Thyroid Hormone Action in Muscle Atrophy
Abstract
1. Introduction
1.1. TH Action
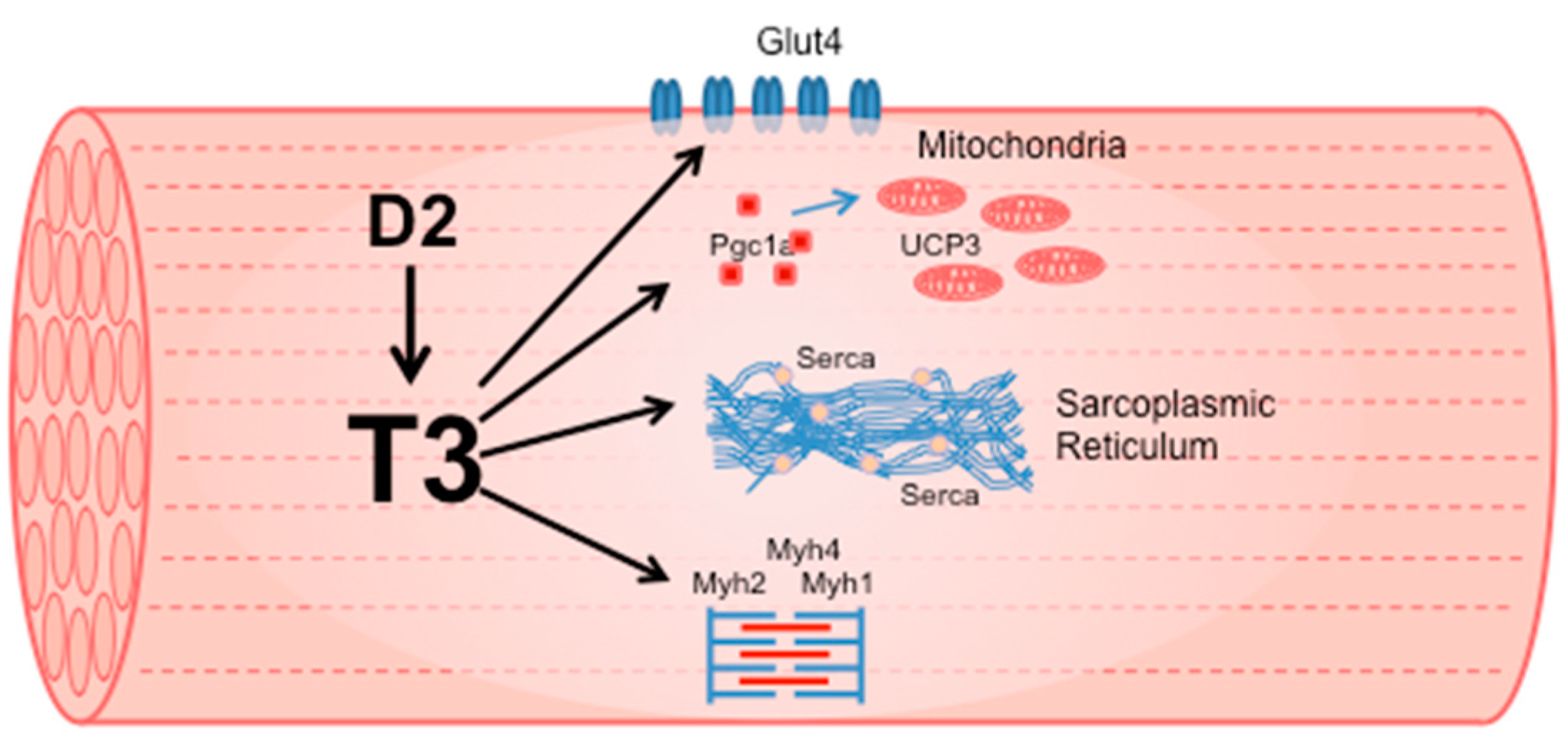
1.2. Effects of TH on Skeletal Muscle Physiology
1.3. Muscle Atrophy and Thyroid Dysfunction
1.4. Effects of TH on Skeletal Muscle Pathophysiology
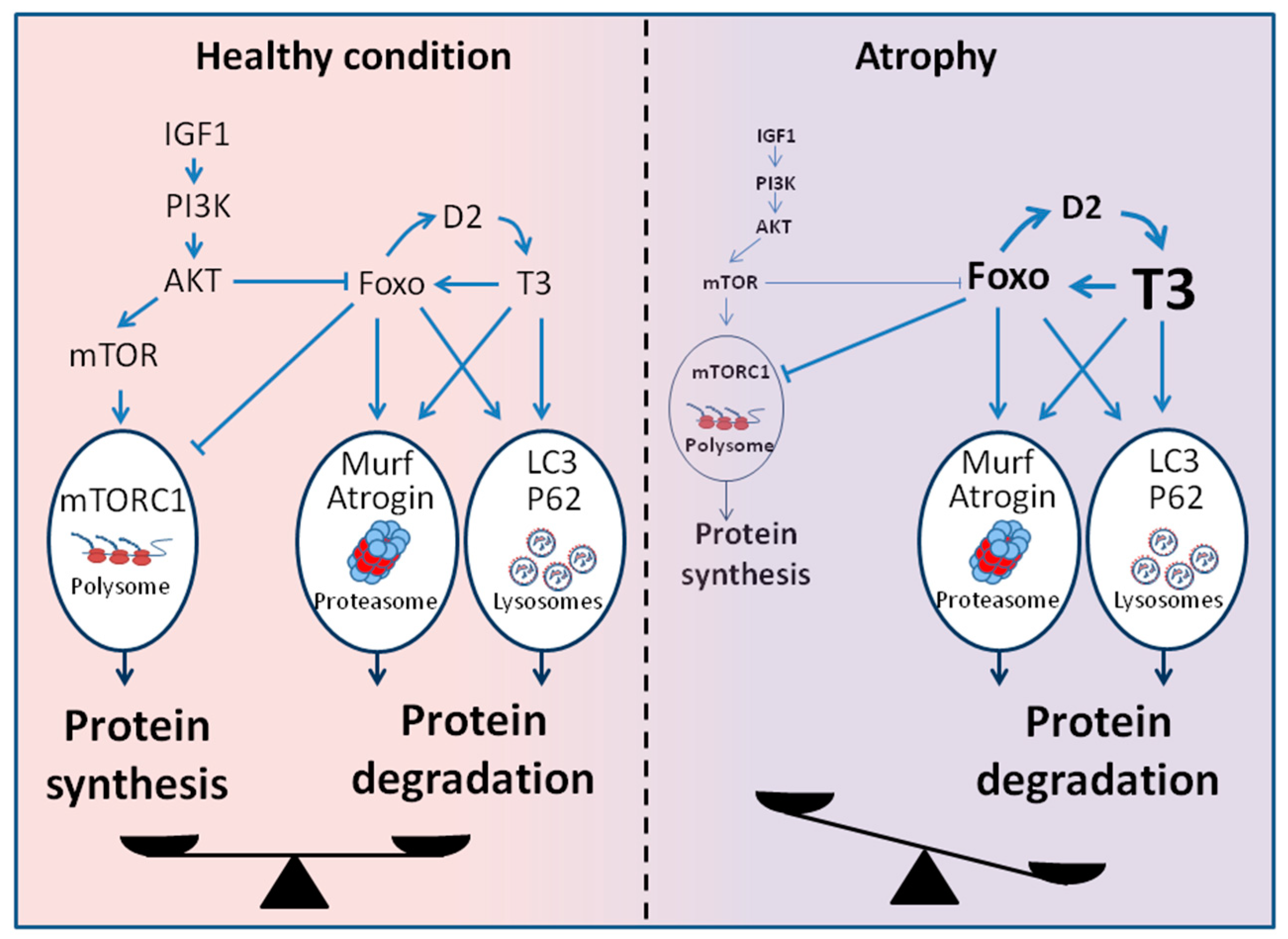
1.5. Common Pathways and Shared Molecular Mechanisms between TH and Muscle Wasting
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Simonides, W.S.; Dentice, M.; Zavacki, A.M.; Larsen, P.R. Thyroid hormones and skeletal muscle—New insights and potential implications. Nat. Rev. Endocrinol. 2014, 10, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Bloise, F.F.; Oliveira, T.S.; Cordeiro, A.; Ortiga-Carvalho, T.M. Thyroid hormones play role in sarcopenia and myopathies. Front. Physiol. 2018, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Duyff, R.F.; Van den Bosch, J.; Laman, D.M.; van Loon, B.J.; Linssen, W.H. Neuromuscular findings in thyroid dysfunction: A prospective clinical and electrodiagnostic study. J. Neurol. Neurosurg. Psychiatry 2000, 68, 750–755. [Google Scholar] [CrossRef]
- Khaleeli, A.A.; Edwards, R.H. Effect of treatment on skeletal muscle dysfunction in hypothyroidism. Clin. Sci. 1984, 66, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Models Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Luongo, C.; Dentice, M.; Salvatore, D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat. Rev. Endocrinol. 2019, 15, 479–488. [Google Scholar] [CrossRef]
- Silva, J.E.; Larsen, P.R. Pituitary nuclear 3,5,3′-triiodothyronine and thyrotropin secretion: An explanation for the effect of thyroxine. Science 1977, 198, 617–620. [Google Scholar] [CrossRef]
- Visser, W.E.; Friesema, E.C.; Visser, T.J. Minireview: Thyroid hormone transporters: The knowns and the unknowns. Mol. Endocrinol. 2011, 25, 1–14. [Google Scholar] [CrossRef]
- Friesema, E.C.; Ganguly, S.; Abdalla, A.; Manning Fox, J.E.; Halestrap, A.P.; Visser, T.J. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J. Biol. Chem. 2003, 278, 40128–40135. [Google Scholar] [CrossRef]
- Brent, G.A. The molecular basis of thyroid hormone action. N. Engl. J. Med. 1994, 331, 847–853. [Google Scholar] [CrossRef]
- Dentice, M.; Marsili, A.; Ambrosio, R.; Guardiola, O.; Sibilio, A.; Paik, J.H.; Minchiotti, G.; DePinho, R.A.; Fenzi, G.; Larsen, P.R.; et al. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J. Clin. Investig. 2010, 120, 4021–4030. [Google Scholar] [CrossRef] [PubMed]
- Dentice, M.; Ambrosio, R.; Damiano, V.; Sibilio, A.; Luongo, C.; Guardiola, O.; Yennek, S.; Zordan, P.; Minchiotti, G.; Colao, A.; et al. Intracellular inactivation of thyroid hormone is a survival mechanism for muscle stem cell proliferation and lineage progression. Cell Metab. 2014, 20, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Luongo, C.; Butruille, L.; Sebillot, A.; Le Blay, K.; Schwaninger, M.; Heuer, H.; Demeneix, B.A.; Remaud, S. Absence of both thyroid hormone transporters MCT8 and OATP1C1 impairs neural stem cell fate in the adult mouse subventricular zone. Stem Cell Rep. 2021, 16, 337–353. [Google Scholar] [CrossRef]
- Gothie, J.D.; Sebillot, A.; Luongo, C.; Legendre, M.; Nguyen Van, C.; Le Blay, K.; Perret-Jeanneret, M.; Remaud, S.; Demeneix, B.A. Adult neural stem cell fate is determined by thyroid hormone activation of mitochondrial metabolism. Mol. Metab. 2017, 6, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Vancamp, P.; Gothie, J.D.; Luongo, C.; Sebillot, A.; Le Blay, K.; Butruille, L.; Pagnin, M.; Richardson, S.J.; Demeneix, B.A.; Remaud, S. Gender-specific effects of transthyretin on neural stem cell fate in the subventricular zone of the adult mouse. Sci. Rep. 2019, 9, 19689. [Google Scholar] [CrossRef] [PubMed]
- Carmody, C.; Ogawa-Wong, A.N.; Martin, C.; Luongo, C.; Zuidwijk, M.; Sager, B.; Petersen, T.; Roginski Guetter, A.; Janssen, R.; Wu, E.Y.; et al. A global loss of Dio2 leads to unexpected changes in function and fiber types of slow skeletal muscle in male mice. Endocrinology 2019, 160, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Martinez, M.E.; Fiering, S.; Galton, V.A.; St Germain, D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J. Clin. Investig. 2006, 116, 476–484. [Google Scholar] [CrossRef]
- Yu, F.; Gothe, S.; Wikstrom, L.; Forrest, D.; Vennstrom, B.; Larsson, L. Effects of thyroid hormone receptor gene disruption on myosin isoform expression in mouse skeletal muscles. Am. J. Physiol. 2000, 278, R1545–R1554. [Google Scholar] [CrossRef]
- Mayerl, S.; Schmidt, M.; Doycheva, D.; Darras, V.M.; Huttner, S.S.; Boelen, A.; Visser, T.J.; Kaether, C.; Heuer, H.; von Maltzahn, J. Thyroid hormone transporters MCT8 and OATP1C1 control skeletal muscle regeneration. Stem Cell Rep. 2018, 10, 1959–1974. [Google Scholar] [CrossRef]
- Simonides, W.S.; van Hardeveld, C. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid 2008, 18, 205–216. [Google Scholar] [CrossRef]
- Janssen, J.W.; van Hardeveld, C.; Kassenaar, A.A. A methodological study in measuring T3 and T4 concentration in red and white skeletal muscle and plasma of euthyroid rats. Acta Endocrinol. 1979, 90, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Marsili, A.; Ramadan, W.; Harney, J.W.; Mulcahey, M.; Castroneves, L.A.; Goemann, I.M.; Wajner, S.M.; Huang, S.A.; Zavacki, A.M.; Maia, A.L.; et al. Type 2 iodothyronine deiodinase levels are higher in slow-twitch than fast-twitch mouse skeletal muscle and are increased in hypothyroidism. Endocrinology 2010, 151, 5952–5960. [Google Scholar] [CrossRef] [PubMed]
- Wulf, A.; Harneit, A.; Kroger, M.; Kebenko, M.; Wetzel, M.G.; Weitzel, J.M. T3-mediated expression of PGC-1alpha via a far upstream located thyroid hormone response element. Mol. Cell. Endocrinol. 2008, 287, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Sagliocchi, S.; Cicatiello, A.G.; Di Cicco, E.; Ambrosio, R.; Miro, C.; Di Girolamo, D.; Nappi, A.; Mancino, G.; De Stefano, M.A.; Luongo, C.; et al. The thyroid hormone activating enzyme, type 2 deiodinase, induces myogenic differentiation by regulating mitochondrial metabolism and reducing oxidative stress. Redox Biol. 2019, 24, 101228. [Google Scholar] [CrossRef]
- Cao, R.Y.; Li, J.; Dai, Q.; Li, Q.; Yang, J. Muscle atrophy: Present and Future. Adv. Exp. Med. Biol. 2018, 1088, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Lesmana, R.; Sinha, R.A.; Singh, B.K.; Zhou, J.; Ohba, K.; Wu, Y.; Yau, W.W.; Bay, B.H.; Yen, P.M. Thyroid hormone stimulation of autophagy is essential for mitochondrial biogenesis and activity in skeletal muscle. Endocrinology 2016, 157, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Zurcher, R.M.; Horber, F.F.; Grunig, B.E.; Frey, F.J. Effect of thyroid dysfunction on thigh muscle efficiency. J. Clin. Endocrinol. Metab. 1989, 69, 1082–1086. [Google Scholar] [CrossRef]
- Sheng, Y.; Ma, D.; Zhou, Q.; Wang, L.; Sun, M.; Wang, S.; Qi, H.; Liu, J.; Ding, G.; Duan, Y. Association of thyroid function with sarcopenia in elderly Chinese euthyroid subjects. Aging Clin. Exp. Res. 2019, 31, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, A.; Valentini, A.; Cianfarani, M.A.; Gasbarra, E.; Tarantino, U.; Federici, M. Low FT3: A possible marker of frailty in the elderly. Clin. Interv. Aging 2017, 12, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.A. Endocrin myopathies and toxic myopathies. In Neuromuscular Function and Disease. Basic, Clinical and Electrodiagnostic Aspects; Brown, W.F., Bolton, C.F., Aminoff, M.J., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 2002; Volume 2, pp. 1399–1402. [Google Scholar]
- Rurale, G.; Di Cicco, E.; Dentice, M.; Salvatore, D.; Persani, L.; Marelli, F.; Luongo, C. Thyroid hormone hyposensitivity: From genotype to phenotype and back. Front. Endocrinol. 2019, 10, 912. [Google Scholar] [CrossRef]
- Grounds, M.D.; Yablonka-Reuveni, Z. Molecular and cell biology of skeletal muscle regeneration. Mol. Cell Biol. Hum. Dis. 1993, 3, 210–256. [Google Scholar] [CrossRef]
- Hawke, T.J.; Garry, D.J. Myogenic satellite cells: Physiology to molecular biology. J. Appl. Physiol. 2001, 91, 534–551. [Google Scholar] [CrossRef] [PubMed]
- da Costa, V.M.; Moreira, D.G.; Rosenthal, D. Thyroid function and aging: Gender-related differences. J. Endocrinol. 2001, 171, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Edstrom, L.; Lindegren, B.; Gorza, L.; Schiaffino, S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. Am. J. Physiol. 1991, 261, C93–C101. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Biral, D.; Campione, M.; Schiaffino, S. An age-related type IIB to IIX myosin heavy chain switching in rat skeletal muscle. Acta Physiol. Scand. 1993, 147, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Bloise, F.F.; Cordeiro, A.; Ortiga-Carvalho, T.M. Role of thyroid hormone in skeletal muscle physiology. J. Endocrinol. 2018, 236, R57–R68. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Muller, U.; Li, X.; Schiaffino, S. Thyroid hormone regulation of myosin heavy chain isoform composition in young and old rats, with special reference to IIX myosin. Acta Physiol. Scand. 1995, 153, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.N.; Chen, C.C.; Hood, D.A. Mitochondria, muscle health, and exercise with advancing age. Physiology 2015, 30, 208–223. [Google Scholar] [CrossRef]
- Li, C.; White, S.H.; Warren, L.K.; Wohlgemuth, S.E. Effects of aging on mitochondrial function in skeletal muscle of American American Quarter Horses. J. Appl. Physiol. 2016, 121, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Casas, F.; Pessemesse, L.; Grandemange, S.; Seyer, P.; Baris, O.; Gueguen, N.; Ramonatxo, C.; Perrin, F.; Fouret, G.; Lepourry, L.; et al. Overexpression of the mitochondrial T3 receptor induces skeletal muscle atrophy during aging. PLoS ONE 2009, 4, e5631. [Google Scholar] [CrossRef]
- Khurana, T.S.; Davies, K.E. Pharmacological strategies for muscular dystrophy. Nat. Rev. Drug Discov. 2003, 2, 379–390. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, L.M.; Anderson, J.E. Hypothyroidism prolongs and increases mdx muscle precursor proliferation and delays myotube formation in normal and dystrophic limb muscle. Biochem. Cell Biol. 1995, 73, 181–190. [Google Scholar] [CrossRef] [PubMed]
- King, D.B.; Entrikin, R.K. Thyroidal involvement in the expression of avian muscular dystrophy. Life Sci. 1991, 48, 909–916. [Google Scholar] [CrossRef]
- Pernitsky, A.N.; McIntosh, L.M.; Anderson, J.E. Hyperthyroidism impairs early repair in normal but not dystrophic mdx mouse tibialis anterior muscle. An in vivo study. Biochem. Cell Biol. 1996, 74, 315–324. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, L.M.; Pernitsky, A.N.; Anderson, J.E. The effects of altered metabolism (hypothyroidism) on muscle repair in the mdx dystrophic mouse. Muscle Nerve 1994, 17, 444–453. [Google Scholar] [CrossRef]
- Tawa, N.E., Jr.; Odessey, R.; Goldberg, A.L. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J. Clin. Investig. 1997, 100, 197–203. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, P.; Alamdari, N.; Smith, I.; Poylin, V.; Menconi, M.; Hasselgren, P.O. Experimental hyperthyroidism in rats increases the expression of the ubiquitin ligases atrogin-1 and MuRF1 and stimulates multiple proteolytic pathways in skeletal muscle. J. Cell. Biochem. 2009, 108, 963–973. [Google Scholar] [CrossRef]
- Nakashima, K.; Ohtsuka, A.; Hayashi, K. Effects of thyroid hormones on myofibrillar proteolysis and activities of calpain, proteasome, and cathepsin in primary cultured chick muscle cells. J. Nutr. Sci. Vitaminol. 1998, 44, 799–807. [Google Scholar] [CrossRef][Green Version]
- Doi, J.; Ohtsubo, A.; Ohtsuka, A.; Hayashi, K. Triiodothyronine but not thyroxine accelerates myofibrillar proteolysis via ATP production in cultured muscle cells. Biosci. Biotechnol. Biochem. 2003, 67, 2451–2454. [Google Scholar] [CrossRef]
- DeMartino, G.N.; Goldberg, A.L. Thyroid hormones control lysosomal enzyme activities in liver and skeletal muscle. Proc. Natl. Acad. Sci. USA 1978, 75, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.C.; Tsai, C.Y.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J. Biomed. Sci. 2019, 26, 24. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Autophagy in skeletal muscle. FEBS Lett. 2010, 584, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef] [PubMed]
| Knockout | Muscular Phenotype | Reference |
|---|---|---|
| Dio2 | Changes in contractile function and fiber type composition. In contrast with local hypothyroidism, mice show increased fast characteristics of soleus and increased myofiber CSA, contraction rate, and fatigue resistance. | [18] |
| Dio3 (Global KO) | Neonatal thyrotoxicosis followed by central hypothyroidism, decrease in body weight, and partial perinatal lethality. | [19] |
| Dio3 (MuSC specific KO) | Massive apoptosis of MuSC, impairment of the initial phases of muscle regeneration, and delay of repair process. | [14] |
| Thra/Thrb, Thra, Thrb | Similar to the absence of thyroid hormone, fast to slow MHC isoform switching and decrease in body and muscle weights. | [20] |
| Slc16a2 (Mct8)/ OATP1C1 | Hyperthyroid state of skeletal muscle, shift from slow to fast fibers, muscle hypoplasia, and impaired muscle regeneration. | [21] |
| Slc16a2 (Mct8) | Hyperthyroid state of skeletal muscle, shift from slow to fast fibers, and impaired muscle regeneration. | [21] |
| Slc16a10 (Mct10) | Increased plasma and muscle and kidney aromatic amino acid. | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Stefano, M.A.; Ambrosio, R.; Porcelli, T.; Orlandino, G.; Salvatore, D.; Luongo, C. Thyroid Hormone Action in Muscle Atrophy. Metabolites 2021, 11, 730. https://doi.org/10.3390/metabo11110730
De Stefano MA, Ambrosio R, Porcelli T, Orlandino G, Salvatore D, Luongo C. Thyroid Hormone Action in Muscle Atrophy. Metabolites. 2021; 11(11):730. https://doi.org/10.3390/metabo11110730
Chicago/Turabian StyleDe Stefano, Maria Angela, Raffaele Ambrosio, Tommaso Porcelli, Gianfranco Orlandino, Domenico Salvatore, and Cristina Luongo. 2021. "Thyroid Hormone Action in Muscle Atrophy" Metabolites 11, no. 11: 730. https://doi.org/10.3390/metabo11110730
APA StyleDe Stefano, M. A., Ambrosio, R., Porcelli, T., Orlandino, G., Salvatore, D., & Luongo, C. (2021). Thyroid Hormone Action in Muscle Atrophy. Metabolites, 11(11), 730. https://doi.org/10.3390/metabo11110730







