Lactate kinetics in handcycling under various exercise modalities and their relationship to performance measures in able-bodied participants. Eur. J. Appl. Physiol. 2018, 118, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Sharma, S. The athlete’s heart. Br. J. Hosp. Med. 2011, 72, 275–281. [Google Scholar] [CrossRef]
- Aubert, A.E.; Seps, B.; Beckers, F. Heart rate variability in athletes. Sports Med. 2003, 33, 889–919. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R.H. Impact of different sports and training on cardiac structure and function. Cardiol. Clin. 1992, 10, 241–256. [Google Scholar] [CrossRef]
- Sharma, S.; Maron, B.J.; Whyte, G.; Firoozi, S.; Elliott, P.M.; McKenna, W.J. Physiologic limits of left ventricular hypertrophy in elite junior athletes: Relevance to differential diagnosis of athlete’s heart and hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 40, 1431–1436. [Google Scholar] [CrossRef]
- Scharhag, J.; Schneider, G.; Urhausen, A.; Rochette, V.; Kramann, B.; Kindermann, W. Athlete’s heart: Right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J. Am. Coll. Cardiol. 2002, 40, 1856–1863. [Google Scholar] [CrossRef]
- Maron, B.J. Structural features of the athlete heart as defined by echocardiography. J. Am. Coll. Cardiol. 1986, 7, 190–203. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Witters, L.A.; Itoi, T.; Barr, R.; Barr, A. Acetyl-CoA carboxylase involvement in the rapid maturation of fatty acid oxidation in the newborn rabbit heart. J. Biol. Chem. 1994, 269, 25871–25878. [Google Scholar] [CrossRef]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed]
- Shrago, E.; Lardy, H.A. Paths of carbon in gluconeogenesis and lipogenesis II. Conversion of precursors to phosphoenolpyruvate in liver cytosol. J. Biol. Chem. 1966, 241, 663–668. [Google Scholar] [CrossRef]
- Bertuzzi, R.; Nascimento, E.M.; Urso, R.P.; Damasceno, M.; Lima-Silva, A.E. Energy system contributions during incremental exercise test. J. Sports Sci. Med. 2013, 12, 454–460. [Google Scholar] [PubMed]
- Simoneau, J.A.; Bouchard, C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am. J. Physiol. 1989, 257, E567–E572. [Google Scholar] [CrossRef] [PubMed]
- Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Murray, T.F.; Hostler, D.P.; Crill, M.T.; Ragg, K.E.; Toma, K. Fiber type composition of the vastus lateralis muscle of young men and women. J. Histochem. Cytochem. 2000, 48, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Bamman, M.M.; Hill, V.J.; Adams, G.R.; Haddad, F.; Wetzstein, C.J.; Gower, B.A.; Ahmed, A.; Hunter, G.R. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 108–116. [Google Scholar] [CrossRef]
- Welle, S.; Tawil, R.; Thornton, C.A. Sex-related differences in gene expression in human skeletal muscle. PLoS ONE 2008, 3, e1385. [Google Scholar] [CrossRef] [PubMed]
- McLay, K.M.; Gilbertson, J.E.; Pogliaghi, S.; Paterson, D.H.; Murias, J.M. Vascular responsiveness measured by tissue oxygen saturation reperfusion slope is sensitive to different occlusion durations and training status. Exp. Physiol. 2016, 101, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Fellahi, J.L.; Butin, G.; Zamparini, G.; Fischer, M.O.; Gérard, J.L.; Hanouz, J.L. Lower limb peripheral NIRS parameters during a vascular occlusion test: An experimental study in healthy volunteers. Ann. Fr. Anesth. Reanim. 2014, 33, e9–e14. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.; Nesterov, S.V.; Kemppainen, J.; Nuutila, P.; Knuuti, J.; Laitio, R.; Kjaer, M.; Boushel, R.; Kalliokoski, K.K. Role of adenosine in regulating the heterogeneity of skeletal muscle blood flow during exercise in humans. J. Appl. Physiol. 2007, 103, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Casado, A.; González-Mohíno, F.; González-Ravé, J.M.; Foster, C. Training Periodization, Methods, Intensity Distribution, and Volume in Highly Trained and Elite Distance Runners: A Systematic Review. Int. J. Sports Physiol. Perform. 2022, 17, 820–833. [Google Scholar] [CrossRef]
- Kenneally, M.; Casado, A.; Gomez-Ezeiza, J.; Santos-Concejero, J. Training intensity distribution analysis by race pace vs. physiological approach in world-class middle- and long-distance runners. Eur. J. Sport Sci. 2021, 21, 819–826. [Google Scholar] [CrossRef]
- Billat, L.V. Interval training for performance: A scientific and empirical practice. Special recommendations for middle- and long-distance running. Part I: Aerobic interval training. Sports Med. 2001, 31, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S. What is best practice for training intensity and duration distribution in endurance athletes? Int. J. Sports Physiol. Perform. 2010, 5, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]


| Parameters | GP | A | GP (Male) | GP (Female) | A (Male) | A (Female) |
|---|---|---|---|---|---|---|
| (n = 15) | (n = 15) | (n = 7) | (n = 8) | (n = 7) | (n = 8) | |
| Age [years] | 33.13 ± 8.99 | 29.47 ± 7.22 | 36.28 ± 10.04 | 30.37 ± 7.52 | 31.85 ± 4.63 | 27.37 ± 8.68 |
| Height [cm] | 171.27 ± 8.50 | 171.67 ± 5.71 | 175.71 ± 7.25 | 167.37 ± 7.90 | 174.42 ± 5.02 | 169.25 ± 5.41 |
| Body mass [kg] | 65.49 ± 10.48 | 65.26 ± 6.73 | 71.01 ± 11.05 | 60.65 ± 7.61 | 69.64 ± 5.64 | 61.42 ± 5.24 |
| Body fat [%] | 16.71 ± 4.69 | 15.07 ± 2.32 | 13.84 ± 4.91 | 18.62 ± 3.76 | 14.88 ± 0.66 | 15.15 ± 2.90 |
| BMI [kg∙m−2] | 22.21 ± 2.11 | 22.10 ± 1.41 | 22.89 ± 2.41 | 21.60 ± 1.74 | 22.85 ± 0.94 | 21.43 ± 1.47 |
| GIET | 1.5 m∙s−1 | 2.0 m∙s−1 | 2.5 m∙s−1 | 3.0 m∙s−1 | 3.5 m∙s−1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GP | A | p (ES) | GP | A | p (ES) | GP | A | p (ES) | GP | A | p (ES) | GP | A | p (ES) | |
| HR [beats∙min−1] | 116 ± 13 | 110 ± 13 | ns | 133 ± 15 | 126 ± 15 | ns | 150 ± 18 | 139 ± 15 | ns | 164 ± 17 | 156 ± 16 | 0.0319 (r = −0.4) | 173 ± 18 | 170 ± 14 | ns |
| % of HRmax | 63 ± 7 | 59 ± 7 | ns | 72 ± 7 | 68 ± 8 | ns | 81 ± 9 | 74 ± 7 | 0.0299 (d = 0.9) | 89 ± 8 | 83 ± 7 | 0.0167 (r = −0.4) | 94 ± 8 | 91 ± 6 | ns |
| O2mean [L∙min−1] | 6.98 ± 1.18 | 6.39 ± 0.69 | ns | 8.70 ± 1.52 | 7.95 ± 0.87 | ns | 10.12 ± 1.80 | 9.38 ± 1.07 | ns | 11.65 ± 2.07 | 10.93 ± 1.31 | ns | 12.93 ± 2.09 | 12.51 ± 1.52 | ns |
| O2mean [mL∙kg−1∙min−1] | 10.68 ± 0.84 | 9.82 ± 0.81 | 0.0079 (d = 1.0) | 13.28 ± 0.90 | 12.20 ± 0.78 | 0.0007 (r = −0.6) | 15.43 ± 0.94 | 14.39 ± 0.87 | 0.0036 (d = 1.2) | 17.77± 0.96 | 16.75 ± 0.95 | 0.0068 (d = 1.1) | 19.91± 0.95 | 19.17 ± 1.14 | ns |
| METs (O2mean) | 3.05 ± 0.24 | 2.81 ± 0.23 | 0.0063 (r = −0.5) | 3.80± 0.26 | 3.49 ± 0.22 | 0.0009 (r = −0.6) | 4.41 ± 0.27 | 4.11 ± 0.25 | 0.0037 (d = 1.2) | 5.08± 0.28 | 4.78 ± 0.27 | 0.0068 (d = 1.1) | 5.69± 0.27 | 5.48 ± 0.33 | ns |
| CO2mean [L∙min−1] | 5.97 ± 1.19 | 5.35 ± 0.91 | ns | 8.02 ± 1.53 | 7.11 ± 1.05 | ns | 9.55 ± 1.82 | 8.46 ± 1.14 | ns | 11.43 ± 2.21 | 10.07 ± 1.39 | ns | 13.09 ± 2.70 | 11.93 ± 1.70 | ns |
| CO2mean [mL∙kg−1∙min−1] | 9.12 ± 1.18 | 8.22 ± 1.47 | 0.0367 (r = −0.4) | 12.23 ± 1.12 | 10.92 ± 1.56 | 0.0016 (r = −0.6) | 14.54 ± 1.11 | 12.99 ± 1.55 | 0.0008 (r = −0.6) | 17.41 ± 1.49 | 15.45 ± 1.67 | 0.0020 (d = 1.2) | 20.07 ± 1.94 | 18.29 ± 2.12 | 0.0378 (d = 0.9) |
| Glucose [mmol∙L−1] | 4.83 ± 0.40 | 4.57 ± 0.31 | ns | 4.71 ± 0.40 | 4.47 ± 0.26 | ns | 4.40 ± 0.86 | 4.51 ± 0.28 | ns | 5.11 ± 0.63 | 4.56 ± 0.33 | ns | 5.16 ± 0.24 | 4.86 ± 0.63 | ns |
| La− [mmol∙L−1] | 1.21 ± 0.31 | 0.81 ± 0.30 | 0.0016 (r = −0.6) | 1.34 ± 0.41 | 0.73 ± 0.22 | <0.0001 (d = 1.8) | 1.86 ± 0.76 | 0.88 ± 0.28 | <0.0001 (d = 1.7) | 3.33 ± 1.82 | 1.39 ± 0.58 | <0.0001 (r = −0.7) | 4.80 ± 1.85 | 2.60 ± 1.40 | 0.0017 (d = 1.3) |
| FATox [g∙min−1] | 1.60 ± 0.86 | 1.64 ± 0.89 | ns | 1.26 ± 0.85 | 1.43 ± 0.94 | ns | 0.99 ± 0.92 | 1.53 ± 0.98 | ns | 0.47 ± 1.27 | 1.44 ± 1.19 | 0.0141 (r = −0.6) | −0.05 ± 1.33 | 0.99 ± 1.53 | 0.0159 (r = −0.6) |
| CHOox [g∙min−1] | 4.73 ± 2.43 | 3.79 ± 2.58 | ns | 8.57 ± 2.93 | 6.81 ± 2.84 | ns | 10.96 ± 3.33 | 8.38 ± 2.83 | 0.0304 (d = 0.8) | 14.61 ± 4.69 | 10.74 ± 3.43 | 0.0155 (d = 0.9) | 18.85 ± 6.28 | 14.10 ± 4.44 | 0.0237 (d = 0.9) |
| WPCr [kJ] | 6.37 ± 2.90 | 4.17 ± 1.53 | 0.0186 (r = −0.1) | 5.94 ± 2.44 | 5.95 ± 2.06 | ns | 6.64 ± 4.15 | 7.44 ± 3.20 | ns | 9.67 ± 7.86 | 7.47 ± 2.24 | ns | 13.74 ± 11.66 | 9.43 ± 5.69 | ns |
| WGly [kJ] | 1.30 ± 0.97 | 0.37 ± 0.75 | 0.0002 (r = −0.6) | 0.67 ± 0.78 | 0.11 ± 0.21 | 0.0107 (d = 0.9) | 2.17 ± 1.67 | 0.73 ± 0.71 | 0.0046 (d = 1.1) | 5.94 ± 4.39 | 2.05 ± 1.30 | 0.0006 (r = −0.6) | 9.19 ± 4.96 | 4.94 ± 3.48 | 0.0165 (d = 0.9) |
| WOxi [kJ] | 103.06 ± 21.55 | 93.59 ± 16.21 | ns | 139.06 ± 27.83 | 126.16 ± 18.39 | ns | 168.71 ± 32.66 | 156.11 ± 21.76 | ns | 200.74 ± 38.57 | 188.45 ± 25.53 | ns | 228.19 ± 39.33 | 221.57 ± 28.69 | ns |
| WTOTAL [kJ] | 109.91 ± 21.66 | 98.91 ± 15.89 | ns | 144.80 ± 27.10 | 133.23 ± 19.16 | ns | 177.73 ± 32.33 | 164.26 ± 22.35 | ns | 216.34 ± 44.83 | 197.97 ± 26.15 | ns | 251.13 ± 43.07 | 235.93 ± 32.82 | ns |
| WPCr [%] | 5.22 ± 2.75 | 5.18 ± 2.77 | ns | 3.66 ± 1.69 | 5.23 ± 1.80 | 0.0030 (r = −0.5) | 4.01 ± 2.86 | 4.58 ± 1.92 | ns | 4.30 ± 2.36 | 3.79 ± 1.09 | ns | 5.37 ± 4.22 | 3.88 ± 2.00 | ns |
| WGly [%] | 1.17 ± 0.78 | 0.35 ± 0.64 | 0.0003 (r = −0.6) | 0.49 ± 0.59 | 0.08 ± 0.16 | 0.0157 (r = −0.5) | 1.24 ± 0.98 | 0.43 ± 0.37 | 0.0068 (r = −0.5) | 2.76 ± 2.06 | 1.04 ± 0.70 | 0.0048 (r = −0.5) | 3.61 ± 1.96 | 2.05 ± 1.36 | 0.0229 (d = 0.9) |
| WOxi [%] | 93.61 ± 2.97 | 94.47 ± 2.66 | ns | 95.85 ± 1.60 | 94.69 ± 1.81 | 0.0270 (r = −0.4) | 94.76 ± 2.70 | 94.99 ± 2.10 | ns | 93.04 ± 2.45 | 95.17 ± 1.42 | 0.0069 (d = 1.0) | 91.02 ± 5.56 | 94.07 ± 2.82 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.-H.; Park, J.-H.; Park, S.-Y.; Park, Y. Energetic Contributions Including Gender Differences and Metabolic Flexibility in the General Population and Athletes. Metabolites 2022, 12, 965. https://doi.org/10.3390/metabo12100965
Yang W-H, Park J-H, Park S-Y, Park Y. Energetic Contributions Including Gender Differences and Metabolic Flexibility in the General Population and Athletes. Metabolites. 2022; 12(10):965. https://doi.org/10.3390/metabo12100965
Chicago/Turabian StyleYang, Woo-Hwi, Jeong-Hyun Park, So-Young Park, and Yongdoo Park. 2022. "Energetic Contributions Including Gender Differences and Metabolic Flexibility in the General Population and Athletes" Metabolites 12, no. 10: 965. https://doi.org/10.3390/metabo12100965
APA StyleYang, W.-H., Park, J.-H., Park, S.-Y., & Park, Y. (2022). Energetic Contributions Including Gender Differences and Metabolic Flexibility in the General Population and Athletes. Metabolites, 12(10), 965. https://doi.org/10.3390/metabo12100965










