Impact of Sustained Exogenous Irisin Myokine Administration on Muscle and Myocyte Integrity in Sprague Dawley Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Pump Implantation and Irisin Administration
2.3. Sampling
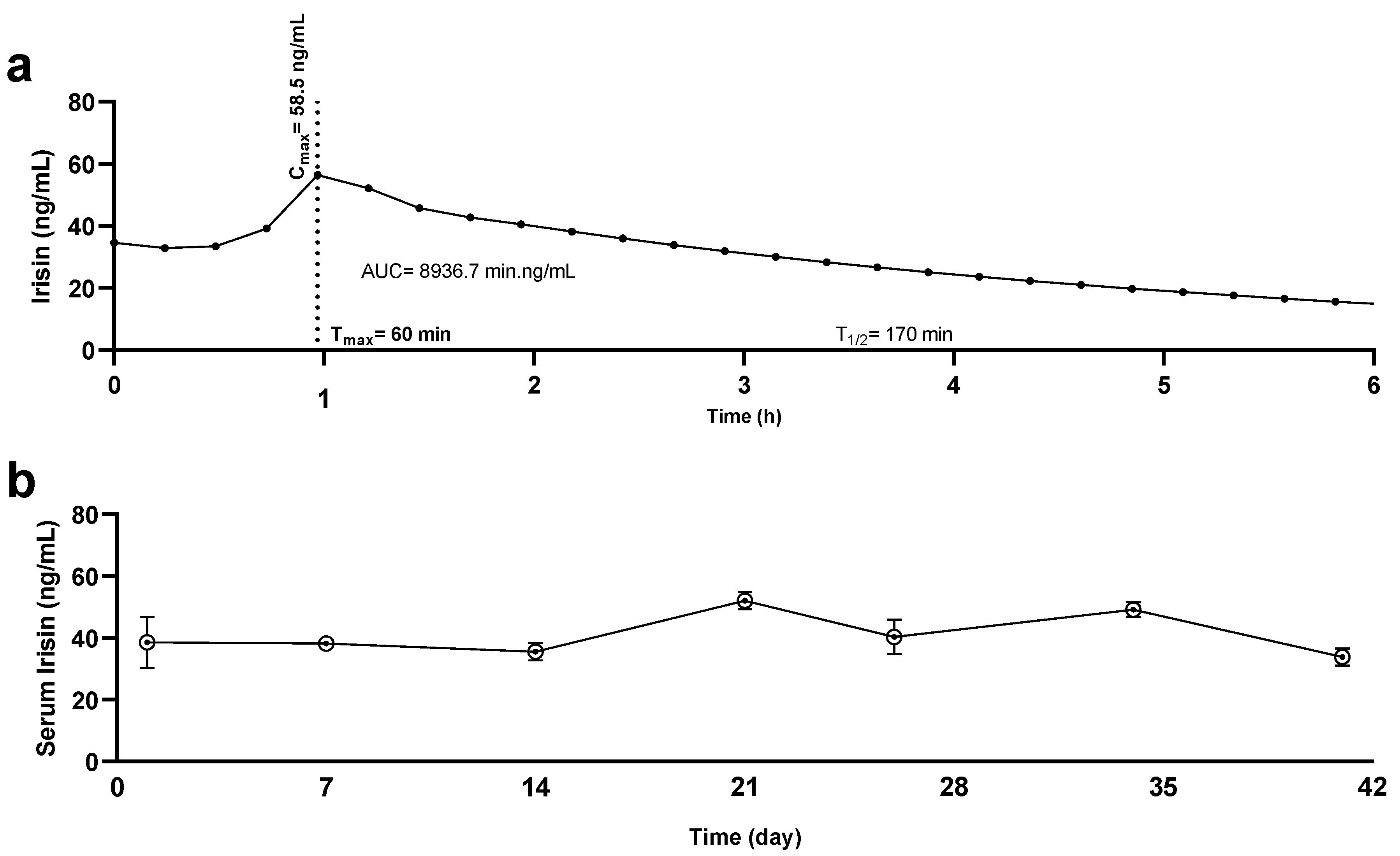
2.4. Irisin Quantification and Kinetics
2.5. Superposition Principle Simulation Modeling
2.6. Histopathology
2.7. RNA Isolation, cDNA Synthesis, and RTq-PCR
3. Results
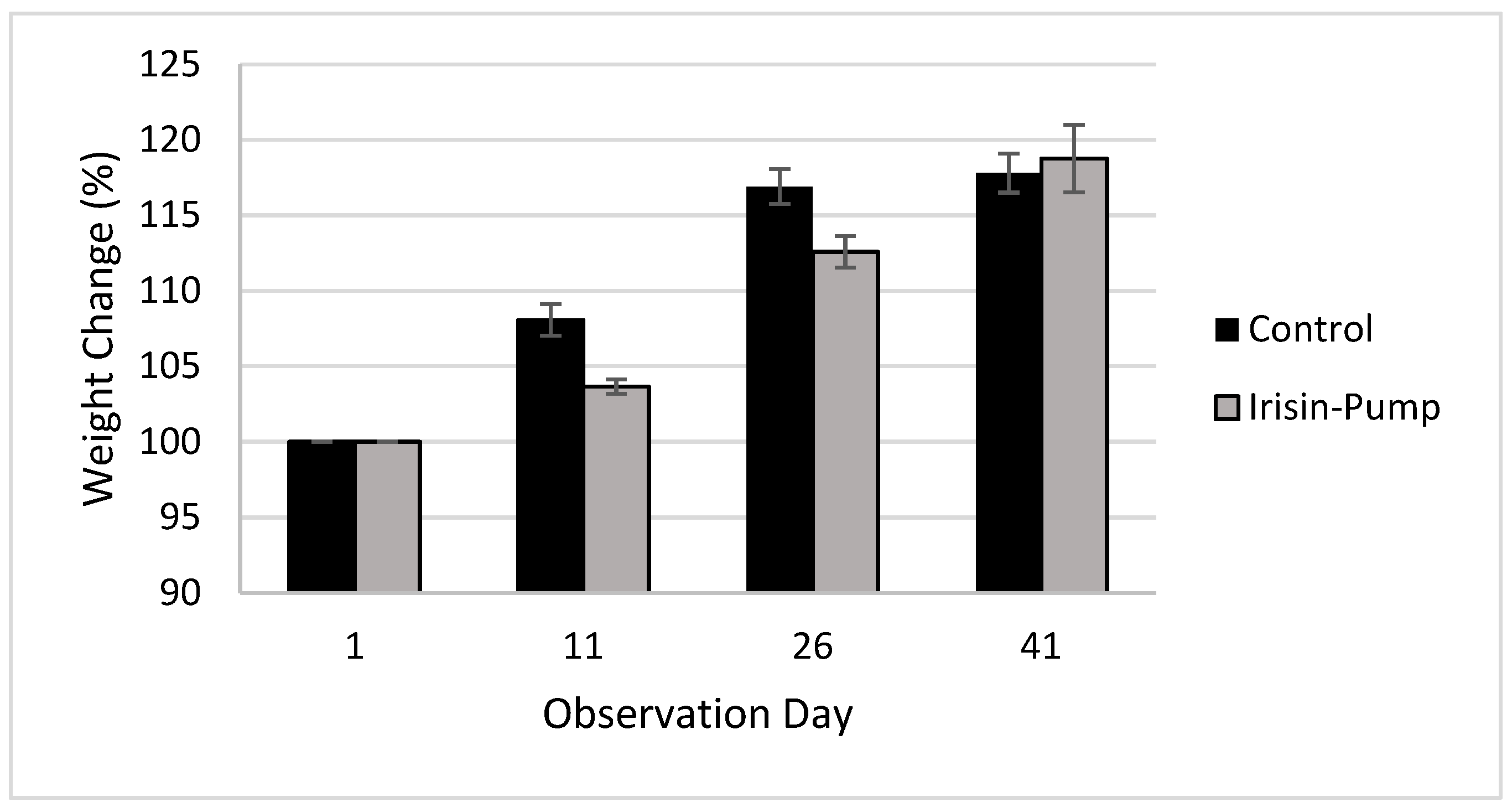
3.1. Body Weights
3.2. Clinical Biochemistry
3.3. Quantification of Serum Irisin
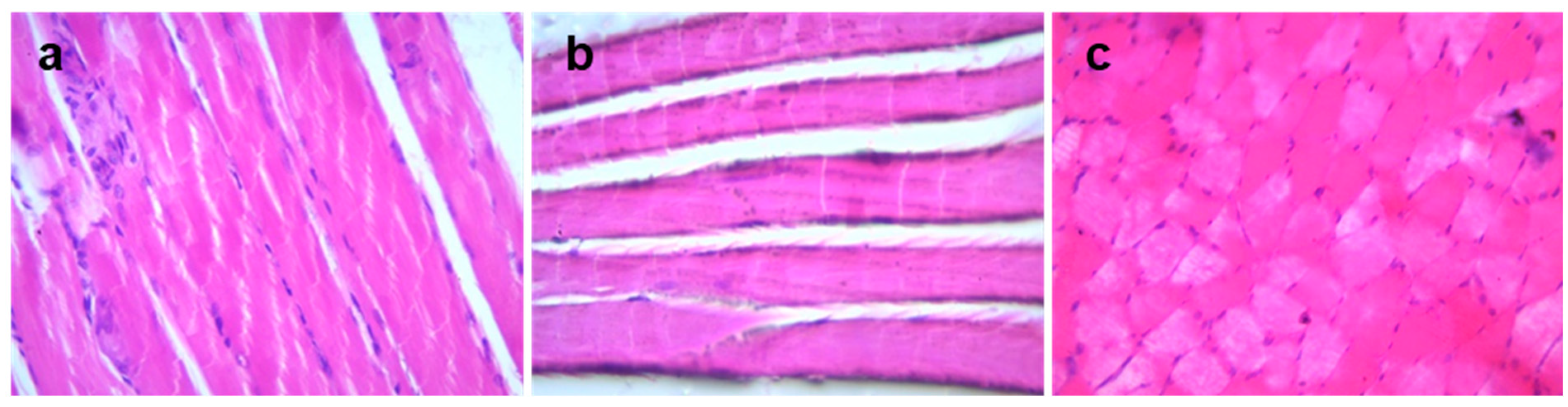
3.4. Histopathology
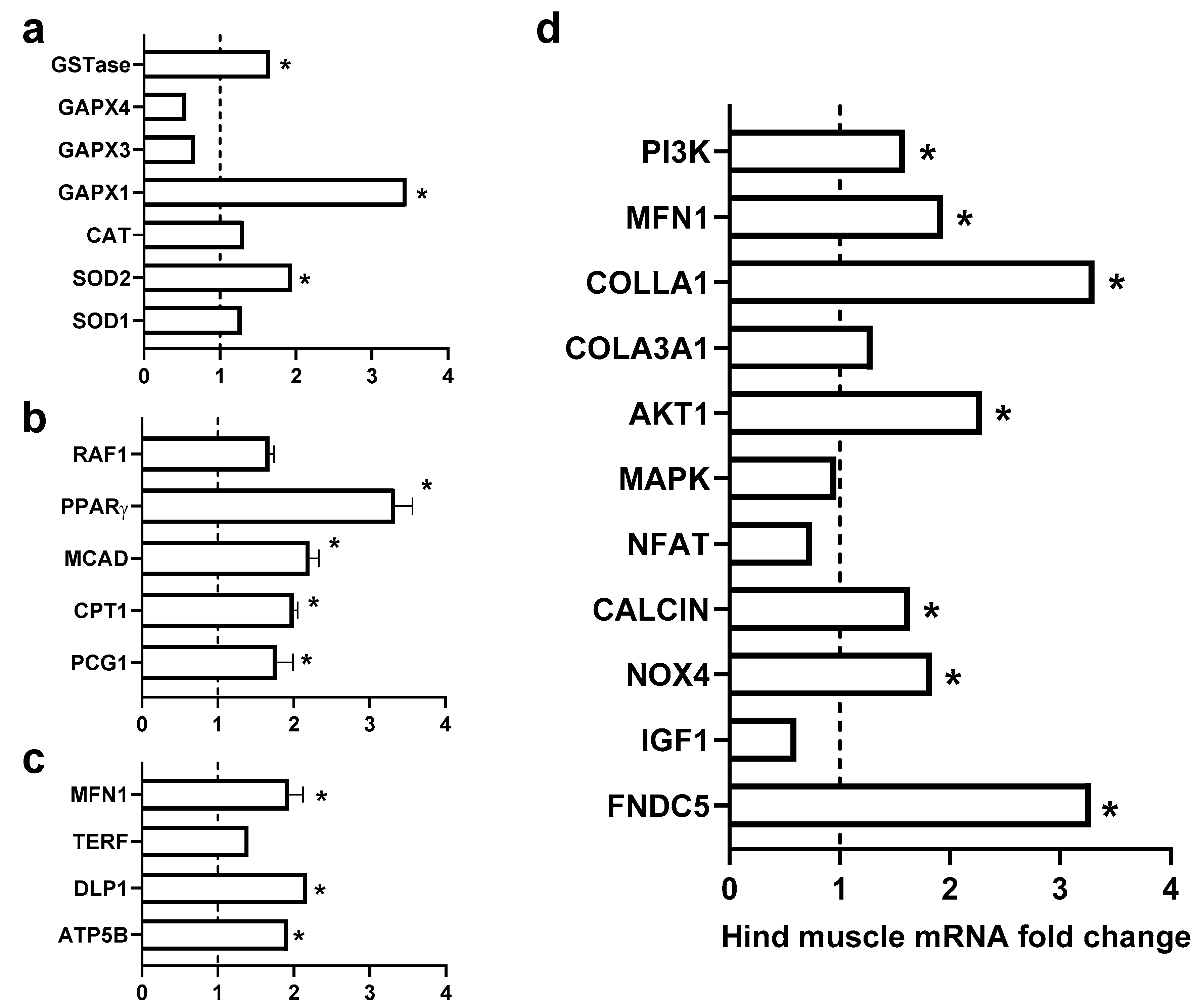
3.5. Gene Expression Levels
4. Discussion
4.1. Role of Exogenous Irisin in Oxidative Stress
4.2. Role of Exogenous Irisin in Fatty Acid β-Oxidation FAO
4.3. Role of Exogenous Irisin in Mitochondrial Fusion
4.4. Role of Exogenous Irisin in Muscle Mass and Aging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grygiel-Górniak, B.; Puszczewicz, M. A review on irisin, a new protagonist that mediates muscle-adipose-bone-neuron connectivity. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4687–4693. [Google Scholar]
- Alzoughool, F.; Al-Zghoul, M.B.; Al-Nassan, S.; Alanagreh, L.; Mufleh, D.; Atoum, M. The optimal therapeutic irisin dose intervention in animal model: A systematic review. Vet. World 2020, 13, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Liu, J.; Song, N.; Huang, Y.; Chen, Y. Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci. Rep. 2018, 8, 15247. [Google Scholar] [CrossRef] [PubMed]
- Uysal, N.; Yuksel, O.; Kizildag, S.; Yuce, Z.; Gümüş, H.; Karakilic, A.; Guvendi, G.; Koc, B.; Kandis, S.; Ates, M. Regular aerobic exercise correlates with reduced anxiety and incresed levels of irisin in brain and white adipose tissue. Neurosci. Lett. 2018, 676, 92–97. [Google Scholar] [CrossRef]
- Alzoughool, F.; Al-Zghoul, M.B.; Ghanim, B.Y.; Gollob, M.; Idkaidek, N.; Qinna, N.A. The Role of Interventional Irisin on Heart Molecular Physiology. Pharmaceuticals 2022, 15, 863. [Google Scholar] [CrossRef]
- Bashar, S.M.; El-Sherbeiny, S.M.S.; Boraie, M.Z. Correlation between the Blood Level of Irisin and the Severity of Acute Myocardial Infarction in Exercise-Trained Rats. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 59–71. [Google Scholar] [CrossRef]
- Berzosa, C.; Cebrián, I.; Fuentes-Broto, L.; Gómez-Trullén, E.; Piedrafita, E.; Martínez-Ballarín, E.; López-Pingarrón, L.; Reiter, R.J.; García, J.J. Acute exercise increases plasma total antioxidant status and antioxidant enzyme activities in untrained men. J. Biomed. Biotechnol. 2011, 2011, 540458. [Google Scholar] [CrossRef]
- Lambertucci, R.H.; Levada-Pires, A.C.; Rossoni, L.V.; Curi, R.; Pithon-Curi, T.C. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech. Ageing Dev. 2007, 128, 267–275. [Google Scholar] [CrossRef]
- Miyazaki, H.; Oh-Ishi, S.; Ookawara, T.; Kizaki, T.; Toshinai, K.; Ha, S.; Haga, S.; Ji, L.L.; Ohno, H. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur. J. Appl. Physiol. 2001, 84, 1–6. [Google Scholar] [CrossRef]
- Batirel, S.; Bozaykut, P.; Altundag, E.M.; Ozer, N.K.; Mantzoros, C.S. The Effect of Irisin on Antioxidant System in Liver. Free Radic. Biol. Med. 2014, 75, S16. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pocheć, E. The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/Ho-1/Hmgb1 Axis. Antioxidants 2021, 10, 88. [Google Scholar] [CrossRef]
- Ghanim, B.Y.; Qinna, N.A. Nrf2/ARE Axis Signalling in Hepatocyte Cellular Death. Mol. Biol. Rep. 2022, 49, 4039–4053. [Google Scholar] [CrossRef]
- Ji, L.L. Antioxidants and oxidative stress in exercise. Proc. Soc. Exp. Biol. Med. 1999, 222, 283–292. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhou, J.-C.; Wu, Y.-Y.; Ren, F.-Z.; Lei, X.G. Role of glutathione peroxidase 1 in glucose and lipid metabolism-related diseases. Free Radic. Biol. Med. 2018, 127, 108–115. [Google Scholar] [CrossRef]
- Udomsinprasert, R.; Pongjaroenkit, S.; Wongsantichon, J.; Oakley, A.J.; Prapanthadara, L.-A.; Wilce, M.C.J.; Ketterman, A.J. Identification, characterization and structure of a new Delta class glutathione transferase isoenzyme. Biochem. J. 2005, 388, 763–771. [Google Scholar] [CrossRef]
- Xirouchaki, C.E.; Jia, Y.; McGrath, M.J.; Greatorex, S.; Tran, M.; Merry, T.L.; Hong, D.; Eramo, M.J.; Broome, S.C.; Woodhead, J.S.T.; et al. Skeletal muscle NOX4 is required for adaptive responses that prevent insulin resistance. Sci. Adv. 2021, 7, eabl4988. [Google Scholar] [CrossRef]
- Olpin, S.E. Pathophysiology of fatty acid oxidation disorders and resultant phenotypic variability. J. Inherit. Metab. Dis. 2013, 36, 645–658. [Google Scholar] [CrossRef]
- Zheng, F.; Cai, Y. Concurrent exercise improves insulin resistance and nonalcoholic fatty liver disease by upregulating PPAR-γ and genes involved in the beta-oxidation of fatty acids in ApoE-KO mice fed a high-fat diet. Lipids Health Dis. 2019, 18, 6. [Google Scholar] [CrossRef]
- Chen, H.; Chan, D.C. Physiological functions of mitochondrial fusion. Ann. N. Y. Acad. Sci. 2010, 1201, 21–25. [Google Scholar] [CrossRef]
- Chen, H.; Chan, D.C. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum. Mol. Genet. 2009, 18, R169–R176. [Google Scholar] [CrossRef]
- Detmer, S.A.; Chan, D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007, 8, 870–879. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C.; Akimoto, T.; Blaauw, B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. J. Neuromuscul. Dis. 2021, 8, 169–183. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Gami, M.S.; Wolkow, C.A. Studies of Caenorhabditis elegans DAF-2/insulin signaling reveal targets for pharmacological manipulation of lifespan. Aging Cell 2006, 5, 31–37. [Google Scholar] [CrossRef]
| Clinical Biochemistry Parameters | Control | Irisin Pump |
|---|---|---|
| Myoglobin (nmol/L) | 110.4 ± 30.4 | 129.6 ± 41.8 |
| Creatine kinases (U/L) | 364 ± 25.2 | 256.8 ± 39.5 * |
| Troponin-T (ng/mL) | 1655.5 ± 568.3 | 1713.5 ± 482.6 |
| Lactate dehydrogenase (LDH) (U/L) | 1335.4 ± 223.3 | 808.2 ± 156.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzoughool, F.; Al-Zghoul, M.B.; Ghanim, B.Y.; Atoum, M.; Aljawarneh, Y.; Idkaidek, N.; Qinna, N.A. Impact of Sustained Exogenous Irisin Myokine Administration on Muscle and Myocyte Integrity in Sprague Dawley Rats. Metabolites 2022, 12, 939. https://doi.org/10.3390/metabo12100939
Alzoughool F, Al-Zghoul MB, Ghanim BY, Atoum M, Aljawarneh Y, Idkaidek N, Qinna NA. Impact of Sustained Exogenous Irisin Myokine Administration on Muscle and Myocyte Integrity in Sprague Dawley Rats. Metabolites. 2022; 12(10):939. https://doi.org/10.3390/metabo12100939
Chicago/Turabian StyleAlzoughool, Foad, Mohammad Borhan Al-Zghoul, Bayan Y. Ghanim, Manar Atoum, Yousef Aljawarneh, Nasir Idkaidek, and Nidal A. Qinna. 2022. "Impact of Sustained Exogenous Irisin Myokine Administration on Muscle and Myocyte Integrity in Sprague Dawley Rats" Metabolites 12, no. 10: 939. https://doi.org/10.3390/metabo12100939
APA StyleAlzoughool, F., Al-Zghoul, M. B., Ghanim, B. Y., Atoum, M., Aljawarneh, Y., Idkaidek, N., & Qinna, N. A. (2022). Impact of Sustained Exogenous Irisin Myokine Administration on Muscle and Myocyte Integrity in Sprague Dawley Rats. Metabolites, 12(10), 939. https://doi.org/10.3390/metabo12100939







