N-3 Fatty Acid Supplementation Impacts Protein Metabolism Faster Than it Lowers Proinflammatory Cytokines in Advanced Breast Cancer Patients: Natural 15N/14N Variations during a Clinical Trial
Abstract
1. Introduction
2. Material and Methods
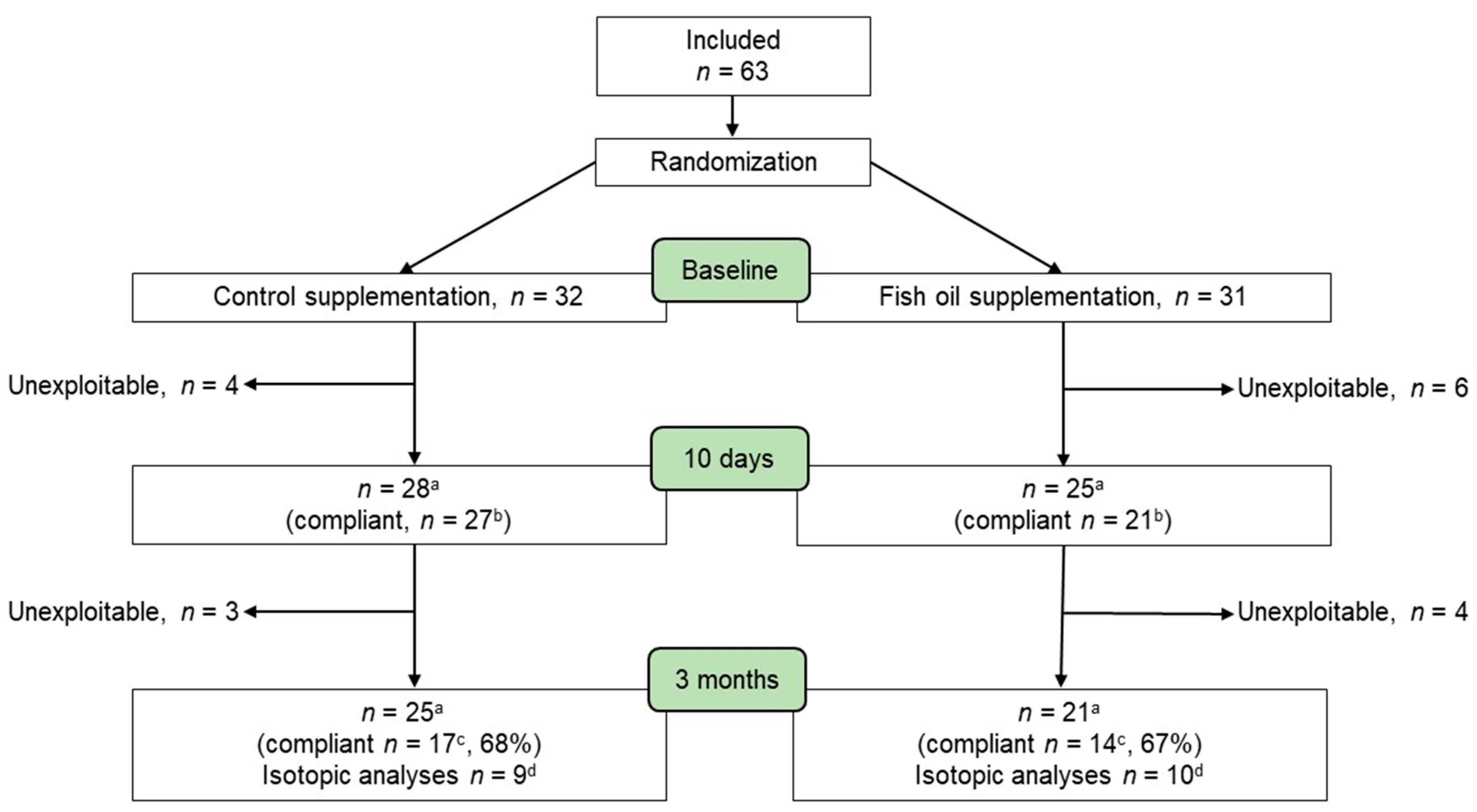
2.1. Study Design and Participants
2.2. Supplements
2.3. Fatty Acid Profile
2.4. Proinflammatory Cytokines
2.5. Isotopic Analyses
2.6. Statistical Analyses
3. Results
3.1. Compliance with Fish Oil Supplementation Increases Plasma EPA and DHA
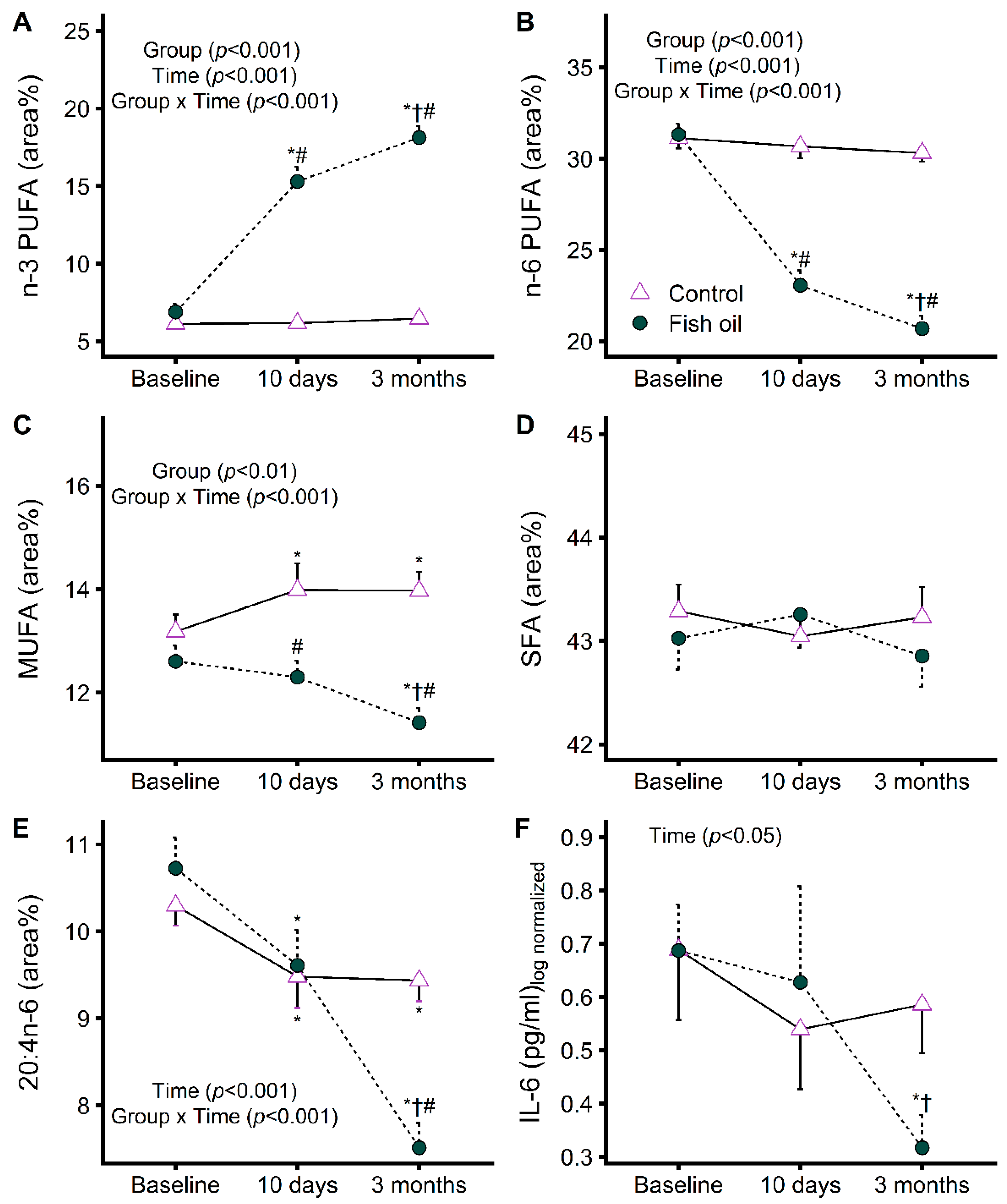
3.2. Impact of Increasing EPA and DHA Levels on Plasma Fatty Acid Profile and Proinflammatory Cytokines
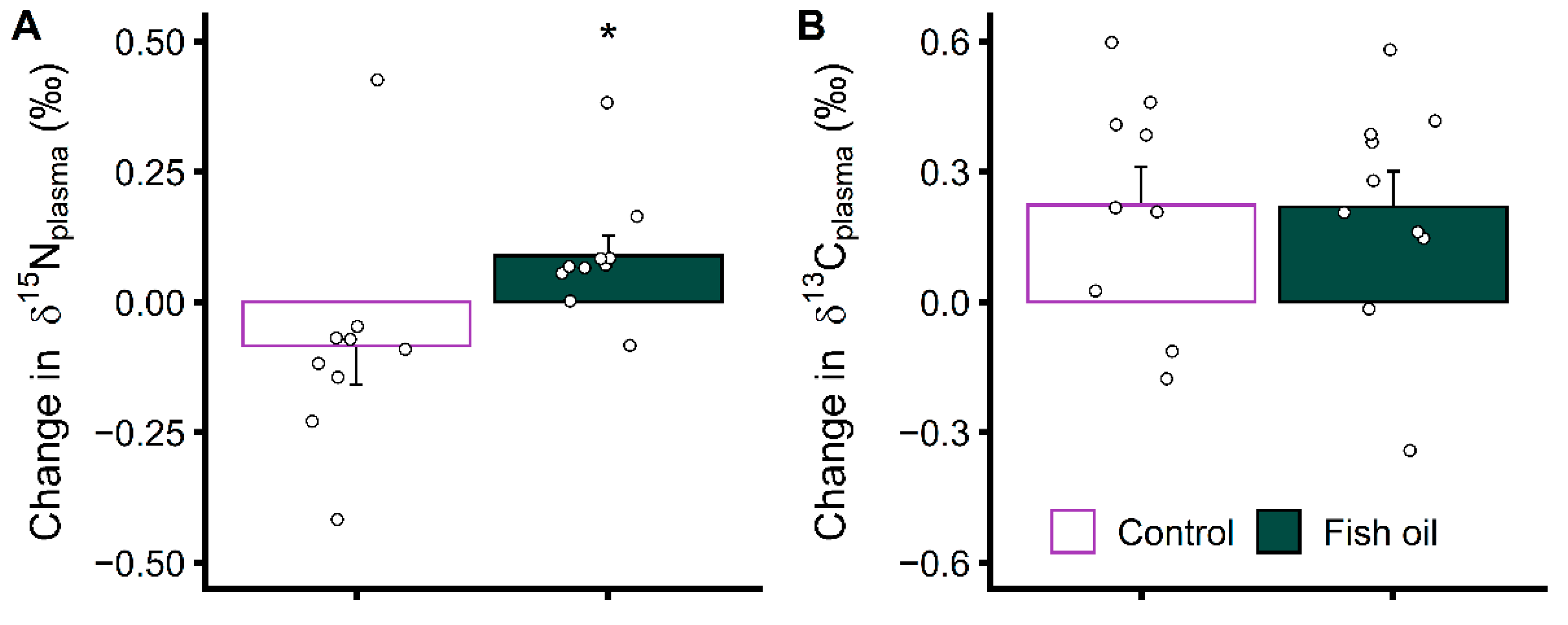
3.3. Increasing EPA and DHA Levels Rapidly Impacts Protein Metabolism
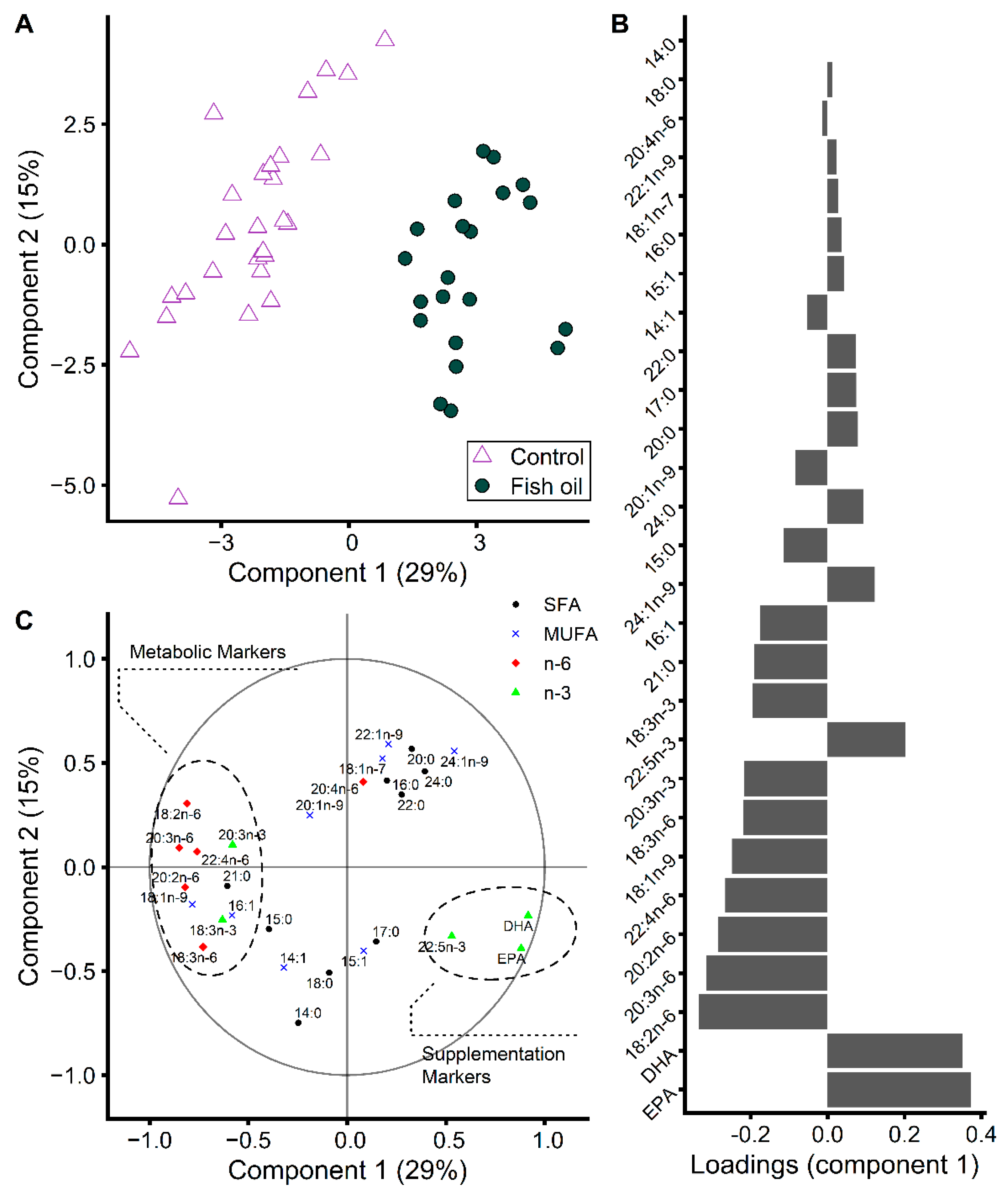
3.4. During EPA and DHA Supplementation, More Fatty Acids Reflect Metabolic Regulations Than Their Intake Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newell, M.; Mazurak, V.; Postovit, L.M.; Field, C.J. N-3 Long-Chain Polyunsaturated Fatty Acids, Eicosapentaenoic and Docosahexaenoic Acid, and the Role of Supplementation during Cancer Treatment: A Scoping Review of Current Clinical Evidence. Cancers 2021, 13, 1206. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.; Giraudeau, B.; Couet, C.; Le Floch, O. Improving Outcome of Chemotherapy of Metastatic Breast Cancer by Docosahexaenoic Acid: A Phase II Trial. Br. J. Cancer 2009, 101, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Heinrich, J.; Koletzko, B. Role of FADS1 and FADS2 Polymorphisms in Polyunsaturated Fatty Acid Metabolism. Metabolism 2010, 59, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Chilton, F.H.; Dutta, R.; Reynolds, L.M.; Sergeant, S.; Mathias, R.A.; Seeds, M.C. Precision Nutrition and Omega-3 Polyunsaturated Fatty Acids: A Case for Personalized Supplementation Approaches for the Prevention and Management of Human Diseases. Nutrients 2017, 9, 1165. [Google Scholar] [CrossRef]
- Serini, S.; Calviello, G. Omega-3 PUFA Responders and Non-Responders and the Prevention of Lipid Dysmetabolism and Related Diseases. Nutrients 2020, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G.; Steensberg, A.; Fischer, C.; Keller, C.; Møller, K.; Moseley, P.; Pedersen, B.K. Interleukin-6 Markedly Decreases Skeletal Muscle Protein Turnover and Increases Nonmuscle Amino Acid Utilization in Healthy Individuals. J. Clin. Endocrinol. Metab. 2008, 93, 2851–2858. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary Omega-3 Fatty Acid Supplementation Increases the Rate of Muscle Protein Synthesis in Older Adults: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef]
- Buoite Stella, A.; Gortan Cappellari, G.; Barazzoni, R.; Zanetti, M. Update on the Impact of Omega 3 Fatty Acids on Inflammation, Insulin Resistance and Sarcopenia: A Review. Int. J. Mol. Sci. 2018, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 Polyunsaturated Fatty Acids Augment the Muscle Protein Anabolic Response to Hyperinsulinaemia–Hyperaminoacidaemia in Healthy Young and Middle-Aged Men and Women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Witard, O.C.; Combet, E.; Gray, S.R. Long-Chain n-3 Fatty Acids as an Essential Link between Musculoskeletal and Cardio-Metabolic Health in Older Adults. Proc. Nutr. Soc. 2020, 79, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Deger, S.M.; Hung, A.M.; Ellis, C.D.; Booker, C.; Bian, A.; Chen, G.; Abumrad, N.N.; Ikizler, T.A. High Dose Omega-3 Fatty Acid Administration and Skeletal Muscle Protein Turnover in Maintenance Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2016, 11, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Votruba, S.B.; Shaw, P.A.; Oh, E.J.; Venti, C.A.; Bonfiglio, S.; Krakoff, J.; O’Brien, D.M. Associations of Plasma, RBCs, and Hair Carbon and Nitrogen Isotope Ratios with Fish, Meat, and Sugar-Sweetened Beverage Intake in a 12-Wk Inpatient Feeding Study. Am. J. Clin. Nutr. 2019, 110, 1306–1315. [Google Scholar] [CrossRef]
- Yun, H.Y.; Lampe, J.W.; Tinker, L.F.; Neuhouser, M.L.; Beresford, S.A.; Niles, K.R.; Mossavar-Rahmani, Y.; Snetselaar, L.G.; Van Horn, L.; Prentice, R.L. Serum Nitrogen and Carbon Stable Isotope Ratios Meet Biomarker Criteria for Fish and Animal Protein Intake in a Controlled Feeding Study of a Women’s Health Initiative Cohort. J. Nutr. 2018, 148, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Mantha, O.L. Effect of Organic Food Intake on Nitrogen Stable Isotopes. Nutrients 2020, 12, 2965. [Google Scholar] [CrossRef] [PubMed]
- Huneau, J.-F.; Mantha, O.L.; Hermier, D.; Mathé, V.; Galmiche, G.; Mariotti, F.; Fouillet, H. Natural Isotope Abundances of Carbon and Nitrogen in Tissue Proteins and Amino Acids as Biomarkers of the Decreased Carbohydrate Oxidation and Increased Amino Acid Oxidation Induced by Caloric Restriction under a Maintained Protein Intake in Obese Rats. Nutrients 2019, 11, 1087. [Google Scholar] [CrossRef] [PubMed]
- Mantha, O.L. Differential Changes to Splanchnic and Peripheral Protein Metabolism during the Diet-Induced Development of Metabolic Syndrome in Rats. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E175–E186. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, K.; Jousse, C.; Fafournoux, P.; Schiphorst, A.-M.; Grand, M.; Robins, R.J.; Hankard, R.; De Luca, A. Protein Restricted Diet during Gestation and/or Lactation in Mice Affects 15N Natural Isotopic Abundance of Organs in the Offspring: Effect of Diet 15N Content and Growth. PLoS ONE 2018, 13, e0205271. [Google Scholar] [CrossRef] [PubMed]
- Macko, S.A.; Estep, M.L.F.; Engel, M.H.; Hare, P. Kinetic Fractionation of Stable Nitrogen Isotopes during Amino Acid Transamination. Geochim. Et Cosmochim. Acta 1986, 50, 2143–2146. [Google Scholar] [CrossRef]
- Poupin, N.; Mariotti, F.; Huneau, J.-F.; Hermier, D.; Fouillet, H. Natural Isotopic Signatures of Variations in Body Nitrogen Fluxes: A Compartmental Model Analysis. PLoS Comput. Biol. 2014, 10, e1003865. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, C.Z.; Jones, C.M.; Yost, R.A.; Garrett, T.J.; Bowden, J.A. Optimization of Folch, Bligh-Dyer, and Matyash Sample-to-Extraction Solvent Ratios for Human Plasma-Based Lipidomics Studies. Anal. Chim. Acta 2018, 1037, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Chas, M.; Goupille, C.; Arbion, F.; Bougnoux, P.; Pinault, M.; Jourdan, M.L.; Chevalier, S.; Ouldamer, L. Low Eicosapentaenoic Acid and Gamma-Linolenic Acid Levels in Breast Adipose Tissue Are Associated with Inflammatory Breast Cancer. Breast 2019, 45, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Tea, I.; Martineau, E.; Antheaume, I.; Lalande, J.; Mauve, C.; Gilard, F.; Barillé-Nion, S.; Blackburn, A.C.; Tcherkez, G. 13 C and 15 N Natural Isotope Abundance Reflects Breast Cancer Cell Metabolism. Sci. Rep. 2016, 6, 34251. [Google Scholar] [CrossRef] [PubMed]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. MixOmics: An R Package for ‘omics Feature Selection and Multiple Data Integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short-and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Jannas-Vela, S.; Roke, K.; Boville, S.; Mutch, D.M.; Spriet, L.L. Lack of Effects of Fish Oil Supplementation for 12 Weeks on Resting Metabolic Rate and Substrate Oxidation in Healthy Young Men: A Randomized Controlled Trial. PLoS ONE 2017, 12, e0172576. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.L.; Spriet, L.L. Omega-3 Fatty Acid Supplementation for 12 Weeks Increases Resting and Exercise Metabolic Rate in Healthy Community-Dwelling Older Females. PLoS ONE 2015, 10, e0144828. [Google Scholar] [CrossRef]
- Couet, C.; Delarue, J.; Ritz, P.; Antoine, J.; Lamisse, F. Effect of Dietary Fish Oil on Body Fat Mass and Basal Fat Oxidation in Healthy Adults. Int. J. Obes. 1997, 21, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Mostad, I.L.; Bjerve, K.S.; Bjorgaas, M.R.; Lydersen, S.; Grill, V. Effects of N− 3 Fatty Acids in Subjects with Type 2 Diabetes: Reduction of Insulin Sensitivity and Time-Dependent Alteration from Carbohydrate to Fat Oxidation. Am. J. Clin. Nutr. 2006, 84, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Weech, M.; Vafeiadou, K.; Hasaj, M.; Todd, S.; Yaqoob, P.; Jackson, K.G.; Lovegrove, J.A. Development of a Food-Exchange Model to Replace Saturated Fat with MUFAs and n–6 PUFAs in Adults at Moderate Cardiovascular Risk. J. Nutr. 2014, 144, 846–855. [Google Scholar] [CrossRef]
- Markey, O.; Vasilopoulou, D.; Kliem, K.E.; Koulman, A.; Fagan, C.C.; Summerhill, K.; Wang, L.Y.; Grandison, A.S.; Humphries, D.J.; Todd, S. Plasma Phospholipid Fatty Acid Profile Confirms Compliance to a Novel Saturated Fat-Reduced, Monounsaturated Fat-Enriched Dairy Product Intervention in Adults at Moderate Cardiovascular Risk: A Randomized Controlled Trial. Nutr. J. 2017, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-Related Effects of Eicosapentaenoic Acid on Innate Immune Function in Healthy Humans: A Comparison of Young and Older Men. Am. J. Clin. Nutr. 2006, 83, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T. Interorgan Amino Acid Transport and Its Regulation. J. Nutr. 2003, 133, 2068S–2072S. [Google Scholar] [CrossRef] [PubMed]
- Tessari, P.; Garibotto, G. Interorgan Amino Acid Exchange. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Levitt, D.G.; Levitt, M.D. Human Serum Albumin Homeostasis: A New Look at the Roles of Synthesis, Catabolism, Renal and Gastrointestinal Excretion, and the Clinical Value of Serum Albumin Measurements. Int. J. Gen. Med. 2016, 9, 229. [Google Scholar] [CrossRef]
- Smith, G.I.; Patterson, B.W.; Mittendorfer, B. Human Muscle Protein Turnover—Why Is It so Variable? J. Appl. Physiol. 2011, 110, 480–491. [Google Scholar] [CrossRef]
- Metges, C.C.; Petzke, K.J. Measurement Of15N/14N Isotopic Composition in Individual Plasma Free Amino Acids of Human Adults at Natural Abundance by Gas Chromatography–Combustion Isotope Ratio Mass Spectrometry. Anal. Biochem. 1997, 247, 158–164. [Google Scholar] [CrossRef]
- Karakas, S.E.; Perroud, B.; Kind, T.; Palazoglu, M.; Fiehn, O. Changes in Plasma Metabolites and Glucose Homeostasis during Omega-3 Polyunsaturated Fatty Acid Supplementation in Women with Polycystic Ovary Syndrome. BBA Clin. 2016, 5, 179–185. [Google Scholar] [CrossRef]
- Huffman, D.M.; Altena, T.S.; Mawhinney, T.P.; Thomas, T.R. Effect of N-3 Fatty Acids on Free Tryptophan and Exercise Fatigue. Eur. J. Appl. Physiol. 2004, 92, 584–591. [Google Scholar] [CrossRef]
- Brown, K.M.; Sharma, S.; Baker, E.; Hawkins, W.; van der Merwe, M.; Puppa, M.J. Delta-6-Desaturase (FADS2) Inhibition and Omega-3 Fatty Acids in Skeletal Muscle Protein Turnover. Biochem. Biophys. Rep. 2019, 18, 100622. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.M.; Kempen, L.J.; Hardy, R.S.; Langen, R.C. Inflammation and Skeletal Muscle Wasting during Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty Acid Composition of Adipose Tissue and Blood in Humans and Its Use as a Biomarker of Dietary Intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
- Cho, H.P.; Nakamura, M.; Clarke, S.D. Cloning, Expression, and Fatty Acid Regulation of the Human Δ-5 Desaturase. J. Biol. Chem. 1999, 274, 37335–37339. [Google Scholar] [CrossRef]
- Garg, M.L.; Sebokova, E.; Thomson, A.B.; Clandinin, M.T. Δ 6-Desaturase Activity in Liver Microsomes of Rats Fed Diets Enriched with Cholesterol and/or ω 3 Fatty Acids. Biochem. J. 1988, 249, 351–356. [Google Scholar] [CrossRef]
- Garg, M.L.; Thomson, A.B.; Clandinin, M.T. Effect of Dietary Cholesterol and/or Ω3 Fatty Acids on Lipid Composition and Δ5-Desaturase Activity of Rat Liver Microsomes. J. Nutr. 1988, 118, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, E.N.; Lund, J.S.; Rørtveit, T.; Rustan, A.C. Effect of Dietary N− 3 and N− 6 Fatty Acids on Fatty Acid Desaturation in Rat Liver. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1991, 1082, 57–62. [Google Scholar] [CrossRef]
- Gonzalez-Soto, M.; Mutch, D.M. Diet Regulation of Long-Chain PUFA Synthesis: Role of Macronutrients, Micronutrients, and Polyphenols on Δ-5/Δ-6 Desaturases and Elongases 2/5. Adv. Nutr. 2021, 12, 980–994. [Google Scholar] [CrossRef]
- Vessby, B.; Gustafsson, I.-B.; Tengblad, S.; Berglund, L. Indices of Fatty Acid Desaturase Activity in Healthy Human Subjects: Effects of Different Types of Dietary Fat. Br. J. Nutr. 2013, 110, 871–879. [Google Scholar] [CrossRef]
- Cormier, H.; Rudkowska, I.; Lemieux, S.; Couture, P.; Julien, P.; Vohl, M.-C. Effects of FADS and ELOVL Polymorphisms on Indexes of Desaturase and Elongase Activities: Results from a Pre-Post Fish Oil Supplementation. Genes Nutr. 2014, 9, 437. [Google Scholar] [CrossRef]
- Al-Hilal, M.; AlSaleh, A.; Maniou, Z.; Lewis, F.J.; Hall, W.L.; Sanders, T.A.; O’Dell, S.D. Genetic Variation at the FADS1-FADS2 Gene Locus Influences Delta-5 Desaturase Activity and LC-PUFA Proportions after Fish Oil Supplement [S]. J. Lipid Res. 2013, 54, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhou, D.; Pan, Y.-X.; Wang, X.; Nakamura, M.T. Compensatory Induction of Fads1 Gene Expression in Heterozygous Fads2-Null Mice and by Diet with a High n-6/n-3 PUFA Ratio [S]. J. Lipid Res. 2016, 57, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.M.; Howard, T.D.; Ruczinski, I.; Kanchan, K.; Seeds, M.C.; Mathias, R.A.; Chilton, F.H. Tissue-Specific Impact of FADS Cluster Variants on FADS1 and FADS2 Gene Expression. PLoS ONE 2018, 13, e0194610. [Google Scholar] [CrossRef] [PubMed]



| Control Supplement | Fish Oil Supplement | |
|---|---|---|
| Nutritional composition | per 200 mL can | |
| Energy | 300 Kcal | 300 Kcal |
| Proteins | 15 g | 15 g |
| Carbohydrates | 36 g | 36 g |
| Lipids | 12 g | 12 g |
| Saturated FA | 6.6 g | 3 g |
| Monounsaturated FA | 3.8 g | 5 g |
| PUFA | 1.1 g | 4 g |
| 18:2n-6 | 1 g | 1 g |
| 18:3n-3 | 0.5 g | 0.5 g |
| EPA | 0 g | 0.88 g |
| DHA | 0 g | 0.52 g |
| Isotopic composition | ||
| δ15N | 5.3‰ | 5.3‰ |
| δ13C | −25.4‰ | −24.6‰ |
| Control (n = 32) | Fish oil (n = 31) | p | |
|---|---|---|---|
| Age (y) | 57.0 ± 2.1 | 60.6 ± 2.1 | 0.23 |
| Body weight (kg) | 69.2 ± 2.2 | 66.3 ± 1.9 | 0.32 |
| BMI | 26.6 ± 0.8 | 25.5 ± 0.7 | 0.31 |
| Menopausal status | |||
| Non-menopausal | 4/29 (14%) | 4/30 (13%) | 1.0 |
| Peri-menopausal | 3/29 (10%) | 2/30 (7%) | 0.67 |
| Menopausal | 22/29 (76%) | 24/30 (80%) | 0.76 |
| Not available | 3 | 1 | |
| Disease severity at diagnosis * | |||
| Node status | |||
| N0 | 7/27 (26%) | 5/28 (18%) | 0.53 |
| N1 | 15/27 (56%) | 16/28 (57%) | 1.0 |
| N2 | 3/27 (11%) | 2/28 (7%) | 0.67 |
| N3 | 2/27 (7%) | 5/28 (18%) | 0.42 |
| Not available | 5 | 3 | |
| SBR grade | |||
| I | 9/32 (28%) | 2/28 (7%) | <0.05 |
| II | 18/32 (56%) | 17/28 (61%) | 0.80 |
| III | 5/32 (16%) | 9/28 (32%) | 0.22 |
| Not available | 0 | 3 | |
| Metastases at inclusion | |||
| Number of metastases † | 2.2 ± 0.2 | 2.3 ± 0.2 | 0.69 |
| Pleuropulmonary region | 10/32 (31%) | 10/31 (32%) | 1.0 |
| Liver | 16/32 (50%) | 16/31 (52%) | 1.0 |
| Node | 15/32 (47%) | 8/31 (26%) | 0.12 |
| Bone | 24/32 (75%) | 25/31 (81%) | 0.76 |
| Baseline | 10 Days | 3 Months | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Fish Oil | Control | Fish Oil | Control | Fish Oil | Group | Time | Group × Time | |
| δ15N | 9.2 ± 0.1 | 9.0 ± 0.1 | 9.1 ± 0.1 | 9.1 ± 0.1 | 9.1 ± 0.1 | 9.1 ± 0.1 | 0.75 | 0.98 | 0.10 |
| δ13C | −22.2 ± 0.2 | −22.1 ± 0.1 | −22.0 ± 0.2 | −21.9 ± 0.1 | −21.7 ± 0.2 *,† | −21.7 ± 0.1 * | 0.55 | <0.001 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantha, O.L.; Hankard, R.; Tea, I.; Schiphorst, A.-M.; Dumas, J.-F.; Berger, V.; Goupille, C.; Bougnoux, P.; De Luca, A. N-3 Fatty Acid Supplementation Impacts Protein Metabolism Faster Than it Lowers Proinflammatory Cytokines in Advanced Breast Cancer Patients: Natural 15N/14N Variations during a Clinical Trial. Metabolites 2022, 12, 899. https://doi.org/10.3390/metabo12100899
Mantha OL, Hankard R, Tea I, Schiphorst A-M, Dumas J-F, Berger V, Goupille C, Bougnoux P, De Luca A. N-3 Fatty Acid Supplementation Impacts Protein Metabolism Faster Than it Lowers Proinflammatory Cytokines in Advanced Breast Cancer Patients: Natural 15N/14N Variations during a Clinical Trial. Metabolites. 2022; 12(10):899. https://doi.org/10.3390/metabo12100899
Chicago/Turabian StyleMantha, Olivier L., Régis Hankard, Illa Tea, Anne-Marie Schiphorst, Jean-François Dumas, Virginie Berger, Caroline Goupille, Philippe Bougnoux, and Arnaud De Luca. 2022. "N-3 Fatty Acid Supplementation Impacts Protein Metabolism Faster Than it Lowers Proinflammatory Cytokines in Advanced Breast Cancer Patients: Natural 15N/14N Variations during a Clinical Trial" Metabolites 12, no. 10: 899. https://doi.org/10.3390/metabo12100899
APA StyleMantha, O. L., Hankard, R., Tea, I., Schiphorst, A.-M., Dumas, J.-F., Berger, V., Goupille, C., Bougnoux, P., & De Luca, A. (2022). N-3 Fatty Acid Supplementation Impacts Protein Metabolism Faster Than it Lowers Proinflammatory Cytokines in Advanced Breast Cancer Patients: Natural 15N/14N Variations during a Clinical Trial. Metabolites, 12(10), 899. https://doi.org/10.3390/metabo12100899







