The Level of Remnant Cholesterol and Implications for Lipid-Lowering Strategy in Hospitalized Patients with Acute Coronary Syndrome in China: Findings from the Improving Care for Cardiovascular Disease in China—Acute Coronary Syndrome Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Measurement of Lipid Profiles
2.4. Definition of Variables
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
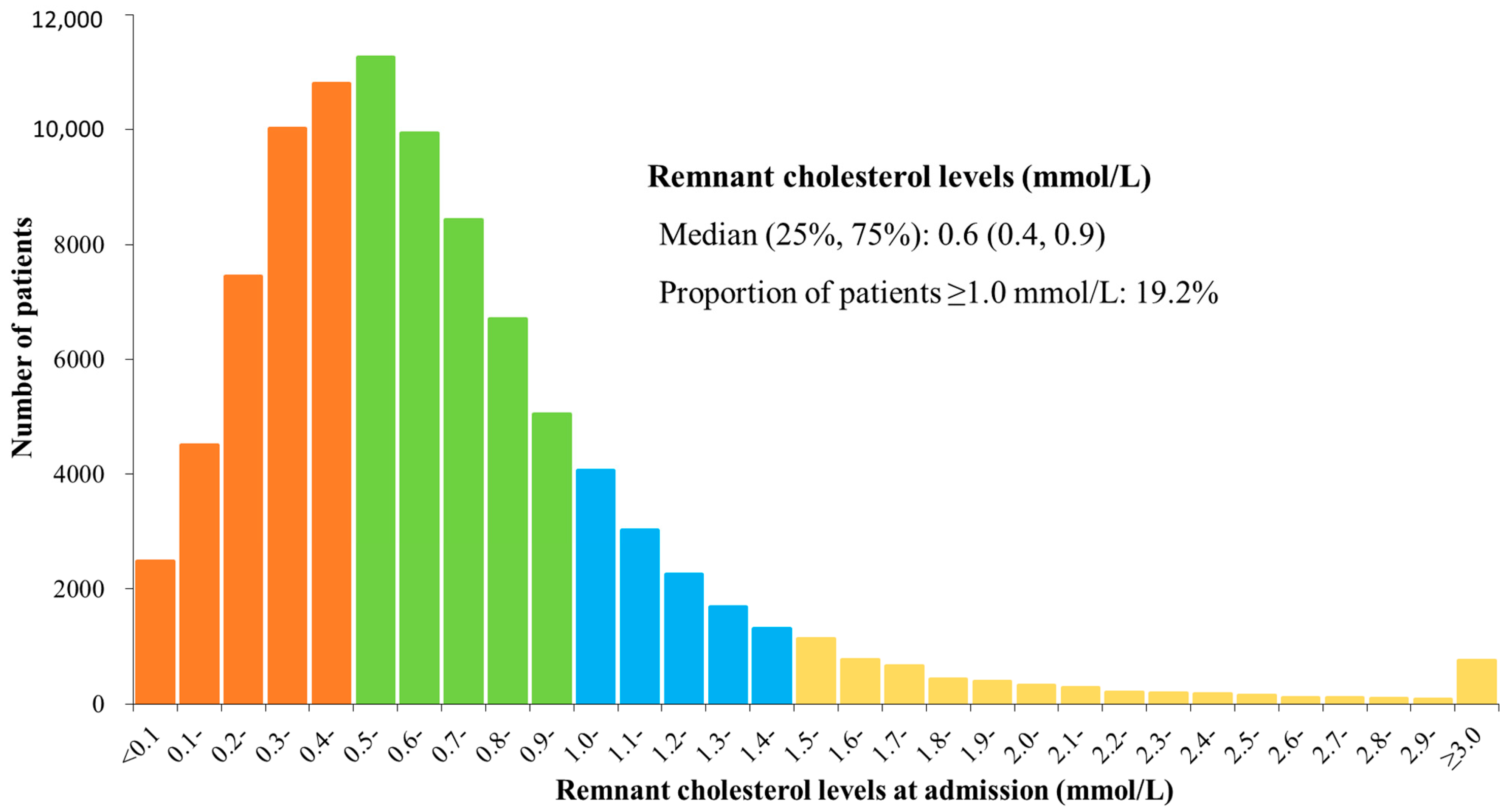
3.2. Distribution of Remnant Cholesterol
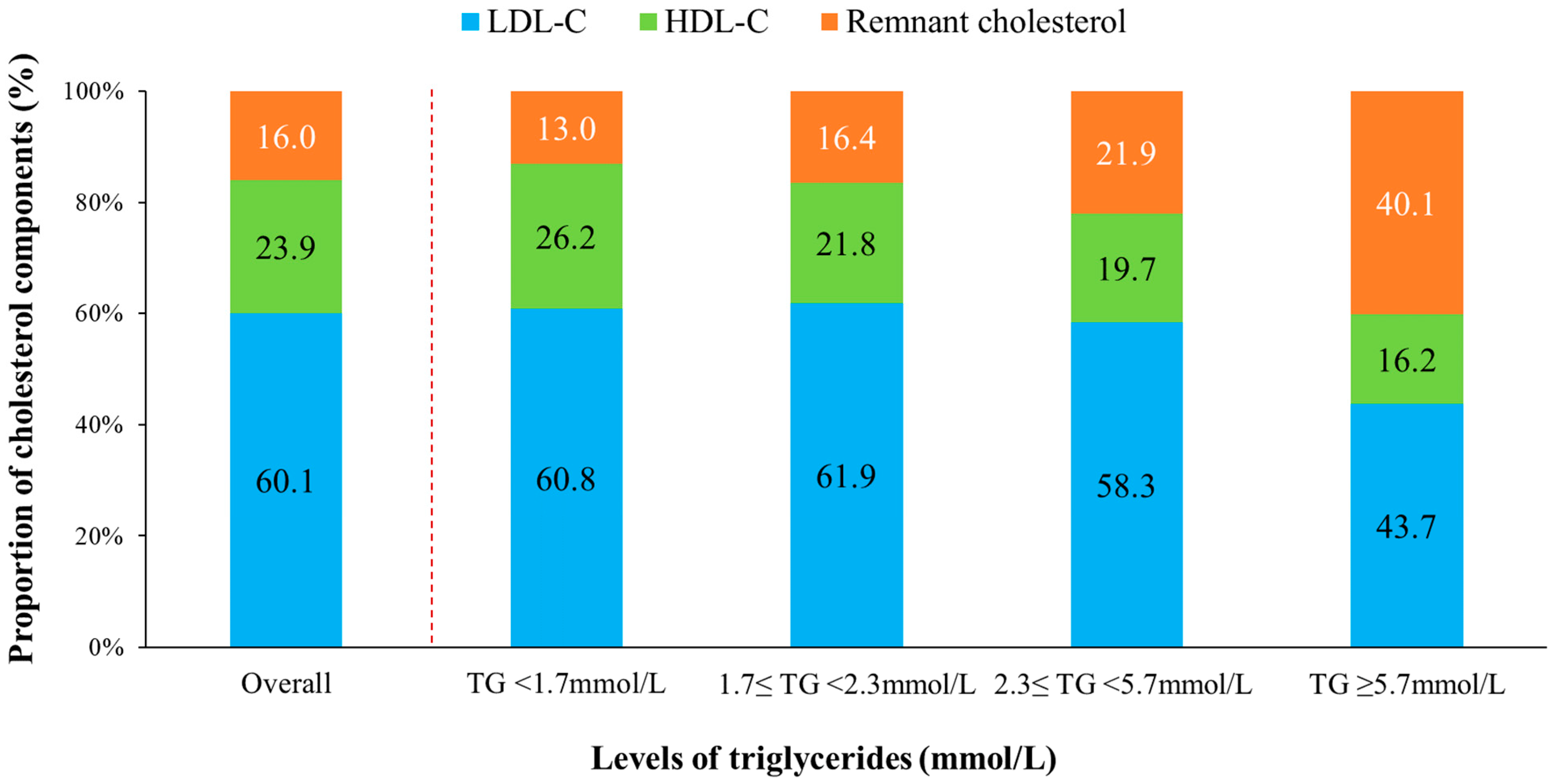
3.3. Proportion of Remnant Cholesterol in TC
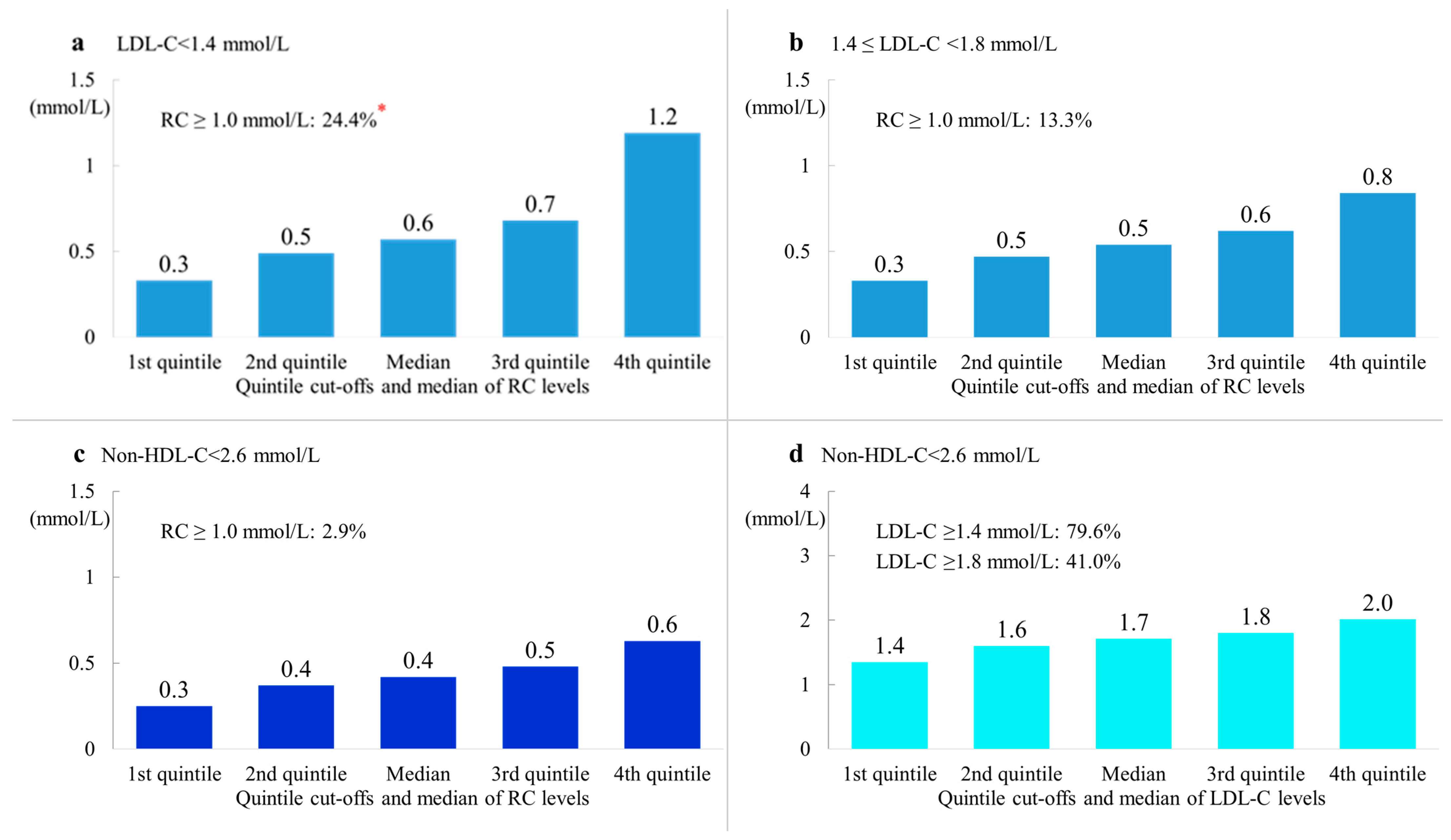
3.4. Remnant Cholesterol Concentrations in Patients with Different LDL-C or Non-HDL-C Concentrations
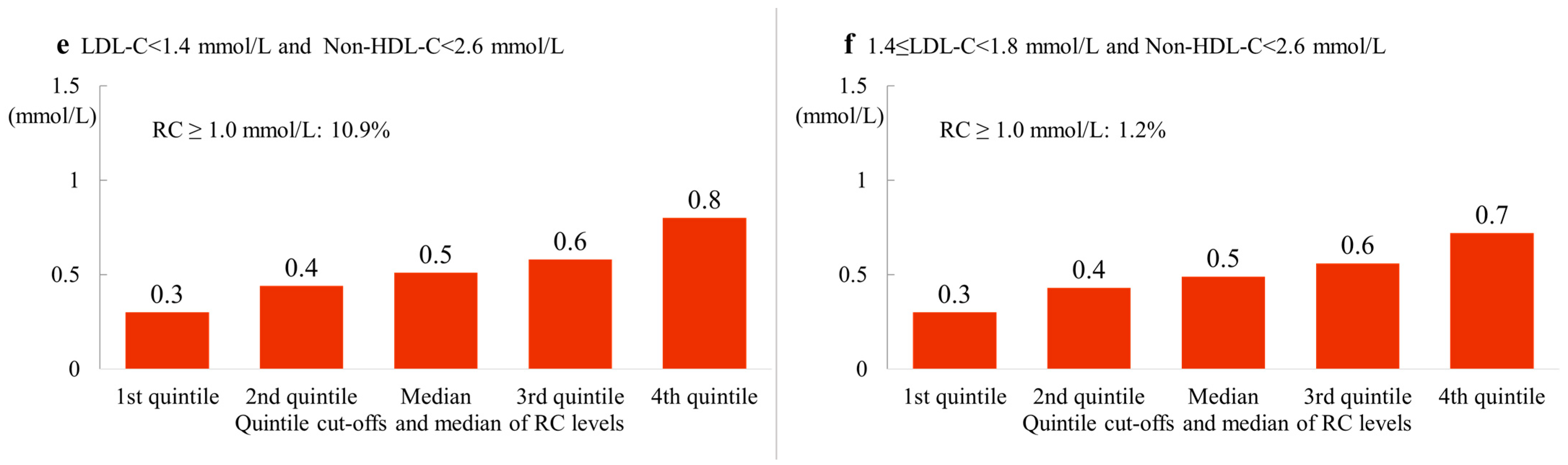
3.5. Remnant Cholesterol Concentrations in Patients with LDL-C and/or Non-HDL-C Concentrations within the Treatment Target
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [PubMed]
- Joint Committee on the Revision of Guidelines for prevention and treatment of dyslipidemia in Chinese adults, Guidelines for prevention and treatment of dyslipidemia in Chinese adults (2016 revision). Chin. Circ. J. 2016, 31, 937–950.
- National Clinical Guideline Center. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease; National Institute for Health and Care Excellence (UK); Copyright © National Clinical Guideline Centre: London, UK, 2014. [Google Scholar]
- Handelsman, Y.; Jellinger, P.S.; Guerin, C.K.; Bloomgarden, Z.T.; Brinton, E.A.; Budoff, M.J.; Davidson, M.H.; Einhorn, D.; Fazio, S.; Fonseca, V.A.; et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the management of dyslipidemia and prevention of cardiovascular disease algorithm—2020 executive summary. Endocr. Pract. 2020, 26, 1196–1224. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; Aguilar-Salinas, C.A.; Averna, M.; Ference, B.A.; Gaudet, D.; Hegele, R.A.; Kersten, S.; et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef]
- Varbo, A.; Nordestgaard, B.G. Remnant lipoproteins. Curr. Opin. Lipidol. 2017, 28, 300–307. [Google Scholar] [CrossRef]
- Sandesara, P.B.; Virani, S.S.; Fazio, S.; Shapiro, M.D. The forgotten lipids: Triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr. Rev. 2019, 40, 537–557. [Google Scholar] [CrossRef]
- Jørgensen, A.B.; Frikke-Schmidt, R.; West, A.S.; Grande, P.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 2013, 34, 1826–1833. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013, 128, 1298–1309. [Google Scholar] [CrossRef]
- Grundy, S.M. An International Atherosclerosis Society Position Paper: Global recommendations for the management of dyslipidemia. J. Clin. Lipidol. 2013, 7, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National lipid association recommendations for patient-centered management of dyslipidemia: Part 1—full report. J. Clin. Lipidol. 2015, 9, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Taskinen, M.R.; Björnson, E.; Packard, C.J. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat. Rev. Cardiol. 2022, 19, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Liu, J.; Liu, J.; Smith, S.C., Jr.; Huo, Y.; Fonarow, G.C.; Ma, C.; Ge, J.; Taubert, K.A.; Morgan, L.; et al. Rationale and design of the Improving Care for Cardiovascular Disease in China (CCC) project: A national effort to prompt quality enhancement for acute coronary syndrome. Am. Heart J. 2016, 179, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Castañer, O.; Pintó, X.; Subirana, I.; Amor, A.J.; Ros, E.; Hernáez, Á.; Martínez-González, M.; Corella, D.; Salas-Salvadó, J.; Estruch, R.; et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J. Am. Coll. Cardiol. 2020, 76, 2712–2724. [Google Scholar] [CrossRef]
- Langsted, A.; Madsen, C.M.; Nordestgaard, B.G. Contribution of remnant cholesterol to cardiovascular risk. J. Intern. Med. 2020, 288, 116–127. [Google Scholar] [CrossRef]
- Shao, Q.; Yang, Z.; Wang, Y.; Li, Q.; Han, K.; Liang, J.; Shen, H.; Liu, X.; Zhou, Y.; Ma, X.; et al. Elevated remnant cholesterol is associated with adverse cardiovascular outcomes in patients with acute coronary syndrome. J. Atheroscler. Thromb. 2022, 63397. [Google Scholar] [CrossRef]
- Jepsen, A.M.; Langsted, A.; Varbo, A.; Bang, L.E.; Kamstrup, P.R.; Nordestgaard, B.G. Increased remnant cholesterol explains part of residual risk of all-cause mortality in 5414 patients with ischemic heart disease. Clin. Chem. 2016, 62, 593–604. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Fayyad, R.; Boekholdt, S.M.; Hovingh, G.K.; Kastelein, J.J.; Melamed, S.; Barter, P.; Waters, D.D.; Ray, K.K. Triglyceride-rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT trial. Circulation 2018, 138, 770–781. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Wootton, R.; Lewis, B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Proctor, S.D.; Vine, D.F.; Mamo, J.C. Arterial retention of apolipoprotein B(48)- and B(100)-containing lipoproteins in atherogenesis. Curr. Opin. Lipidol. 2002, 13, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Smith, G.D.; Timpson, N.J.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Remnant cholesterol, low-density lipoprotein cholesterol, and blood pressure as mediators from obesity to ischemic heart disease. Circ. Res. 2015, 116, 665–673. [Google Scholar] [CrossRef]
- Soran, H.; Schofield, J.D.; Adam, S.; Durrington, P.N. Diabetic dyslipidaemia. Curr. Opin. Lipidol. 2016, 27, 313–322. [Google Scholar] [CrossRef]
| Characteristics | All (n = 94,896) | Male (n = 69,674) | Female (n = 25,222) | p Value |
|---|---|---|---|---|
| n (%) or Mean ± SD | n (%) or Mean ± SD | n (%) or Mean ± SD | ||
| Age, years | 63.3 ± 12.4 | 61.2 ± 12.4 | 69.0 ± 10.6 | <0.001 |
| Type of ACS | ||||
| STEMI | 40,297 (42.5) | 27,128 (38.9) | 13,169 (52.2) | <0.001 |
| NSTE-ACS | 54,599 (57.5) | 42,546 (61.1) | 12,053 (47.8) | |
| Glucose level, mmol/L | 6.9 ± 3.0 | 6.8 ± 2.8 | 7.2 ± 3.3 | <0.001 |
| TG, mmol/L * | 1.4 (1.0, 2.0) | 1.4 (1.0, 2.0) | 1.5 (1.1, 2.1) | <0.001 |
| LDL-C at admission, mmol/L | 2.7 ± 1.0 | 2.7 ± 0.9 | 2.8 ± 1.0 | |
| LDL-C < 1.4 mmol/L | 6022 (6.4) | 4560 (6.5) | 1462 (5.8) | <0.001 |
| 1.4 ≤ LDL-C < 1.8 mmol/L | 9312 (9.8) | 7081 (10.2) | 2231 (8.9) | |
| 1.8 ≤ LDL-C < 2.6 mmol/L | 30,980 (32.7) | 23,316 (33.5) | 7664 (30.4) | |
| LDL-C ≥ 2.6 mmo/L | 48,582 (51.2) | 34,717 (49.8) | 13,865 (55.0) | |
| non-HDL-C at admission (mmol/L) | 3.4 ± 1.1 | 3.4 ± 1.1 | 3.6 ± 1.2 | |
| non-HDL-C < 2.6 mmol/L | 22,116 (23.3) | 16,920 (24.3) | 5196 (20.6) | <0.001 |
| 2.6 ≤ non-HDL-C < 3.4 mmol/L | 28,120 (29.6) | 21,231 (30.5) | 6889 (27.3) | |
| 3.4 ≤ non-HDL-C < 4.1 mmo/L | 21,756 (22.9) | 15,752 (22.6) | 6004 (23.8) | |
| non-HDL-C ≥ 4.1 mmo/L | 22,904 (24.1) | 15,771 (22.6) | 7133 (28.3) | |
| HDL-C at admission, mmol/L | 1.1 ± 0.3 | 1.0 ± 0.3 | 1.2 ± 0.3 | <0.001 |
| RC at admission, mmol/L * | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.9) | 0.6 (0.4, 0.9) | |
| RC ≥ 1.0 mmol/L | 18,221 (19.2) | 12,799 (18.4) | 5422 (21.5) | <0.001 |
| Smoking | 38,265 (40.3) | 36,301 (52.1) | 1964 (7.8) | <0.001 |
| BMI (kg/m2) # | 24.4 ± 3.4 | 24.5 ± 3.3 | 24.0 ± 3.6 | <0.001 |
| Comorbidities | ||||
| Hypertension | 63,689 (67.1) | 44,819 (64.3) | 18,870 (74.8) | <0.001 |
| Diabetes | 41,201 (43.4) | 28,746 (41.3) | 12,455 (49.4) | <0.001 |
| Prior CVD | 26,011 (27.4) | 18,116 (26) | 7895 (31.3) | <0.001 |
| Prior heart failure | 2235 (2.4) | 1285 (1.8) | 950 (3.8) | <0.001 |
| Prior chronic renal failure | 1659 (1.8) | 1130 (1.6) | 529 (2.1) | <0.001 |
| Statin use in the past two weeks before admission | 16,730 (17.6) | 11,882 (17.1) | 4848 (19.2) | <0.001 |
| Proportion of Patients with Remnant Cholesterol Levels ≥ 1.0 mmol/L among Patients Reached Different Treatment Goals (%) | Proportion of Patients with LDL-C above the Target among Patients with Non-HDL-C < 2.6 mmol/L (%) | ||||||
|---|---|---|---|---|---|---|---|
| LDL-C < 1.4 mmol/L | 1.4 ≤ LDL-C < 1.8 mmol/L | Non-HDL-C < 2.6 mmol/L | LDL-C < 1.4 + Non-HDL-C < 2.6 mmol/L | 1.4 ≤ LDL-C <1.8 + Non-HDL-C < 2.6 mmol/L | LDL-C ≥ 1.4 mmol/L | LDL-C ≥ 1.8 mmol/L | |
| ALL | 24.4 | 13.3 | 2.9 | 10.9 | 1.2 | 76.9 | 41.0 |
| Type of ACS | |||||||
| STEMI | 27.0 | 14.1 | 2.7 | 11.4 | 1.2 | 80.0 | 44.8 |
| NSTE-ACS | 22.4 | 12.4 | 3.2 | 10.5 | 1.2 | 73.8 | 37.2 |
| Prior CVD | |||||||
| Yes | 17.5 | 11.2 | 2.9 | 8.7 | 1.2 | 72.5 | 35.4 |
| No | 29.5 | 14.5 | 3.0 | 12.6 | 1.2 | 79.6 | 44.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Wang, M.; Liu, J.; Liu, J.; Hao, Y.; Zhao, D.; on behalf of CCC-ACS Investigators. The Level of Remnant Cholesterol and Implications for Lipid-Lowering Strategy in Hospitalized Patients with Acute Coronary Syndrome in China: Findings from the Improving Care for Cardiovascular Disease in China—Acute Coronary Syndrome Project. Metabolites 2022, 12, 898. https://doi.org/10.3390/metabo12100898
Yang N, Wang M, Liu J, Liu J, Hao Y, Zhao D, on behalf of CCC-ACS Investigators. The Level of Remnant Cholesterol and Implications for Lipid-Lowering Strategy in Hospitalized Patients with Acute Coronary Syndrome in China: Findings from the Improving Care for Cardiovascular Disease in China—Acute Coronary Syndrome Project. Metabolites. 2022; 12(10):898. https://doi.org/10.3390/metabo12100898
Chicago/Turabian StyleYang, Na, Miao Wang, Jing Liu, Jun Liu, Yongchen Hao, Dong Zhao, and on behalf of CCC-ACS Investigators. 2022. "The Level of Remnant Cholesterol and Implications for Lipid-Lowering Strategy in Hospitalized Patients with Acute Coronary Syndrome in China: Findings from the Improving Care for Cardiovascular Disease in China—Acute Coronary Syndrome Project" Metabolites 12, no. 10: 898. https://doi.org/10.3390/metabo12100898
APA StyleYang, N., Wang, M., Liu, J., Liu, J., Hao, Y., Zhao, D., & on behalf of CCC-ACS Investigators. (2022). The Level of Remnant Cholesterol and Implications for Lipid-Lowering Strategy in Hospitalized Patients with Acute Coronary Syndrome in China: Findings from the Improving Care for Cardiovascular Disease in China—Acute Coronary Syndrome Project. Metabolites, 12(10), 898. https://doi.org/10.3390/metabo12100898







