Preliminary Observations on Skeletal Muscle Adaptation and Plasticity in Homer 2-/- Mice
Abstract
:1. Introduction
2. Results
2.1. SOL, EDL, and FDB Morphometry in WT and Homer 2-/- Mice
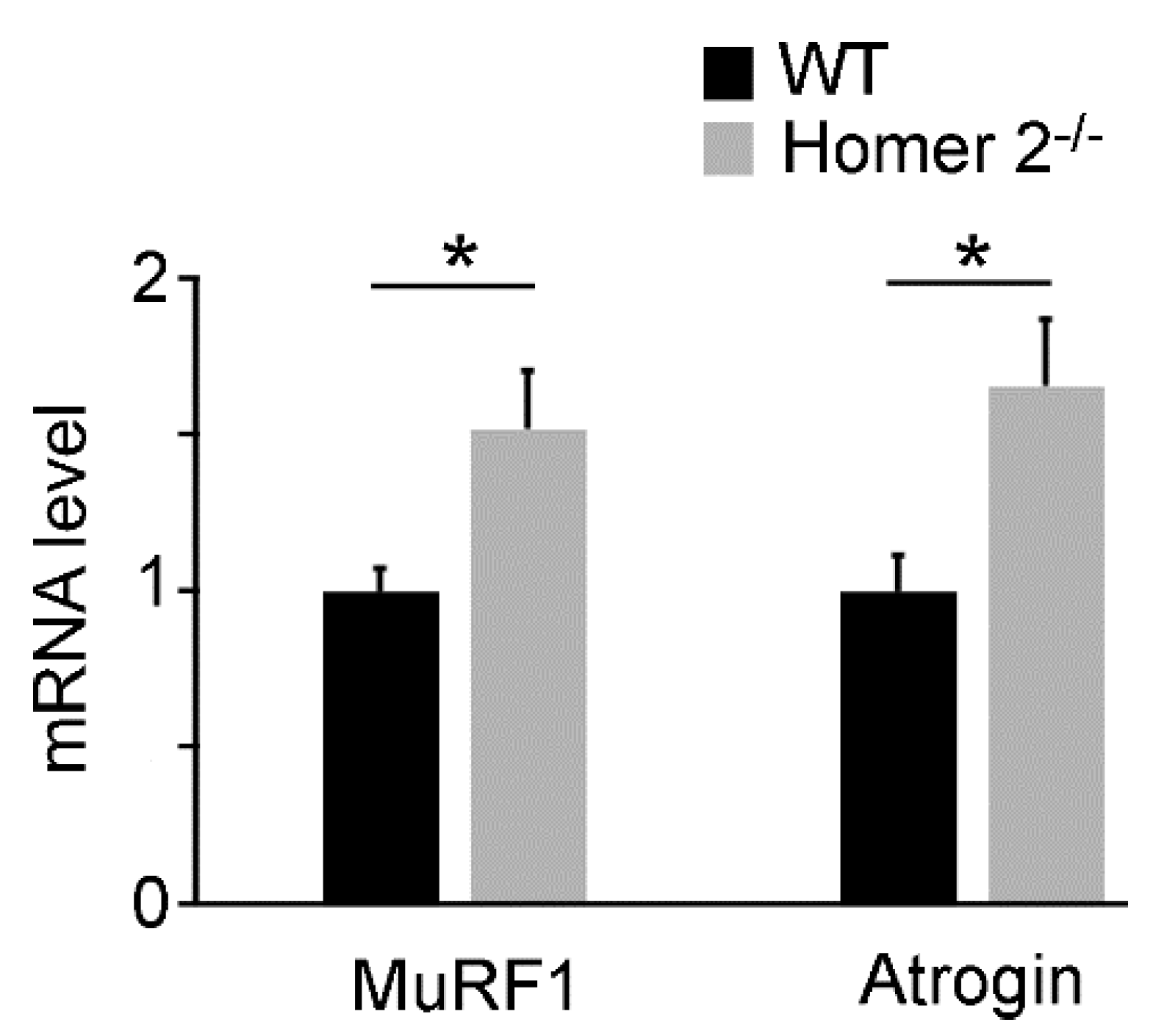
2.2. Quantitative RT-PCR Analysis of MuRF1 and Atrogin-1 Genes in SOL of WT and Homer 2-/- Mice
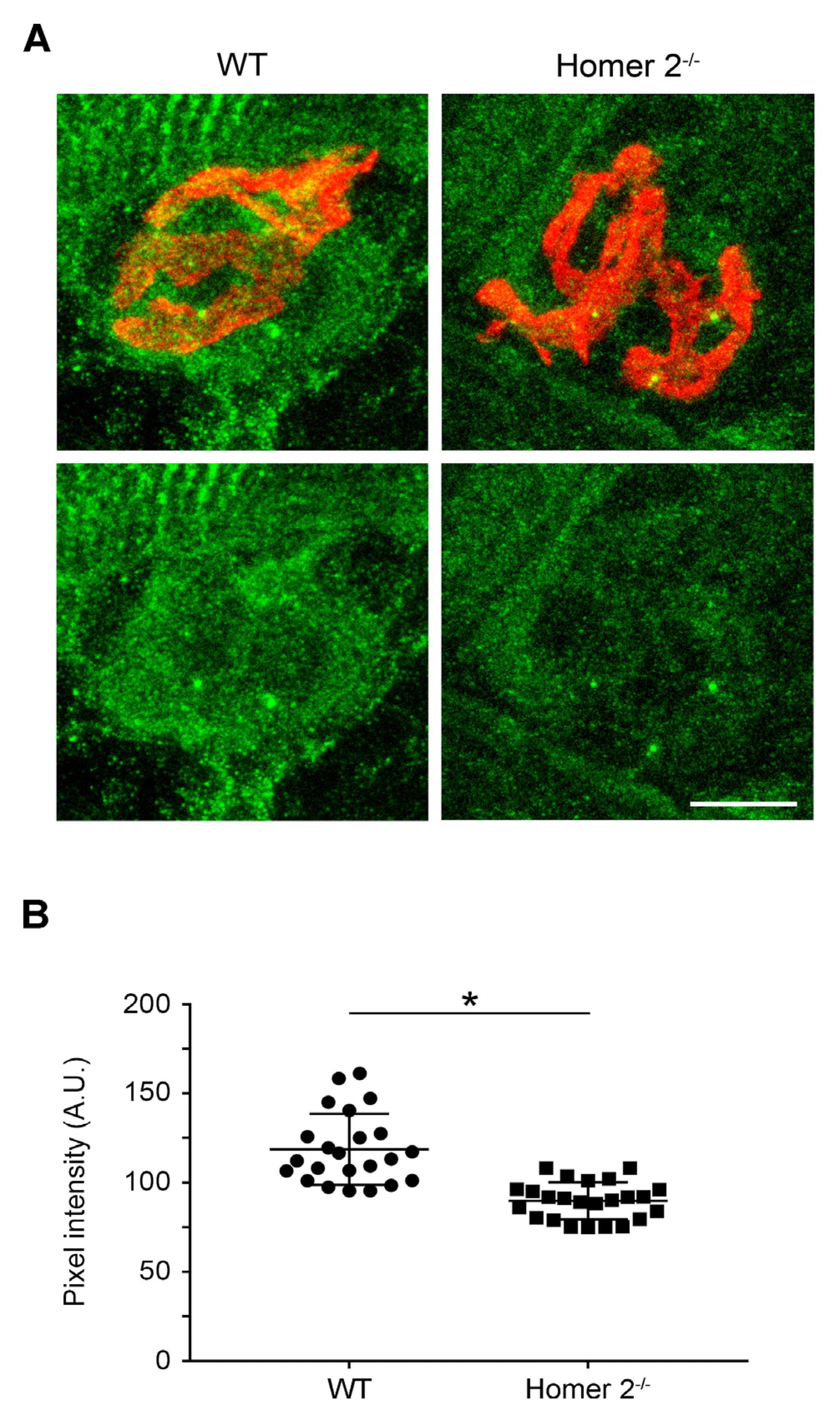
2.3. Homer Immunoreactivity in SOL from WT and Homer 2-/- Mice
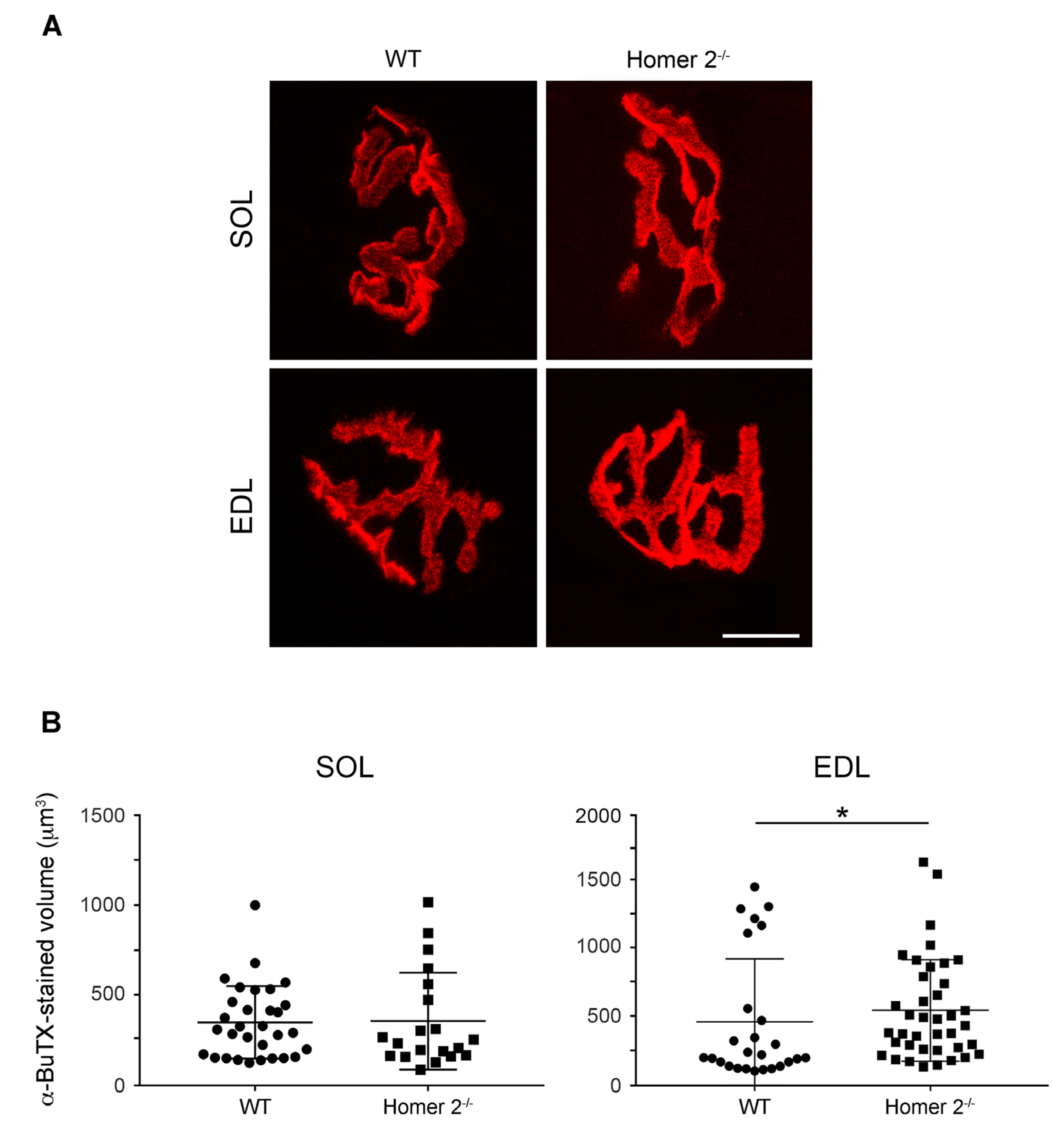
2.4. Endplate Volume in Intact SOL and EDL from WT and Homer 2-/- Mice
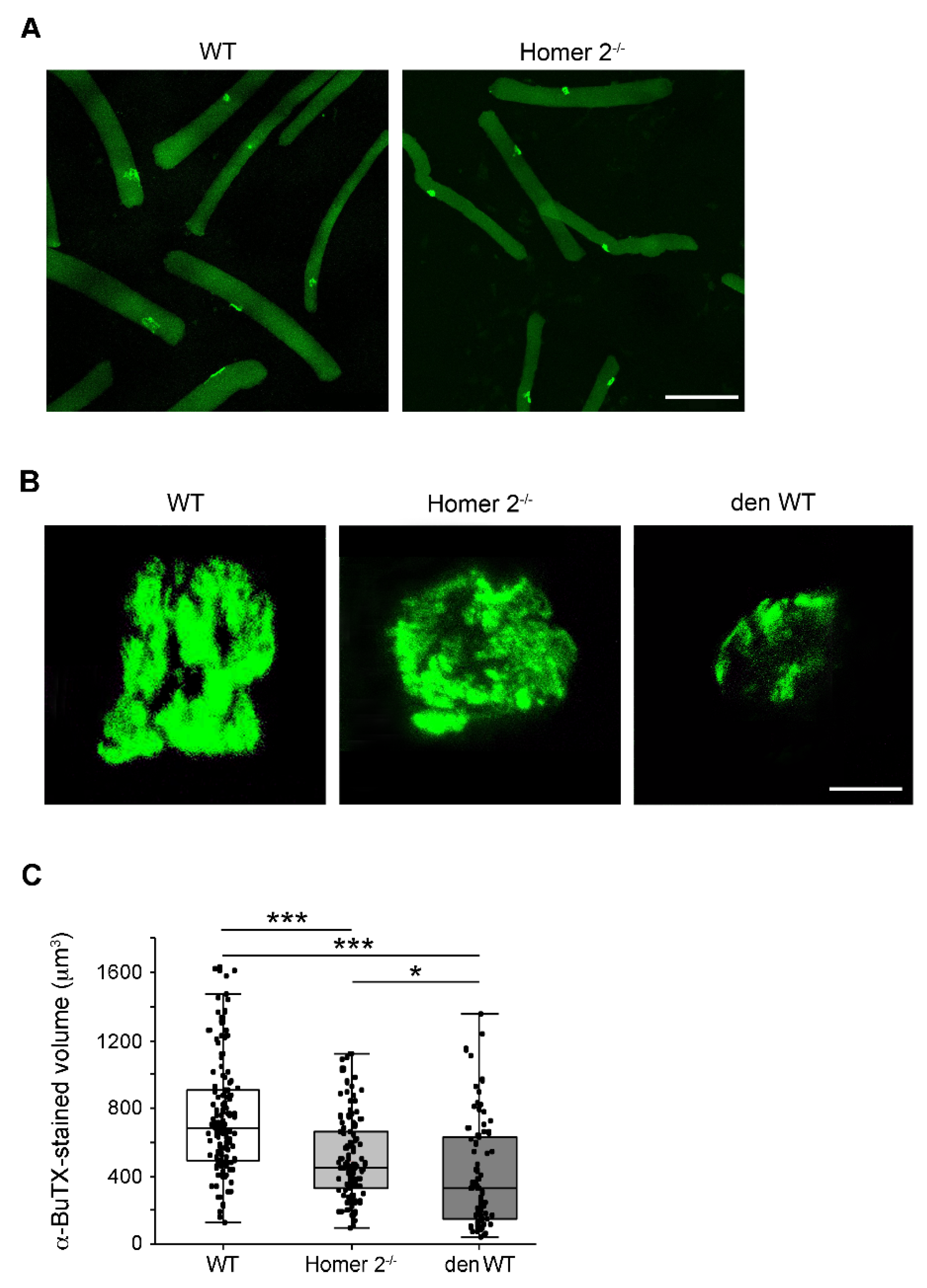
2.5. The Endplate Volume in Single FDB Fibres from WT and Homer 2-/- Mice
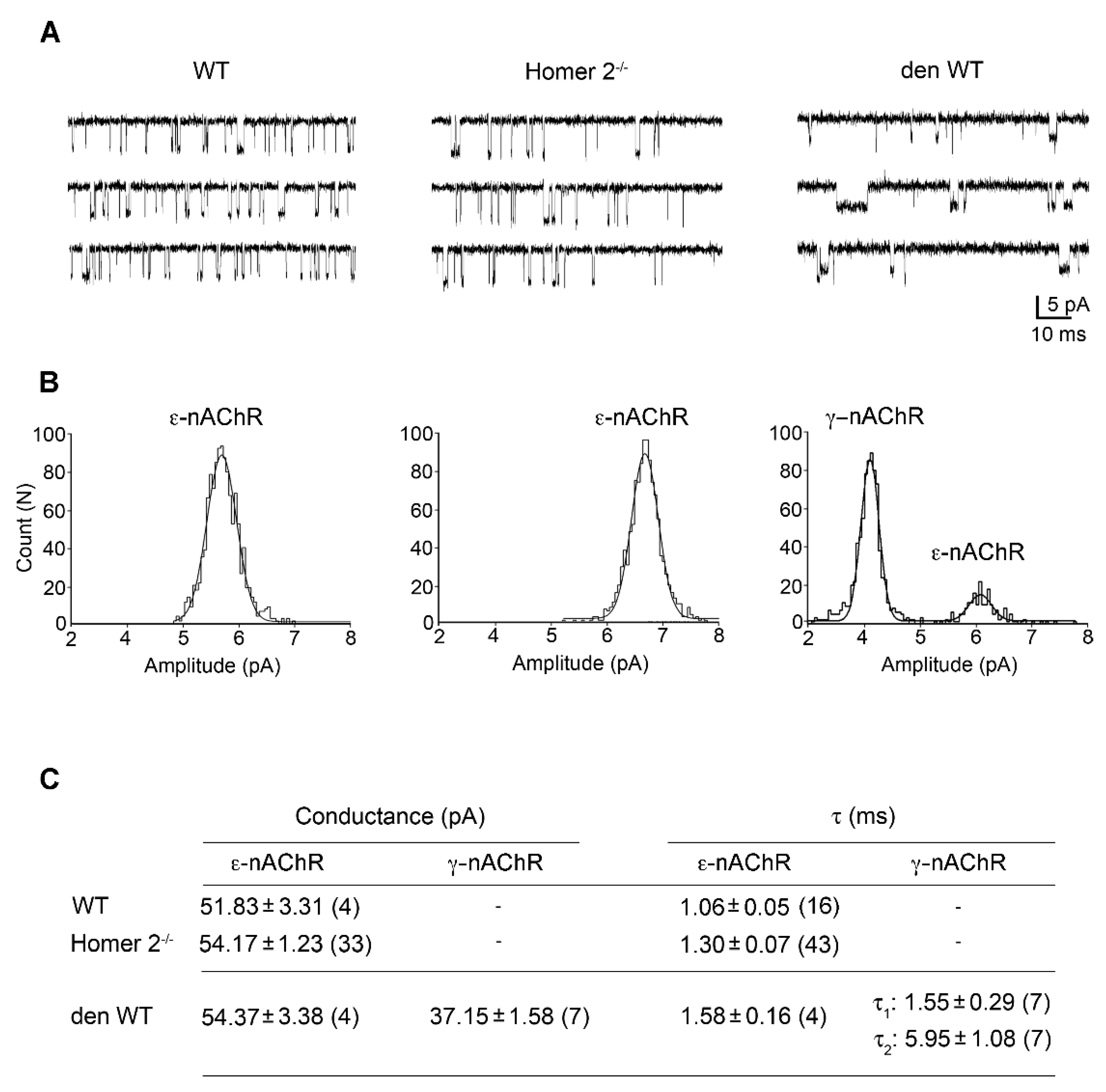
2.6. Patch Clamp Experiments in Single FDB Fibres from Homer 2-/- Mice
3. Discussion
4. Materials and Methods
4.1. Mice and Transgenic Mice
4.2. Morphometry
4.3. RNA Extraction and Quantitative RT-PCR
| MuRF-1 (Trim63) | Fw 5′-ACCTGCTGGTGGAAAACATC-3′ |
| Rv 5′-CTTCGTGTTCCTTGCACATC-3′ | |
| Atrogin-1 (Fbxo32) | Fw 5′-GCAAACACTGCCACATTCTCTC-3′ |
| Rv 5′-CTTGAGGGGAAAGTGAGACG-3′ | |
| Homer 1a | Fw 5′-GAAGTCGCAGGAGAAGATGG-3′ |
| Rv 5′-GAACTTCCATATTTATCCA-3′ | |
| Homer 1b/c | Fw 5′- GAAGTCGCAGGAGAAGATGG-3′ |
| Rv 5′-TAGCTCAGCCTCCCAGTGTT-3′ | |
| PPIA | Fw 5′-AGCATGTGGTCTTTGGGAAGGTG-3′ |
| Rv: 5′-CTTCTTGCTGGTCTTGCCATTCC-3′ | |
| TBP1 | Fw: 5′-TCAAACCCAGAATTGTTCTCC-3′ |
| Rv: 5′-AACTATGTGGTCTTCCTGAATCC-3′ | |
| HPRT1 | Fw: 5′-CTCATGGACTGATTATGGACAGGAC-3′ |
| Rv: 5′-GCAGGTCAGCAAAGAACTTATAGCC-3′ |
4.4. Staining and Image Analysis of SOL and EDL Muscles
4.5. Isolation of Muscles Fibres from FDB Muscles
4.6. nAChRs Staining and Image Analysis of Isolated FDB Fibres
4.7. nAChRs Single Channel Recordings in Isolated FDB Fibres
Author Contributions
Funding
Institutional Review Board Statement
Informed consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stibert, J.A.; Tabatabaei, N.; Howkins, A.F.; Hawke, T.; Worley, P.F.; Williams, R.S.; Rosenberg, P. Homer modulates NFAT-dependent signaling during muscle differentiation. Dev. Biol. 2005, 287, 213–224. [Google Scholar] [CrossRef]
- Stiber, J.A.; Zhang, Z.S.; Burch, J.; Eu, J.P.; Zhang, S.; Truskey, G.A.; Seth, M.; Yamaguchi, N.; Meissner, G.; Shah, R.; et al. Mice lacking Homer1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol. Cell Biol. 2008, 28, 2637–2647. [Google Scholar] [CrossRef] [Green Version]
- Bortoloso, E.; Pilati, N.; Megighian, A.; Tibaldo, E.; Sandonà, D.; Volpe, P. Transition of Homer isoforms during skeletal muscle rigeneration. Am. J. Physiol. Cell Physiol. 2006, 290, C711–C718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortoloso, E.; Megighian, A.; Furlan, S.; Gorza, L.; Volpe, P. Homer 2 antagonizes protein degradation in slow-twitch skeletal muscles. Am. J. Physiol. Cell Physiol. 2013, 304, C68–C77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salanova, M.; Volpe, P.; Blottner, D. Homer protein family regulation in skeletal muscle and neuromuscular adaptation. IUBMB Life 2013, 65, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Fagni, L.; Worley, P.F.; Ango, F. Homer as both a scaffold and transduction molecule. Sci. STKE 2002, 137, re8. [Google Scholar] [CrossRef]
- Duncan, R.S.; Hwang, S.Y.; Koulen, P. Effects of Vesl/Homer proteins on intracellular signaling. Exp. Biol. Med. 2005, 230, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Jardin, I.; López, J.J.; Berna-Erro, A.; Salido, G.M.; Rosado, J.A. Homer proteins in Ca2+ entry. IUBMB Life 2013, 65, 497–504. [Google Scholar] [CrossRef]
- Feng, W.; Tu, J.; Pouliquin, P.; Cabrales, E.; Shen, X.; Dulhunty, A.; Worley, P.F.; Allen, P.D.; Pessah, I.N. Dynamic regulation of ryanodine receptor type 1 (RyR1) channel activity by Homer 1. Cell Calcium 2008, 43, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Volpe, P.; Sandri, C.; Valle, G.; Nori, A.; Bortoloso, E. Topology of Homer 1a and Homer 1c in C2C12 myotubes and transgenic skeletal muscle fibers. Biochem. Biophys. Res. Commun. 2004, 316, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Kiselyov, K.; Shin, D.M.; Chen, J.; Shcheynikov, N.; Kang, S.H.; Dehoff, M.H.; Schwarz, M.K.; Seeburg, P.H.; Muallem, S.; et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 2003, 114, 777–789. [Google Scholar] [CrossRef] [Green Version]
- Salanova, M.; Bortoloso, E.; Schiffl, G.; Gutsmann, M.; Belavý, D.L.; Felsenberg, D.; Furlan, S.; Volpe, P.; Blottner, D. Expression and regulation of Homer in human skeletal muscle during neuromuscular junction adaptation to disuse and exercise. FASEB J. 2011, 25, 4312–4325. [Google Scholar] [CrossRef]
- Buonanno, A.; Fields, R.D. Gene regulation by patterned electrical activity during neural and skeletal muscle development. Curr. Opin. Neurobiol. 1999, 9, 110–120. [Google Scholar] [CrossRef]
- Sanes, J.R.; Lichtman, J.W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2001, 11, 791–805. [Google Scholar] [CrossRef]
- Lin, S.; Landmann, L.; Ruegg, M.A.; Brenner, H.R. The role of nerve- versus muscle-derived factors in mammalian neuromuscular junction formation. J. Neurosci. 2008, 28, 3333–3340. [Google Scholar] [CrossRef] [Green Version]
- Zayas, R.; Groshong, J.S.; Gomez, C.M. Inositol-1,4,5-triphosphate receptors mediate activity-induced synaptic Ca2+ signals in muscle fibers and Ca2+ overload in slow-channel syndrome. Cell Calcium 2007, 41, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bhattacharyya, B.J.; Lin, H.; Gomez, C.M. Skeletal muscle IP3R1 receptors amplify physiological and pathological synaptic calcium signals. J. Neurosci. 2011, 31, 15269–15283. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.M.; Dehoff, M.; Luo, X.; Hyeok Kang, S.; Tu, J.; Nayak, S.K.; Ross, E.M.; Worley, P.F.; Muallem, S. Homer 2 tunes G protein–coupled receptors stimulus intensity by regulating RGS proteins and PLCβ GAP activities. J. Cell Biol. 2003, 162, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Briguet, A.; Courdier-Fruh, I.; Foster, M.; Meier, T.; Magyar, J.P. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord. 2004, 14, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, Y.-Q.; Yuan, Y.-Y.; Xu, P.-W.; Zhang, C.; Fan, L.; Wang, L.-N.; Yin, C.; Zhang, L.; Cai, X.-C.; et al. Succinate induces skeletal muscle fiber remodeling via SUNCR1 signaling. EMBO Rep. 2019, 20, e47892. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.V.; Waddell, D.S.; Siu, R.; Stein, M.; Dewey, S.; Furlow, J.D.; Bodine, S.C. Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation. FASEB J. 2012, 26, 2986–2999. [Google Scholar] [CrossRef] [Green Version]
- Boehm, I.; Alhindi, A.; Leite, A.S.; Logie, C.; Gibbs, A.; Murray, O.; Farrukh, R.; Pirie, R.; Proudfoot, C.; Clutton, R.; et al. Comparative anatomy of the mammalian neuromuscular junction. J. Anat. 2020, 237, 827–836. [Google Scholar] [CrossRef]
- Tarpey, M.D.; Amorese, A.J.; Balestrieri, N.P.; Ryan, T.E.; Schmidt, C.A.; McClung, J.M.; Spangenburg, E.E. Characterization and utilization of the flexor digitorum brevis for assessing skeletal muscle function. Skelet. Muscle 2018, 8, 14. [Google Scholar] [CrossRef]
- Henderson, L.P.; Lechleiter, J.D.; Brehm, P. Single channel properties of newly synthesized acetylcholine receptors following denervation of mammalian skeletal muscle. J. Gen. Physiol. 1987, 89, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Chan, J.P.; Uong, J.; Palipsis, W.A.; Wright, D.J.; Shah, S.B.; Ward, S.R.; Lee, T.Q.; Steward, O. Human motor endplate remodeling after traumatic nerve injury. J. Neurosurg. 2020, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, J.; Bermedo-García, F.; Pérez, V.; Mella, J.; Hanna, P.; Herzberg, D.; Tejero, R.; López Manzaneda, M.; Tabares, L.; Henríquez, P. The Mouse Levator Auris Longus Muscle: An amenable model system to study the role of postsynaptic proteins to the maintenance and regeneration of the neuromuscular synapse. Front. Cell Neurosci. 2020, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Grohovaz, F.; Lorenzon, P.; Ruzzier, F.; Zorec, R. Properties of acetylcholine receptors in adult rat skeletal muscle fibers in culture. J. Membr. Biol. 1993, 136, 31–42. [Google Scholar] [CrossRef]
- Bernareggi, A.; Ren, E.; Giniatullin, A.; Luin, E.; Sciancalepore, M.; Giniatullin, R.; Lorenzon, P. Adenosine promotes endplate nAChR channel activity in adult mouse skeletal muscle fibers via low affinity P1 receptors. Neuroscience 2018, 383, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Pallafacchina, G.; Blaauw, B.; Schiaffino, S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr. Metab. Cardiovasc. Dis. 2013, 23, S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Gambara, G.; Salanova, M.; Ciciliot, S.; Furlan, S.; Gutsmann, M.; Schiffl, G.; Ungethuem, U.; Volpe, P.; Gunga, H.-C.; Blottner, D. Microgravity-induced transcriptome adaptation in mouse paraspinal longissimus dorsi muscle highlights insulin resistance-linked genes. Front. Physiol. 2017, 8, 279. [Google Scholar] [CrossRef] [Green Version]

| Muscle | WT | Homer 2-/- | Δ | p |
|---|---|---|---|---|
| SOL CSA (µm2) | 1779.00 ± 30.98 | 1353.00 ± 23.92 | −23.92% | <0.0001 |
| (924) | (622) | |||
| EDL CSA (µm2) | 1681.44 ± 31.22 | 1722.00 ± 40.63 | +2.41% | 0.4162 |
| (829) | (745) | |||
| FDB CSA (µm2) | 400.00 ± 9.57 | 414.50 ± 8.51 | +7.42% | 0.2606 |
| (670) | (950) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzon, P.; Furlan, S.; Ravara, B.; Bosutti, A.; Massaria, G.; Bernareggi, A.; Sciancalepore, M.; Trautmann, G.; Block, K.; Blottner, D.; et al. Preliminary Observations on Skeletal Muscle Adaptation and Plasticity in Homer 2-/- Mice. Metabolites 2021, 11, 642. https://doi.org/10.3390/metabo11090642
Lorenzon P, Furlan S, Ravara B, Bosutti A, Massaria G, Bernareggi A, Sciancalepore M, Trautmann G, Block K, Blottner D, et al. Preliminary Observations on Skeletal Muscle Adaptation and Plasticity in Homer 2-/- Mice. Metabolites. 2021; 11(9):642. https://doi.org/10.3390/metabo11090642
Chicago/Turabian StyleLorenzon, Paola, Sandra Furlan, Barbara Ravara, Alessandra Bosutti, Gabriele Massaria, Annalisa Bernareggi, Marina Sciancalepore, Gabor Trautmann, Katharina Block, Dieter Blottner, and et al. 2021. "Preliminary Observations on Skeletal Muscle Adaptation and Plasticity in Homer 2-/- Mice" Metabolites 11, no. 9: 642. https://doi.org/10.3390/metabo11090642
APA StyleLorenzon, P., Furlan, S., Ravara, B., Bosutti, A., Massaria, G., Bernareggi, A., Sciancalepore, M., Trautmann, G., Block, K., Blottner, D., Worley, P. F., Zampieri, S., Salanova, M., & Volpe, P. (2021). Preliminary Observations on Skeletal Muscle Adaptation and Plasticity in Homer 2-/- Mice. Metabolites, 11(9), 642. https://doi.org/10.3390/metabo11090642









