Central Taurine Attenuates Hyperthermia and Isolation Stress Behaviors Augmented by Corticotropin-Releasing Factor with Modifying Brain Amino Acid Metabolism in Neonatal Chicks
Abstract
:1. Introduction
2. Results
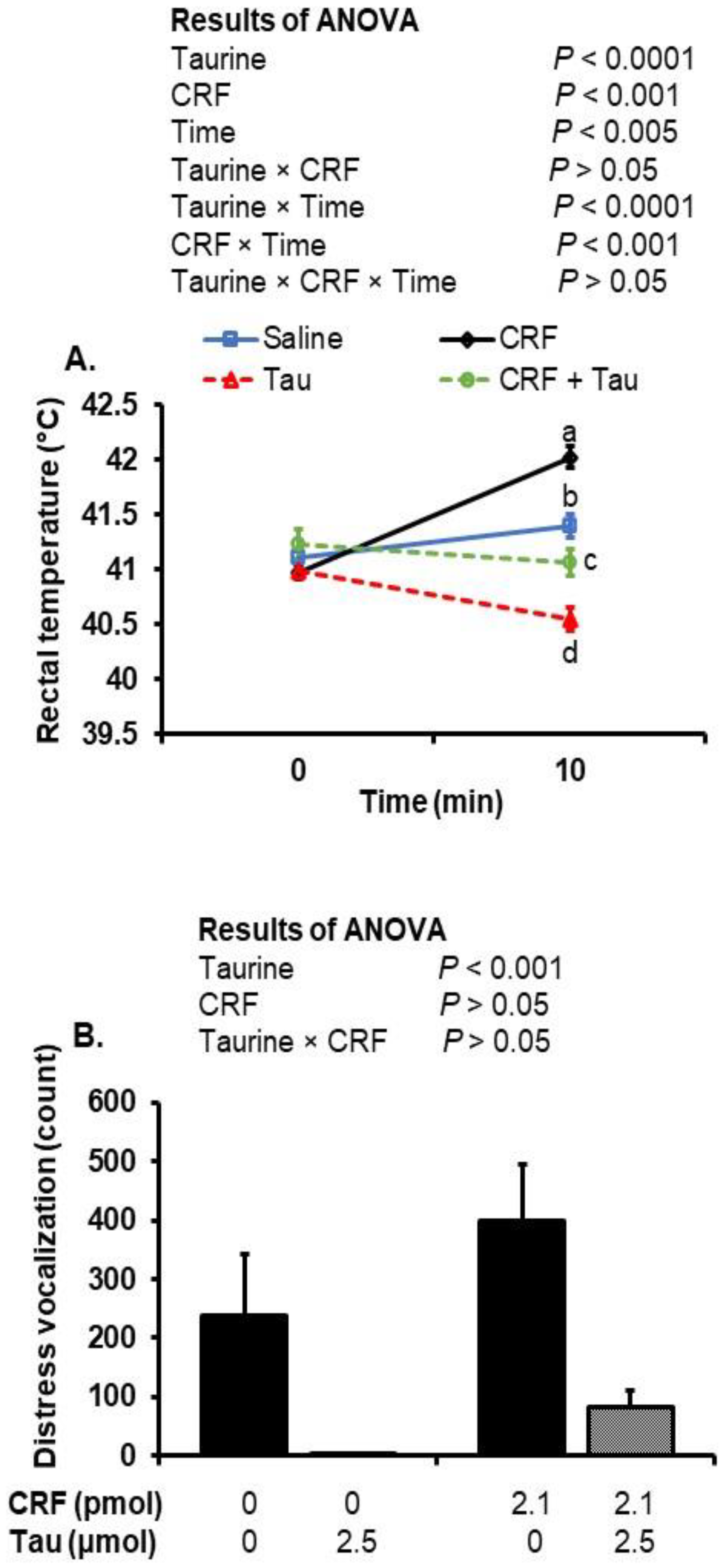
2.1. Changes in Rectal Temperature after Social Isolation and ICV Injection of Taurine and CRF
2.2. Changes in Distress Vocalizations after Social Isolation Stress and Injection of Taurine and CRF
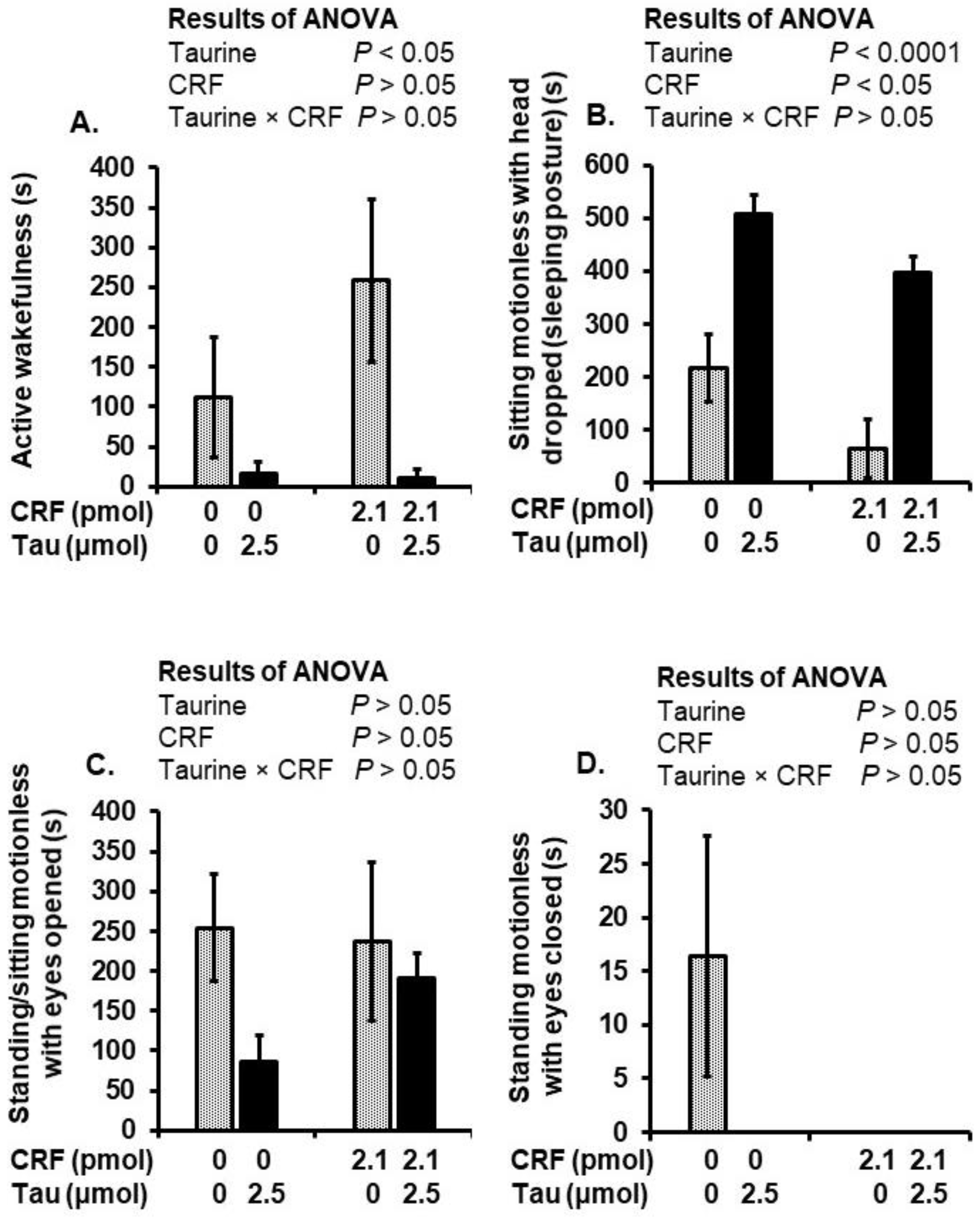
2.3. Behavioral Alterations after Social Isolation Stress and Injection of Taurine and CRF
2.4. Brain Amino Acid Changes after Social Isolation Stress and Injection of Taurine and CRF
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Injection Procedure
4.3. Experimental Design
4.4. Brain Amino Acid Analysis
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golla, A.; Østby, H.; Kermen, F. Chronic unpredictable stress induces anxiety-like behaviors in young zebrafish. Sci. Rep. 2020, 10, 10339. [Google Scholar] [CrossRef]
- Hwang, T.J.; Rabheru, K.; Peisah, C.; Reichman, W.; Ikeda, M. Loneliness, and social isolation during the COVID-19 pandemic. Int. Psychogeriatr. 2020, 32, 1217–1220. [Google Scholar] [CrossRef]
- Sokolowska, J.; Ayton, P.; Brandstätter, E. Editorial: Coronavirus Disease (COVID-19): Psychological Reactions to the Pandemic. Front. Psychol. 2021, 12, 745941. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M. Screening of central functions of amino acids and their metabolites for sedative and hypnotic effects using chick models. Eur. J. Pharmacol. 2015, 762, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Feltenstein, M.W.; Lambdin, L.C.; Ganzera, M.; Ranjith, H.; Dharmaratne, W.; Nanayakkara, N.P.; Khan, I.A.; Sufka, K.J. Anxiolytic properties of Piper methysticum extract samples and fractions in the chick social-separation-stress procedure. Phytother. Res. 2003, 17, 210–216. [Google Scholar] [CrossRef]
- Erwan, E.; Tomonaga, S.; Yoshida, J.; Nagasawa, M.; Ogino, Y.; Denbow, D.M.; Furuse, M. Central administration of L- and D-aspartate attenuates stress behaviors by social isolation and CRF in neonatal chicks. Amino Acids 2012, 43, 1969–1976. [Google Scholar] [CrossRef]
- Feltenstein, M.W.; Warnick, J.E.; Guth, A.N.; Sufka, K.J. The chick separation stress paradigm: A validation study. Pharmacol. Biochem. Behav. 2004, 77, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hamasu, K.; Haraguchi, T.; Kabuki, Y.; Adachi, N.; Tomonaga, S.; Sato, H.; Denbow, D.M.; Furuse, M. L-Proline is a sedative regulator of acute stress in the brain of neonatal chicks. Amino Acids 2009, 37, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Corticotropin-releasing factor, norepinephrine, and stress. Biol. Psychiatry 1999, 46, 1167–1180. [Google Scholar] [CrossRef]
- Wellman, L.L.; Yang, L.; Sanford, L.D. Effects of corticotropin releasing factor (CRF) on sleep and temperature following predictable controllable and uncontrollable stress in mice. Front. Neurosci. 2015, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Owens, M.J.; Nemeroff, C.B. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol. Rev. 1991, 43, 425–473. [Google Scholar] [PubMed]
- Furuse, M.; Matsumoto, M.; Saito, N.; Sugahara, K.; Hasegawa, S. The central corticotropin-releasing factor and glucagon-like peptide-1 in food intake of the neonatal chick. Eur. J. Pharmacol. 1997, 339, 211–214. [Google Scholar] [CrossRef]
- Arborelius, L.; Owens, M.J.; Plotsky, P.M.; Nemeroff, C.B. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999, 160, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Tachibana, T.; Takagi, T.; Koutoku, T.; Denbow, D.M.; Furuse, M. Centrally administered norepinephrine modifies the behavior induced by corticotrophin-releasing factor in neonatal chicks. J. Neurosci. Res. 2003, 74, 630–636. [Google Scholar] [CrossRef]
- Zhang, R.; Tachibana, T.; Takagi, T.; Koutoku, T.; Denbow, D.M.; Furuse, M. Serotonin modifies corticotrophin-releasing factor-induced behaviors of chicks. Behav. Brain Res. 2004, 151, 47–52. [Google Scholar] [CrossRef]
- Kurata, K.; Shigemi, K.; Tomonaga, S.; Aoki, M.; Morishita, K.; Denbow, D.M.; Furuse, M. L-Ornithine attenuates corticotropin-releasing factor-induced stress responses acting at GABAA receptors in neonatal chicks. Neuroscience 2011, 172, 226–231. [Google Scholar] [CrossRef]
- Nakamori, T.; Morimoto, A.; Murakami, N. Effect of a central CRF antagonist on cardiovascular and thermoregulatory responses induced by stress or IL-1 beta. Am. J. Physiol. 1993, 265, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Fabricio, A.S.; Rae, G.A.; Zampronio, A.R.; D’Orléans-Juste, P.; Souza, G.E. Central endothelin ET(B) receptors mediate IL-1-dependent fever induced by preformed pyrogenic factor and corticotropin-releasing factor in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R164–R171. [Google Scholar] [CrossRef] [Green Version]
- Richard, D.; Lin, Q.; Timofeeva, E. The corticotropin-releasing factor family of peptides and CRF receptors: Their roles in the regulation of energy balance. Eur. J. Pharmacol. 2002, 440, 189–197. [Google Scholar] [CrossRef]
- Ohgushi, A.; Bungo, T.; Shimojo, M.; Masuda, Y.; Denbow, D.M.; Furuse, M. Relationships between feeding and locomotion behaviors after central administration of CRF in chicks. Physiol. Behav. 2001, 72, 287–289. [Google Scholar] [CrossRef]
- Zhang, R.; Nakanishi, T.; Ohgushi, A.; Ando, R.; Yoshimatsu, T.; Denbow, D.M.; Furuse, M. Interaction of corticotropin-releasing factor and glucagon-like peptide-1 on behaviors in chicks. Eur. J. Pharmacol. 2001, 430, 73–78. [Google Scholar] [CrossRef]
- Blache, D.; Terlouw, C.; Maloney, S.K. Physiology. In Animal Welfare, 3rd ed.; Appleby, M.C., Olsson, I.A.S., Galindo, F., Eds.; CABI: Wallingford, UK, 2017; pp. 181–212. [Google Scholar]
- Kataoka, N.; Shima, Y.; Nakajima, K.; Nakamura, K. A central master driver of psychosocial stress responses in the rat. Science 2020, 367, 1105–1112. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Penning, R.; Hellhammer, J.; Verster, J.C.; Klaessens, J.H.; Olivier, B.; Kalkman, C.J. The effect of stress on core and peripheral body temperature in humans. Stress 2013, 16, 520–530. [Google Scholar] [CrossRef]
- Van der Heyden, J.A.; Zethof, T.J.; Olivier, B. Stress-induced hyperthermia in singly housed mice. Physiol. Behav. 1997, 62, 463–470. [Google Scholar] [CrossRef]
- Shigemi, K.; Tsuneyoshi, Y.; Hamasu, K.; Han, L.; Hayamizu, K.; Denbow, D.M.; Furuse, M. l-Serine induces sedative and hypnotic effects acting at GABA(A) receptors in neonatal chicks. Eur. J. Pharmacol. 2008, 599, 86–90. [Google Scholar] [CrossRef]
- Wu, J.Y.; Prentice, H. Role of taurine in the central nervous system. J. Biomed. Sci. 2010, 17, S1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffer, S.W.; Jong, C.J.; Ramila, K.C.; Azuma, J. Physiological roles of taurine in heart and muscle. J. Biomed. Sci. 2010, 17, S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhao, L.; Zhou, Y.; Lu, X.; Wang, Z.; Wang, J.; Li, W. Taurine ameliorated homocysteine induced H9C2 cardiomyocyte apoptosis by modulating endoplasmic reticulum stress. Apoptosis 2017, 22, 647–661. [Google Scholar] [CrossRef]
- Olive, M.F. Interactions between taurine and ethanol in the central nervous system. Amino Acids 2002, 23, 345–357. [Google Scholar] [CrossRef]
- Ochoa-de la Paz, L.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H. Taurine and GABA neurotransmitter receptors, a relationship with therapeutic potential. Expert Rev. Neurother. 2019, 19, 289–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhussiny, M.Z.; Tran, P.V.; Pham, C.V.; Nguyen, L.T.N.; Haraguchi, S.; Gilbert, E.R.; Cline, M.A.; Bungo, T.; Furuse, M.; Chowdhury, V.S. Central GABAA receptor mediates taurine-induced hypothermia and possibly reduces food intake in thermo-neutral chicks and regulates plasma metabolites in heat-exposed chicks. J. Therm. Biol. 2021, 98, 102905. [Google Scholar] [CrossRef]
- Chen, S.W.; Kong, W.X.; Zhang, Y.J.; Li, Y.L.; Mi, X.J.; Mu, X.S. Possible anxiolytic effects of taurine in the mouse elevated plus-maze. Life Sci. 2004, 75, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.X.; Chen, S.W.; Li, Y.L.; Zhang, Y.J.; Wang, R.; Min, L.; Mi, X. Effects of taurine on rat behaviors in three anxiety models. Pharmacol. Biochem. Behav. 2006, 83, 271–276. [Google Scholar] [CrossRef]
- Murakami, T.; Furuse, M. The impact of taurine- and beta-alanine-supplemented diets on behavioral and neurochemical parameters in mice: Antidepressant versus anxiolytic-like effects. Amino Acids 2010, 39, 427–434. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, 37–46. [Google Scholar] [CrossRef]
- Kataoka, N.; Hioki, H.; Kaneko, T.; Nakamura, K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 2014, 20, 346–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, B.A.S.; Zitnik, G.; Foster, C.; van Bockstaele, E.J.; Valentino, R.J. Social stress engages neurochemically-distinct afferents to the rat locus coeruleus depending on coping strategy. eNeuro 2015, 2, 4215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breton-Provencher, V.; Drummond, G.T.; Sur, M. Locus coeruleus norepinephrine in learned behavior: Anatomical modularity and spatiotemporal integration in targets. Front. Neural Circuits 2021, 15, 7. [Google Scholar] [CrossRef]
- Tjoumakaris, S.I.; Rudoy, C.; Peoples, J.; Valentino, R.J.; van Bockstaele, E.J. Cellular interactions between axon terminals containing endogenous opioid peptides or corticotropin-releasing factor in the rat locus coeruleus and surrounding dorsal pontine tegmentum. J. Comp. Neurol. 2003, 466, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Saito, E.S.; Saito, S.; Tomonaga, S.; Denbow, D.M.; Furuse, M. Comparison of brain arginine-vasotocin and corticotrophin-releasing factor for physiological responses in chicks. Neurosci. Lett. 2004, 360, 165–169. [Google Scholar] [CrossRef]
- Tachibana, T.; Takahashi, H.; Oikawa, D.; Denbow, D.M.; Furuse, M. Thyrotropin-releasing hormone increased heat production without the involvement of corticotropin-releasing factor in neonatal chicks. Pharmacol. Biochem. Behav. 2006, 83, 528–532. [Google Scholar] [CrossRef]
- Harris, W.S.; Lipton, J.M. Intracerebroventricular taurine in rabbits: Effects of normal body temperature, endotoxin fever and hyperthermia produced by PGE1 and amphetamine. J. Physiol. 1977, 266, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, J.; Tomonaga, S.; Ogino, Y.; Nagasawa, M.; Kurata, K.; Furuse, M. Intracerebroventricular injection of kynurenic acid attenuates corticotrophin-releasing hormone-augmented stress responses in neonatal chicks. Neuroscience 2012, 220, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Smith, Q.R. Glutamate, and glutamine in the brain transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr. 2000, 130, 1016. [Google Scholar] [CrossRef]
- Bai, W.; Zhou, Y.G. Homeostasis of the intraparenchymal-blood glutamate concentration gradient: Maintenance, imbalance, and regulation. Front. Mol. Neurosci. 2017, 10, 400. [Google Scholar] [CrossRef] [Green Version]
- Cederberg, H.H.; Uhd, N.C.; Brodin, B. Glutamate efflux at the blood–brain barrier: Cellular mechanisms and potential clinical relevance. Arch. Med. Res. 2014, 45, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Yudkoff, M.; Daikhin, Y.; Grunstein, L.; Nissim, I.; Stern, J.; Pleasure, D.; Nissim, I. Astrocyte leucine metabolism: Significance of branched-chain amino acid transamination. J. Neurochem. 1996, 66, 378–385. [Google Scholar] [CrossRef]
- Bixel, M.G.; Hutson, S.M.; Hamprecht, B. Cellular distribution of branched-chain amino acid aminotransferase isoenzymes among rat brain glial cells in culture. J. Histochem. Cytochem. 1997, 45, 685–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutson, S.M.; Lieth, E.; LaNoue, K.F. Function of leucine in excitatory neurotransmitter metabolism in the central nervous system. J. Nutr. 2001, 131, 846. [Google Scholar] [CrossRef] [Green Version]
- Izumi, T.; Kawamura, K.; Ueda, H.; Bungo, T. Central administration of leucine, but not isoleucine and valine, stimulates feeding behavior in neonatal chicks. Neurosci. Lett. 2004, 354, 166–168. [Google Scholar] [CrossRef]
- Wang, T.; Yao, W.; He, Q.; Shao, Y.; Zheng, R.; Huang, F. L-Leucine stimulates glutamate dehydrogenase activity and glutamate synthesis by regulating mTORC1/SIRT4 pathway in pig liver. Anim. Nutr. 2018, 4, 329–337. [Google Scholar] [CrossRef]
- Gupta, A. Metabolism of Proteins and Amino Acids. In Comprehensive Biochemistry for Dentistry; Springer: Singapore, 2019; pp. 377–393. [Google Scholar]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Yamane, H.; Suenaga, R.; Han, L.; Hayamizu, K.; Denbow, D.M.; Furuse, M. Intracerebroventricular injection of glutathione related dipeptides induce sedative and hypnotic effect under an acute stress in neonatal chicks. Lett. Drug Des. Discov. 2007, 4, 368–372. [Google Scholar] [CrossRef]
- Yamane, H.; Tomonaga, S.; Suenaga, R.; Denbow, D.M.; Furuse, M. Intracerebroventricular injection of glutathione and its derivative induces sedative and hypnotic effects under an acute stress in neonatal chicks. Neurosci. Lett. 2007, 418, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Tsuneyoshi, Y.; Denbow, D.; Furuse, M. N-Methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepro-pionate receptors involved in the induction of sedative effects under an acute stress in neonatal chicks. Amino Acids 2009, 37, 733–739. [Google Scholar] [CrossRef]
- Zhu, H.; Blake, S.; Chan, K.T.; Pearson, R.B.; Kang, J. Cystathionine β-Synthase in Physiology and Cancer. Biomed. Res. Int. 2018, 2018, 3205125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, P.V.; Tamura, Y.; Pham, C.V.; Elhussiny, M.Z.; Han, G.; Chowdhury, V.S.; Furuse, M. Neuropeptide Y modifies a part of diencephalic catecholamine but not indolamine metabolism in chicks depending on feeding status. Neuropeptides 2021, 89, 102169. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef] [PubMed]
- Parildar-Karpuzoğlu, H.; Doğru-Abbasoğlu, S.; Balkan, J.; Aykaç-Toker, G.; Uysal, M. Decreases in taurine levels induced by beta-alanine treatment did not affect the susceptibility of tissues to lipid peroxidation. Amino Acids 2007, 32, 115–119. [Google Scholar] [CrossRef]
- Takeuchi, K.; Toyohara, H.; Sakaguchi, M. A hyperosmotic stress-induced mRNA of carp cell encodes Na(+)- and Cl(-)-dependent high affinity taurine transporter. Biochim. Biophys. Acta 2000, 1464, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Mujahid, A.; Furuse, M. Central administration of corticotropin-releasing factor induces thermogenesis by changes in mitochondrial bioenergetics in neonatal chicks. Neuroscience 2008, 155, 845–851. [Google Scholar] [CrossRef]
- Davis, J.L.; Masuoka, D.T.; Gerbrandt, L.K.; Cherkin, A. Autoradiographic distribution of L-proline in chicks after intracerebral injection. Physiol. Behav. 1979, 22, 693–695. [Google Scholar] [CrossRef]
- Tachibana, T.; Sato, M.; Oikawa, D.; Takahashi, H.; Boswell, T.; Furuse, M. Intracerebroventricular injection of neuropeptide Y modifies carbohydrate and lipid metabolism in chicks. Regul. Pept. 2006, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van Luijtelaar, E.L.; Van der Grinten, C.P.; Blokhuis, H.J.; Coenen, A.M. Sleep in the domestic hen (Gallus domesticus). Physiol. Behav. 1987, 41, 409–414. [Google Scholar] [CrossRef]
- Chowdhury, V.S.; Shigemura, A.; Erwan, E.; Ito, K.; Bahry, M.A.; Tran, P.V.; Furuse, M. Oral administration of L-citrulline, but not L-arginine or L-ornithine, acts as a hypothermic agent in chicks. J. Poult. Sci. 2015, 52, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Kuenzel, W.J.; Masson, M. A Stereotaxic Atlas of the Brain of the Chick (Gallus domesticus); The Johns Hopkins University Press: Baltimore, MD, USA, 1988. [Google Scholar]
- Boogers, I.; Plugge, W.; Stokkermans, Y.Q.; Duchateau, A.L. Ultra-performance liquid chromatographic analysis of amino acids in protein hydrolysates using an automated pre-column derivatisation method. J. Chromatogr. A 2008, 1189, 406–409. [Google Scholar] [CrossRef]
- Ito, K.; Bahry, M.A.; Hui, Y.; Furuse, M.; Chowdhury, V.S. Acute heat stress up-regulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acids in chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Pillai, K.S. Transformation of data and outliers. In A Handbook of Applied Statistics in Pharmacology; CRC Press, Taylor & Francis Group: New York, NY, USA, 2013; pp. 37–46. [Google Scholar]
| Free Amino Acids | Saline | Taurine | CRF | Taurine + CRF | p Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taurine | CRF | Taurine × CRF | |||||||||||||
| Essential amino acids | |||||||||||||||
| Leucine | 221 | ± | 6 | 198 | ± | 7 | 212 | ± | 7 | 208 | ± | 2 | p < 0.05 | NS | NS |
| Isoleucine | 115 | ± | 5 | 97 | ± | 4 | 113 | ± | 6 | 100 | ± | 4 | p < 0.005 | NS | NS |
| Nonessential amino acids | |||||||||||||||
| Taurine | 4183 | ± | 57 | 5863 | ± | 162 | 4232 | ± | 101 | 5276 | ± | 263 | p < 0.0001 | NS | NS |
| Tyrosine | 105 | ± | 5 | 101 | ± | 4 | 121 | ± | 7 | 102 | ± | 5 | p < 0.05 | NS | NS |
| Glutamic acid | 6650 | ± | 69 | 6308 | ± | 68 | 6592 | ± | 144 | 6246 | ± | 119 | p < 0.005 | NS | NS |
| Asparagine | 374 | ± | 7 | 349 | ± | 10 | 383 | ± | 7 | 360 | ± | 11 | p < 0.05 | NS | NS |
| Alanine | 893 | ± | 15 | 860 | ± | 21 | 897 | ± | 29 | 838 | ± | 9 | p < 0.05 | NS | NS |
| β-Alanine | 319 | ± | 16 | 298 | ± | 12 | 318 | ± | 8 | 286 | ± | 11 | p < 0.05 | NS | NS |
| Cystathionine | 16 | ± | 1 | 15 | ± | 1 | 16 | ± | 1 | 14 | ± | 1 | p < 0.05 | NS | NS |
| 3-Methylhistidine | 304 | ± | 32 | 277 | ± | 25 | 323 | ± | 21 | 238 | ± | 19 | p < 0.05 | NS | NS |
| Free Amino Acids | Saline | Taurine | CRF | Taurine + CRF | p Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taurine | CRF | Taurine × CRF | |||||||||||||
| Essential amino acids | |||||||||||||||
| Isoleucine | 93 | ± | 4 | 89 | ± | 2 | 95 | ± | 2 | 84 | ± | 4 | p < 0.05 | NS | NS |
| Phenylalanine | 88 | ± | 4 | 84 | ± | 6 | 95 | ± | 6 | 75 | ± | 2 | p < 0.05 | NS | NS |
| Glycine | 2814 | ± | 66 | 2955 | ± | 62 | 2953 | ± | 63 | 3067 | ± | 44 | p < 0.05 | p < 0.05 | NS |
| Nonessential amino acids | |||||||||||||||
| Taurine | 2686 | ± | 66 b | 3903 | ± | 253 a | 2982 | ± | 127 b | 3168 | ± | 90 b | p < 0.0005 | NS | p < 0.005 |
| Tyrosine | 86 | ± | 5 ab | 88 | ± | 4 ab | 107 | ± | 8 a | 81 | ± | 4 b | p < 0.05 | NS | p < 0.05 |
| Cysteine | 100 | ± | 5 | 88 | ± | 4 | 93 | ± | 5 | 86 | ± | 5 | p < 0.05 | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhussiny, M.Z.; Tran, P.V.; Tsuru, Y.; Haraguchi, S.; Gilbert, E.R.; Cline, M.A.; Bungo, T.; Furuse, M.; Chowdhury, V.S. Central Taurine Attenuates Hyperthermia and Isolation Stress Behaviors Augmented by Corticotropin-Releasing Factor with Modifying Brain Amino Acid Metabolism in Neonatal Chicks. Metabolites 2022, 12, 83. https://doi.org/10.3390/metabo12010083
Elhussiny MZ, Tran PV, Tsuru Y, Haraguchi S, Gilbert ER, Cline MA, Bungo T, Furuse M, Chowdhury VS. Central Taurine Attenuates Hyperthermia and Isolation Stress Behaviors Augmented by Corticotropin-Releasing Factor with Modifying Brain Amino Acid Metabolism in Neonatal Chicks. Metabolites. 2022; 12(1):83. https://doi.org/10.3390/metabo12010083
Chicago/Turabian StyleElhussiny, Mohamed Z., Phuong V. Tran, Yuriko Tsuru, Shogo Haraguchi, Elizabeth R. Gilbert, Mark A. Cline, Takashi Bungo, Mitsuhiro Furuse, and Vishwajit S. Chowdhury. 2022. "Central Taurine Attenuates Hyperthermia and Isolation Stress Behaviors Augmented by Corticotropin-Releasing Factor with Modifying Brain Amino Acid Metabolism in Neonatal Chicks" Metabolites 12, no. 1: 83. https://doi.org/10.3390/metabo12010083
APA StyleElhussiny, M. Z., Tran, P. V., Tsuru, Y., Haraguchi, S., Gilbert, E. R., Cline, M. A., Bungo, T., Furuse, M., & Chowdhury, V. S. (2022). Central Taurine Attenuates Hyperthermia and Isolation Stress Behaviors Augmented by Corticotropin-Releasing Factor with Modifying Brain Amino Acid Metabolism in Neonatal Chicks. Metabolites, 12(1), 83. https://doi.org/10.3390/metabo12010083






