The Role of Amino Acids in Tuberculosis Infection: A Literature Review
Abstract
1. Introduction
2. The Importance of Amino Acids in Tuberculosis Infection
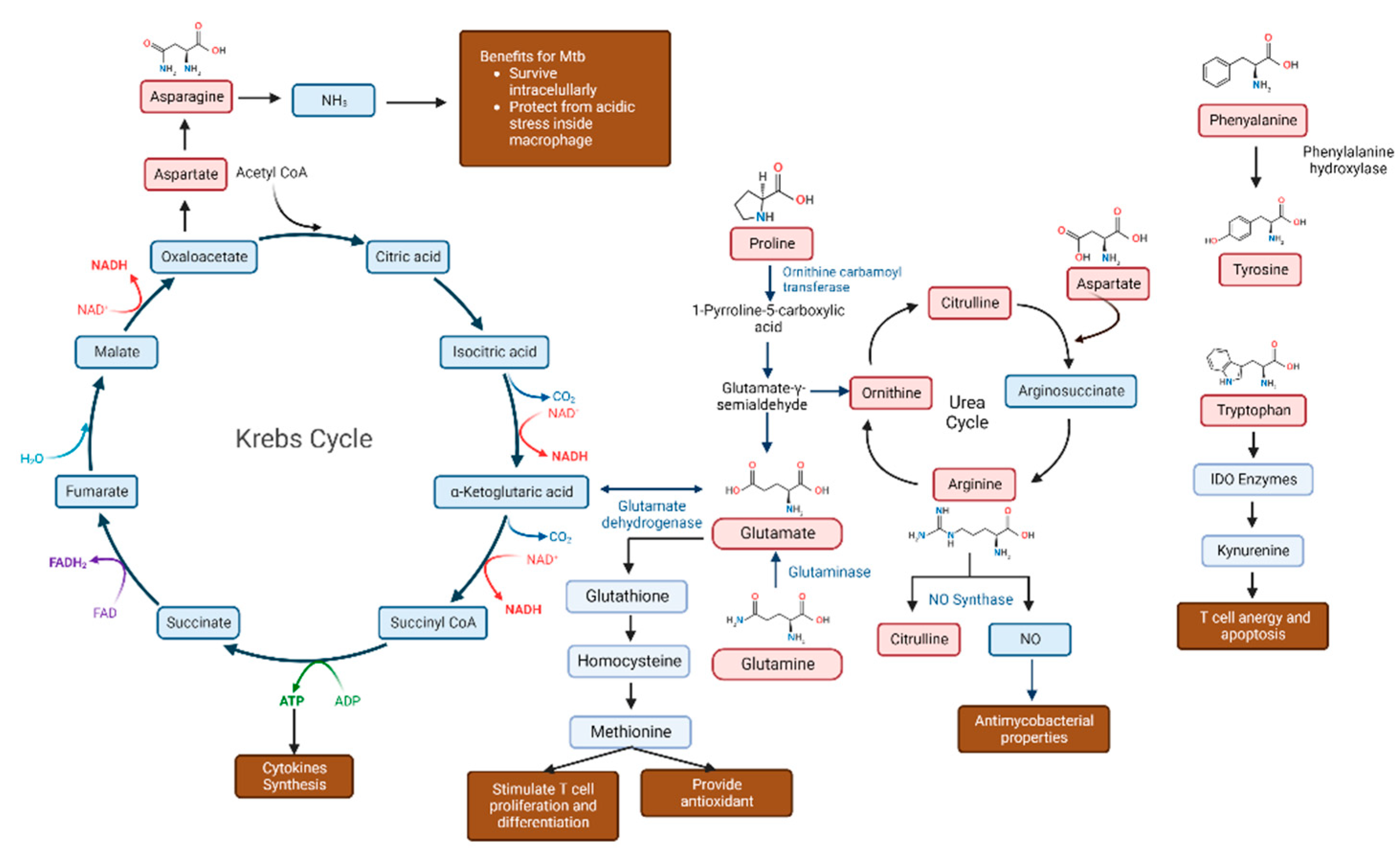
2.1. Amino Acids Are Advantageous for Mycobacterium Tuberculosis
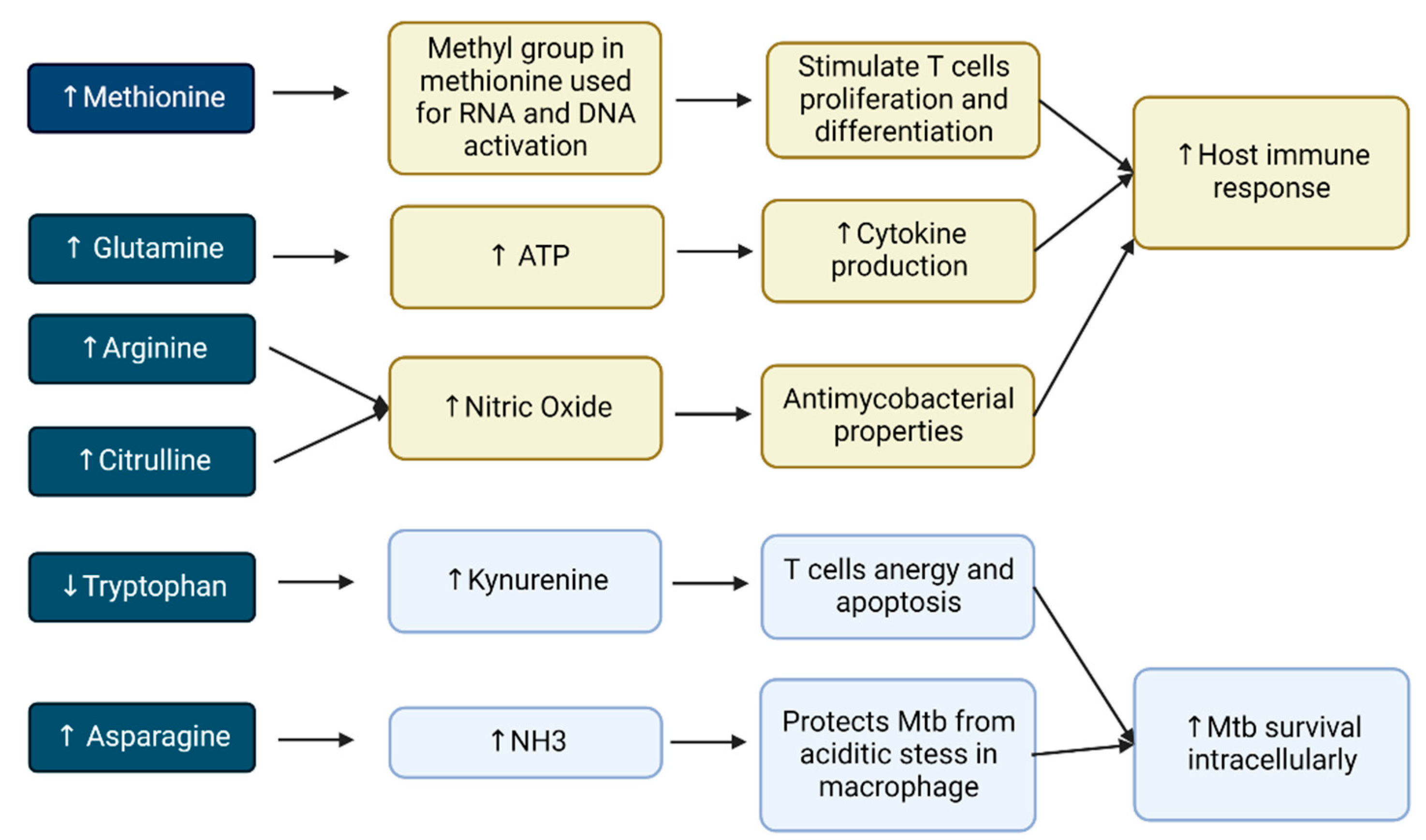
2.2. Amino Acids Are Beneficial to the Host Defense
2.3. Numerous Amino Acids Are Involved in Tuberculosis Infection
2.4. Diverse Roles of Amino Acids in Tuberculosis Infection
2.4.1. Tryptophan
2.4.2. Glutamine
2.4.3. Asparagine
2.4.4. Arginine
2.4.5. Phenylalanine and Tyrosine
2.4.6. Citrulline
2.4.7. Methionine
2.5. Amino-Acid Profile in Multidrug-Resistant TB
2.6. Targeting Amino Acids for Tuberculosis Drug Development
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report. World Health Organization. Available online: https://www.who.int/publications/digital/global-tuberculosis-report-2021 (accessed on 23 July 2022).
- Collins, J.M.; Siddiqa, A.; Jones, D.P.; Liu, K.; Kempker, R.R.; Nizam, A.; Shah, N.S.; Ismail, N.; Ouma, S.G.; Tukvadze, N.; et al. Tryptophan catabolism reflects disease activity in human tuberculosis. JCI Insight 2020, 5, e137131. [Google Scholar] [CrossRef] [PubMed]
- Koeken, V.A.C.M.; Lachmandas, E.; Riza, A.; Matzaraki, V.; Li, Y.; Kumar, V.; Oosting, M.; Joosten, L.A.B.; Netea, M.G.; Van Crevel, R. Role of Glutamine Metabolism in Host Defense against Mycobacterium tuberculosis Infection. J. Infect. Dis. 2019, 219, 1662–1670. [Google Scholar] [CrossRef]
- Kurpad, A.V. The requirements of protein & amino acid during acute & chronic infections. Indian J. Med. Res. 2006, 124, 129–148. [Google Scholar]
- Weiner, J.; Parida, S.K.; Maertzdorf, J.; Black, G.F.; Repsilber, D.; Telaar, A.; Mohney, R.P.; Arndt-Sullivan, C.; Ganoza, C.; Faé, K.C.; et al. Biomarkers of Inflammation, Immunosuppression and Stress Are Revealed by Metabolomic Profiling of Tuberculosis Patients. PLoS ONE 2012, 7, e40221. [Google Scholar] [CrossRef]
- Yani, D.I.; Islam, H.S.; Sari, C.W.M. Diet in The Intensive Phase of Pulmonary Tuberculosis Patients. J. Nurs. Care 2018, 1, 119–128. [Google Scholar] [CrossRef]
- Reid, M.; Forrester, T.; Badaloo, A.; Heird, W.C.; Jahoor, F. Supplementation with aromatic amino acids improves leucine kinetics but not aromatic amino acid kinetics in infants with infection, severe malnutrition, and edema. J. Nutr. 2004, 134, 3004–3010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gould, R.L.; Pazdro, R. Impact of supplementary amino acids, micronutrients, and overall diet on glutathione homeostasis. Nutrients 2019, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Ralph, A.P.; Waramori, G.; Pontororing, G.J.; Kenangalem, E.; Wiguna, A.; Tjitra, E.; Sandjaja; Lolong, D.B.; Yeo, T.W.; Chatfield, M.; et al. L-arginine and Vitamin D Adjunctive Therapies in Pulmonary Tuberculosis: A Randomised, Double-Blind, Placebo-Controlled Trial. PLoS ONE 2013, 8, e70032. [Google Scholar] [CrossRef]
- Ren, Z.; Zhao, F.; Chen, H.; Hu, D.; Yu, W.; Xu, X.; Lin, D.; Luo, F.; Fan, Y.; Wang, H.; et al. Nutritional intakes and associated factors among tuberculosis patients: A cross-sectional study in China. BMC Infect. Dis. 2019, 19, 907. [Google Scholar] [CrossRef]
- Weiner, J.; Maertzdorf, J.; Sutherland, J.S.; Duffy, F.J.; Thompson, E.; Suliman, S.; McEwen, G.; Thiel, B.; Parida, S.K.; Zyla, J.; et al. Metabolite changes in blood predict the onset of tuberculosis. Nat. Commun. 2018, 9, 5208. [Google Scholar] [CrossRef]
- Qualls, J.E.; Murray, P.J. Immunometabolism within the tuberculosis granuloma: Amino acids, hypoxia, and cellular respiration. Semin. Immunopathol. 2016, 38, 139–152. [Google Scholar] [CrossRef]
- Yelamanchi, S.D.; Surolia, A. Targeting amino acid metabolism of Mycobacterium tuberculosis for developing inhibitors to curtail its survival. IUBMB Life 2021, 73, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Gouzy, A.; Poquet, Y.; Neyrolles, O. Nitrogen metabolism in Mycobacterium tuberculosis physiology and virulence. Nat. Rev. Microbiol. 2014, 12, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Yang, J.Y.; Jeon, B.Y.; Yoon, Y.J.; Cho, S.N.; Kang, Y.H.; Ryu, D.H.; Hwang, G.-S. 1H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. J. Proteome Res. 2011, 10, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Borah, K.; Beyß, M.; Theorell, A.; Wu, H.; Basu, P.; Mendum, T.A.; Nöh, K.; Beste, D.J.; McFadden, J. Intracellular Mycobacterium tuberculosis Exploits Multiple Host Nitrogen Sources during Growth in Human Macrophages. Cell Rep. 2019, 29, 3580–3591.e4. [Google Scholar] [CrossRef]
- Albors-Vaquer, A.; Rizvi, A.; Matzapetakis, M.; Lamosa, P.; Coelho, A.V.; Patel, A.B.; Mande, S.C.; Gaddam, S.; Pineda-Lucena, A.; Banerjee, S.; et al. Active and prospective latent tuberculosis are associated with different metabolomic profiles: Clinical potential for the identification of rapid and non-invasive biomarkers. Emerg. Microbes Infect. 2020, 9, 1131–1139. [Google Scholar] [CrossRef]
- Cho, Y.; Park, Y.; Sim, B.; Kim, J.; Lee, H.; Cho, S.-N.; Kang, Y.A.; Lee, S.-G. Identification of serum biomarkers for active pulmonary tuberculosis using a targeted metabolomics approach. Sci. Rep. 2020, 10, 3825. [Google Scholar] [CrossRef]
- Suzuki, Y.; Suda, T.; Asada, K.; Miwa, S.; Suzuki, M.; Fujie, M.; Furuhashi, K.; Nakamura, Y.; Inui, N.; Shirai, T.; et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin. Vaccine Immunol. 2012, 19, 436–442. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, W.S.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Ren, W.; Chen, S.; Yin, J.; Duan, J.; Li, T.; Liu, G.; Feng, Z.; Tan, B.; Yin, Y.; Wu, G. Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J. Nutr. 2014, 144, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Duan, J.; Yin, J.; Liu, G.; Cao, Z.; Xiong, X.; Chen, S.; Li, T.; Yin, Y.; Hou, Y.; et al. Dietary l-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids 2014, 46, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yin, J.; Wu, M.; Liu, G.; Yang, G.; Xion, Y.; Su, D.; Wu, L.; Li, T.; Chen, S.; et al. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS ONE 2014, 9, e88335. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiao, H.; Liu, G.; Chen, S.; Tan, B.; Ren, W.; Bazer, F.W.; Wu, G.; Yin, Y. Glutamine promotes intestinal SIgA secretion through intestinal microbiota and IL-13. Mol. Nutr. Food Res. 2016, 60, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Liu, G.; Chen, S.; Yin, J.; Wang, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Li, T.; et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017, 62, e12394. [Google Scholar] [CrossRef]
- Ren, W.; Liu, G.; Yin, J.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. Amino-acid transporters in T-cell activation and differentiation. Cell Death Dis. 2017, 8, e2757. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J.; et al. Amino acids as mediators of metabolic cross talk between host and pathogen. Front. Immunol. 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Gouzy, A.; Larrouy-Maumus, G.; Wu, T.-D.; Peixoto, A.; Levillain, F.; Lugo-Villarino, G.; Guerquin-Kern, J.-L.; De Carvalho, L.P.S.; Poquet, Y.; Neyrolles, O.; et al. Mycobacterium tuberculosis nitrogen assimilation and host colonization require aspartate. Nat. Chem. Biol. 2013, 9, 674–676. [Google Scholar] [CrossRef]
- Vrieling, F.; Alisjahbana, B.; Sahiratmadja, E.; Van Crevel, R.; Harms, A.C.; Hankemeier, T.; Ottenhoff, T.H.M.; Joosten, S.A. Plasma metabolomics in tuberculosis patients with and without concurrent type 2 diabetes at diagnosis and during antibiotic treatment. Sci. Rep. 2019, 9, 18669. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Raterink, R.-J.; Marín-Juez, R.; Veneman, W.J.; Egbers, K.; Eeden, S.V.D.; Haks, M.C.; Joosten, S.A.; Ottenhoff, T.H.M.; Harms, A.C.; et al. Tuberculosis causes highly conserved metabolic changes in human patients, mycobacteria-infected mice and zebrafish larvae. Sci. Rep. 2020, 10, 11635. [Google Scholar] [CrossRef]
- Zhou, A.; Ni, J.; Xu, Z.; Wang, Y.; Lu, S.; Sha, W.; Karakousis, P.C.; Yao, Y.-F. Application of 1H NMR spectroscopy-based metabolomics to sera of tuberculosis patients. J. Proteome Res. 2013, 12, 4642–4649. [Google Scholar] [CrossRef]
- Frediani, J.; Jones, D.P.; Tukvadze, N.; Uppal, K.; Sanikidze, E.; Kipiani, M.; Tran, V.T.; Hebbar, G.; Walker, D.I.; Kempker, R.R.; et al. Plasma metabolomics in human pulmonary tuberculosis disease: A pilot study. PLoS ONE 2014, 9, e108854. [Google Scholar] [CrossRef] [PubMed]
- Luier, L.; Loots, D.T. Tuberculosis metabolomics reveals adaptations of man and microbe in order to outcompete and survive. Metabolomics 2016, 12, 40. [Google Scholar] [CrossRef]
- Yi, W.-J.; Han, Y.-S.; Wei, L.-L.; Shi, L.-Y.; Huang, H.; Jiang, T.-T.; Li, Z.-B.; Chen, J.; Hu, Y.-T.; Tu, H.-H.; et al. L-Histidine, arachidonic acid, biliverdin, and L-cysteine-glutathione disulfide as potential biomarkers for cured pulmonary tuberculosis. Biomed. Pharm. 2019, 116, 108980. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Shi, L.; Wei, L.; Han, Y.; Yi, W.; Pan, Z. Clinica Chimica Acta Plasma metabolites Xanthine, 4-Pyridoxate, and D -glutamic acid as novel potential biomarkers for pulmonary tuberculosis. Clin. Chim. Acta 2019, 498, 135–142. [Google Scholar] [CrossRef]
- Conde, R.; Laires, R.; Gonçalves, L.G.; Rizvi, A.; Barroso, C.; Villar, M.; Macedo, R.; Simões, M.J.; Gaddam, S.; Lamosa, P.; et al. Discovery of serum biomarkers for diagnosis of tuberculosis by NMR metabolomics including cross-validation with a second cohort. Biomed. J. 2021, 45, 654–664. [Google Scholar] [CrossRef]
- Magdalena, D.; Michal, S.; Marta, S.; Magdalena, K.P.; Anna, P.; Magdalena, G. Targeted metabolomics analysis of serum and Mycobacterium tuberculosis antigen-stimulated blood cultures of pediatric patients with active and latent tuberculosis. Sci. Rep. 2022, 12, 4131. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Lee, K.-C.; Curreem, S.O.T.; Chow, W.-N.; To, K.; Hung, I.F.N.; Ho, D.T.Y.; Sridhar, S.; Li, I.W.S.; Ding, V.S.Y.; et al. Metabolomic profiling of plasma from patients with tuberculosis by use of untargeted mass spectrometry reveals novel biomarkers for diagnosis. J. Clin. Microbiol. 2015, 53, 3750–3759. [Google Scholar] [CrossRef]
- van Laarhoven, A.; Dian, S.; Aguirre-Gamboa, R.; Avila-Pacheco, J.; Ricaño-Ponce, I.; Ruesen, C.; Annisa, J.; Koeken, V.A.C.M.; Chaidir, L.; Li, Y.; et al. Cerebral tryptophan metabolism and outcome of tuberculous meningitis: An observational cohort study. Lancet Infect. Dis. 2018, 18, 526–535. [Google Scholar] [CrossRef]
- Suchard, M.S.; Adu-Gyamfi, C.G.; Cumming, B.M.; Savulescu, D.M. Evolutionary Views of Tuberculosis: Indoleamine 2,3-Dioxygenase Catalyzed Nicotinamide Synthesis Reflects Shifts in Macrophage Metabolism: Indoleamine 2,3-Dioxygenase Reflects Altered Macrophage Metabolism During Tuberculosis Pathogenesis. BioEssays 2020, 42, 1–10. [Google Scholar] [CrossRef]
- Tientcheu, L.D.; Maertzdorf, J.; Weiner, J.; Adetifa, I.M. Differential transcriptomic and metabolic profiles of M. africanum - and M. tuberculosis -infected patients after, but not before drug treatment. Genes Immun. 2016, 16, 347–355. [Google Scholar] [CrossRef]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.; Maguire, T.; Alwine, J. Glutamine metabolism is essential for human cytomegalovirus infection. J. Virol. 2010, 84, 1867–1873. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Novakovic, B.; Ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.-C.; Wang, S.-Y.; et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef]
- Gouzy, A.; Poquet, Y.; Neyrolles, O. Amino acid capture and utilization within the Mycobacterium tuberculosis phagosome. Future Microbiol. 2014, 9, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Huff, J.; Janik, K.; Walter, K.; Keller, C.; Ehlers, S.; Bossmann, S.H.; Niederweis, M. Expression of the ompATb operon accelerates ammonia secretion and adaptation of Mycobacterium tuberculosis to acidic environments. Mol. Mycrobiol. 2011, 80, 900–918. [Google Scholar] [CrossRef] [PubMed]
- Gouzy, A.; Larrouy-Maumus, G.; Bottai, D.; Levillain, F.; Dumas, A.; Wallach, J.B.; Caire-Brandli, I.; de Chastellier, C.; Wu, T.-D.; Poincloux, R.; et al. Mycobacterium tuberculosis Exploits Asparagine to Assimilate Nitrogen and Resist Acid Stress during Infection. PLOS Pathog. 2014, 10, e1003928. [Google Scholar] [CrossRef]
- Rapovy, S.M.; Zhao, J.; Schmidt, S.M.; Bricker, R.L.; Setchell, K.D.R.; Qualls, J.E. Differential requirements for L-citrulline and L-arginine during anti-mycobacterial macrophage activity. J. Immunol. 2015, 195, 3293–3300. [Google Scholar] [CrossRef]
- Das, M.K.; Bishwal, S.C.; Das, A.; Dabral, D.; Badireddy, V.K.; Pandit, B.; Varghese, G.M.; Nanda, R.K. Deregulated tyrosine-phenylalanine metabolism in pulmonary tuberculosis patients. J. Proteome Res. 2015, 14, 1947–1956. [Google Scholar] [CrossRef]
- Mandel, H.; Levy, N.; Izkovitch, S.; Korman, S. Elevated plasma citrulline and arginine due to consumption of Citrullus vulgaris (watermelon). J. Inherit. Metab Dis. 2005, 28, 467–472. [Google Scholar] [CrossRef]
- Lutgens, L.; Lambin, P. Biomarkers for radiation-induced small bowel epithelial damage: An emerging role for plasma Citrulline. World J. Gastroenterol. 2007, 13, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Zerrouk, N.; Aussel, C.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.-C.; Cynober, L.; Sfar, S. Citrulline: From metabolism to therapeutic use. Nutrition 2013, 29, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.M.; McKell, M.; Schmidt, S.M.; Zhao, J.; Crowther, R.R.; Green, L.; Bricker, R.L.; Arnett, E.; Köhler, S.E.; Schlesinger, L.S.; et al. l-Arginine Synthesis from l-Citrulline in Myeloid Cells Drives Host Defense against Mycobacteria In Vivo. J. Immunol. 2019, 202, 1747–1754. [Google Scholar] [CrossRef]
- Sinclair, L.V.; Howden, A.J.M.; Brenes, A.; Spinelli, L.; Hukelmann, J.L.; Macintyre, A.N.; Liu, X.; Thomson, S.; Taylor, P.M.; Rathmell, J.C.; et al. The importance of methionine metabolism. eLife 2019, 8, 8–10. [Google Scholar]
- Lim, J.M.; Kim, G.; Levine, R.L. Methionine in Proteins: It’s Not Just for Protein Initiation Anymore. Neurochem. Res. 2019, 44, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, A.M.; da Silva, D.A.; Ferreira, N.V.; de Pina, L.C.; Evaristo, J.A.; Evaristo, G.P.C.; Nogueira, F.C.S.; Ochs, S.M.; Amaral, J.J.; Ferreira, R.B.; et al. Metabolic profiles of multidrug resistant and extensively drug resistant Mycobacterium tuberculosis unveiled by metabolomics. Tuberculosis 2021, 126, 102043. [Google Scholar] [CrossRef] [PubMed]
- Consalvi, S.; Scarpecci, C.; Biava, M.; Poce, G. Mycobacterial tryptophan biosynthesis: A promising target for tuberculosis drug development? Bioorg. Med. Chem. Lett. 2019, 29, 126731. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Reddy, M.C.; Ioerger, T.R.; Rothchild, A.C.; Dartois, V.; Schuster, B.M.; Trauner, A.; Wallis, D.; Galaviz, S.; Huttenhower, C.; et al. Tryptophan biosynthesis protects mycobacteria from CD4 T cell-mediated killing. Cell 2014, 155, 1296–1308. [Google Scholar] [CrossRef]
| Authors | Subject | Proper-ties | A | P | H | C | Th | G | Gl | As | Tr | V | At | Glt | L | Ty | M | Ci | O | Ar | S | Le | Iso | E |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weiner et al., 2012 [5] | 44 TB 46 latent TB 46 HC | UPLC-MS/MS GC-MS | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||||||||||||
| Weiner et al., 2018 [11] | 4462 progressors * | GC-MS | ↓ | ↓ | ||||||||||||||||||||
| Albors-Vaquer., 2020 [17] | 35 HC 15 active TB 30 household contacts | 1H-NMR | ↓ | ↓ | ||||||||||||||||||||
| Zhou et al., 2013 [32] | 38 TB 39 HC | 1H-NMR spectroscopy | ↓ | ↑ | ↓ | ↑ | ↑ | ↑ | ↑ | |||||||||||||||
| Frediani et al., 2014 [33] | 17 TB | NMR | ↑ | ↑ | ||||||||||||||||||||
| Cho et al., 2020 [18] | 21 active TB 20 latent TB 38 HC | ↓ | ↓ | = | ↑ | ↓ | ||||||||||||||||||
| Vrieling et al., 2019 [30] | 48 TB 20 TB-DM 48 HC | LC-MS/MS | ↑ | ↓ ↓↓ | ↓ ↓↓ | ↓ ↓↓ | ↓ ↓↓ | ↓ ↓↓ | ↑ | ↓ ↓↓ | ↓ ↓↓ | ↓ ↓↓ | ↑ | ↓ ↓↓ | ||||||||||
| Luier and Loots, 2016 [34] | 46 active TB 30 HC | URINE-MS | ↓ | ↑ | ||||||||||||||||||||
| Yi et al., 2019 [35] | 35 HC 35 untreated TB 31 two-month treated TB subjects 29 cured TB subjects | MS | ↑ | ↓ | ↓ | ↑ | ↓ | |||||||||||||||||
| Huang et al., 2019 [36] | 35 TB 35 controls 35 CAP 31 LC | LC-MS/MS | ↓ | |||||||||||||||||||||
| Conde et al., 2022 [37] | 37 PTB 12 EPTB | 1H-NMR | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | |||||||||||||
| Magdalena et al., 2022 [38] | 15 TB children 52 LTBI 20 NMP 149 HC | LC-MS/MS | ↓ | ↑ | ↓ | |||||||||||||||||||
| Shin et al., 2011 [15] | Mice # | 1H-NMR | ↑ | ↑ | ↑ | ↑ | ||||||||||||||||||
| Ding et al. **, 2020 [31] | 20 TB 20 HC zebrafish mice | LC-MS NMR spectroscopy | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amalia, F.; Syamsunarno, M.R.A.A.; Triatin, R.D.; Fatimah, S.N.; Chaidir, L.; Achmad, T.H. The Role of Amino Acids in Tuberculosis Infection: A Literature Review. Metabolites 2022, 12, 933. https://doi.org/10.3390/metabo12100933
Amalia F, Syamsunarno MRAA, Triatin RD, Fatimah SN, Chaidir L, Achmad TH. The Role of Amino Acids in Tuberculosis Infection: A Literature Review. Metabolites. 2022; 12(10):933. https://doi.org/10.3390/metabo12100933
Chicago/Turabian StyleAmalia, Fiki, Mas Rizky A. A. Syamsunarno, Rima Destya Triatin, Siti Nur Fatimah, Lidya Chaidir, and Tri Hanggono Achmad. 2022. "The Role of Amino Acids in Tuberculosis Infection: A Literature Review" Metabolites 12, no. 10: 933. https://doi.org/10.3390/metabo12100933
APA StyleAmalia, F., Syamsunarno, M. R. A. A., Triatin, R. D., Fatimah, S. N., Chaidir, L., & Achmad, T. H. (2022). The Role of Amino Acids in Tuberculosis Infection: A Literature Review. Metabolites, 12(10), 933. https://doi.org/10.3390/metabo12100933






