Efficacy and Safety of Insulin Degludec/Insulin Aspart Compared with a Conventional Premixed Insulin or Basal Insulin: A Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Study Characteristics
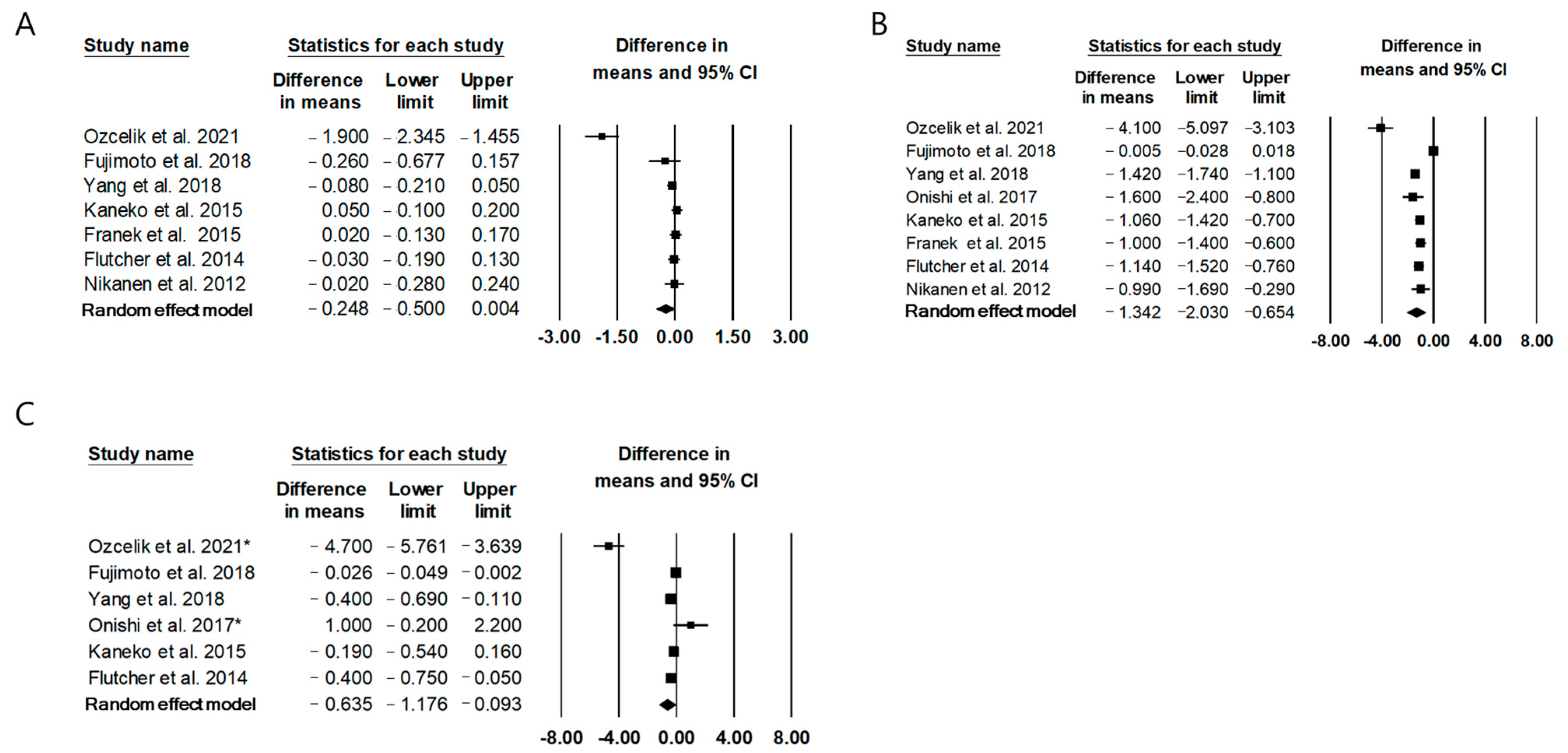
2.2. The Effect of Twice-Daily IDegAsp on Glycemic Control Compared to Conventional Premixed Insulin
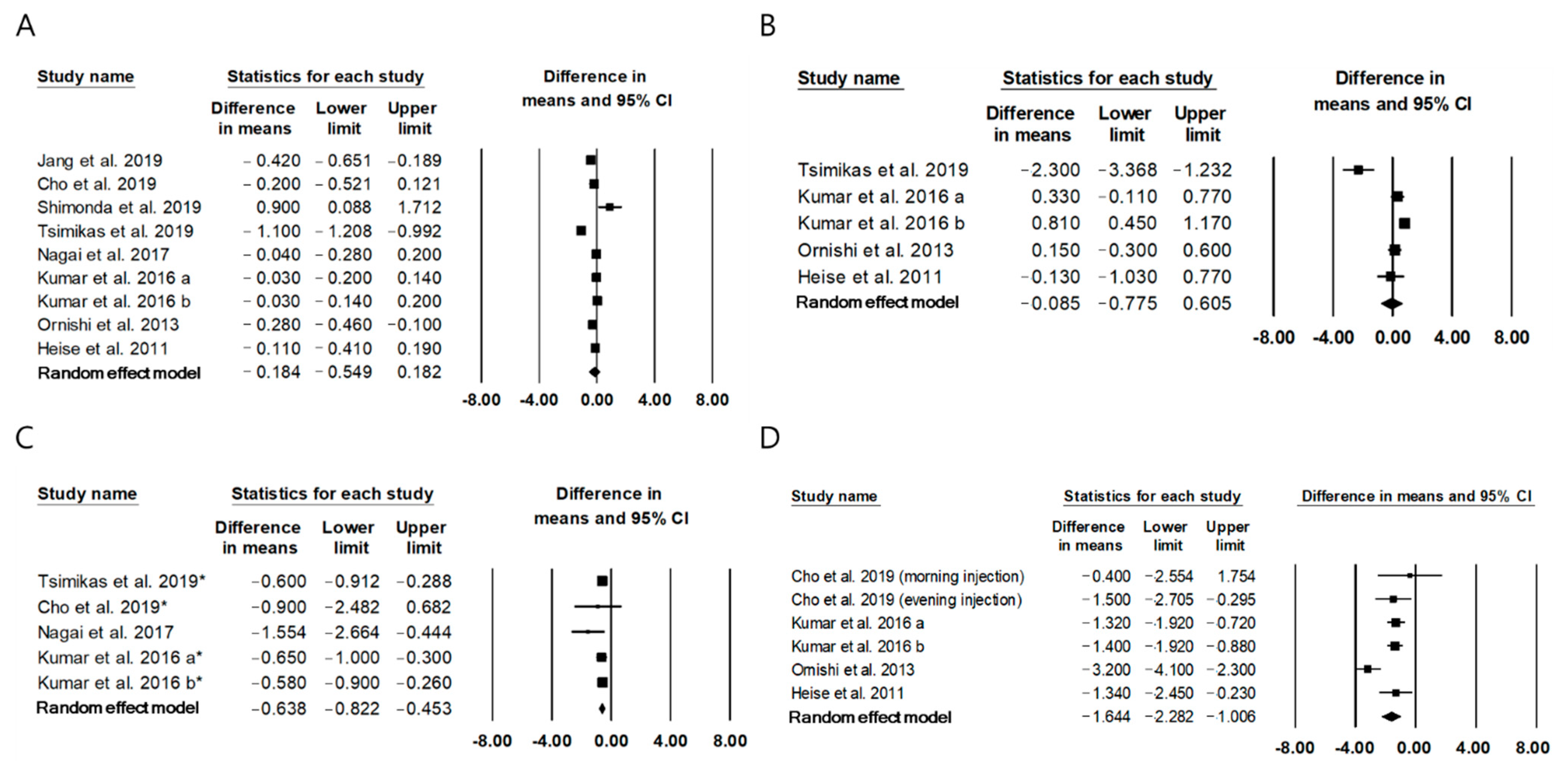
2.3. The Effect of Once-Daily IDegAsp on Glycemic Control Compared to Basal Insulin
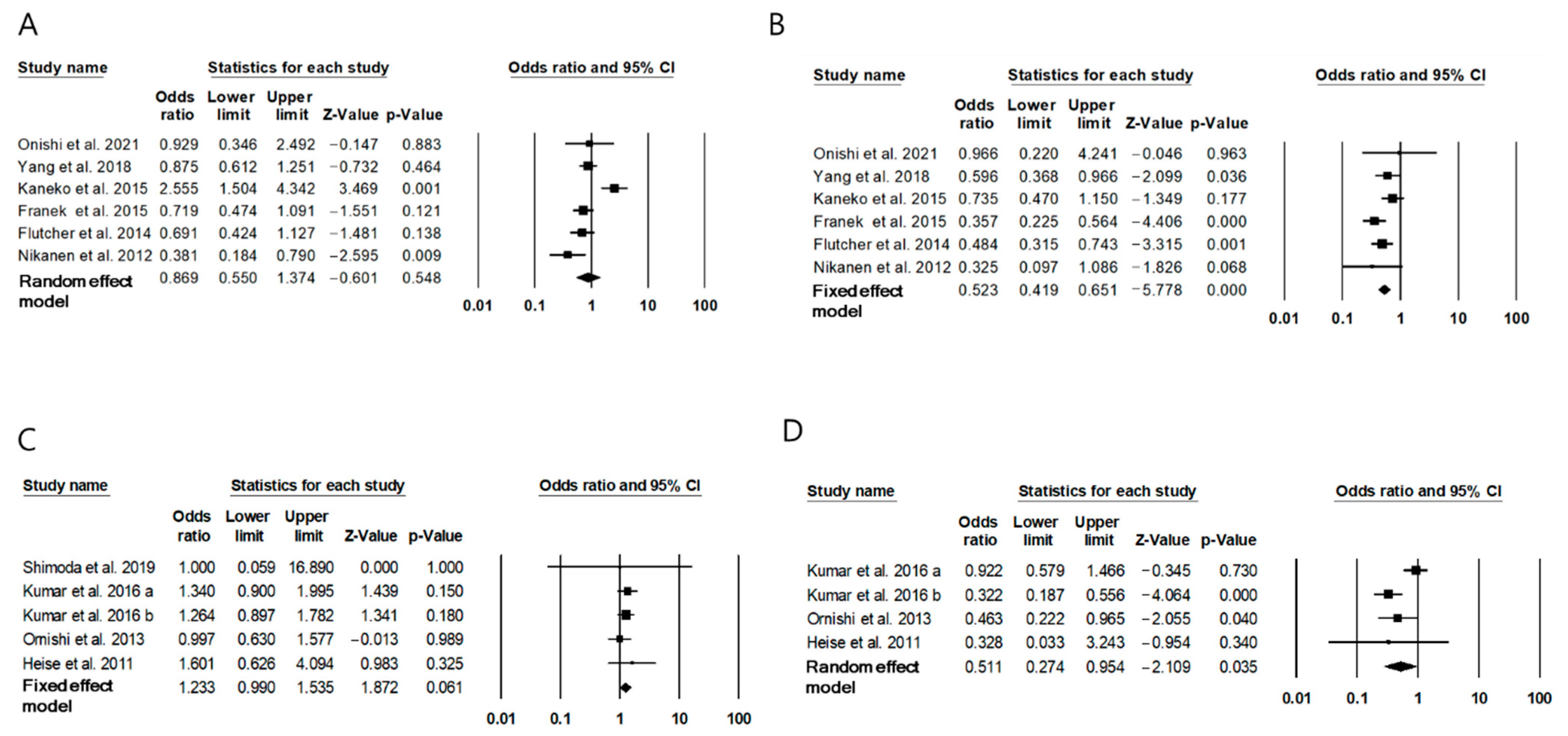
2.4. Risk of Hypoglycemia with IDegAsp
3. Discussion
4. Materials and Methods
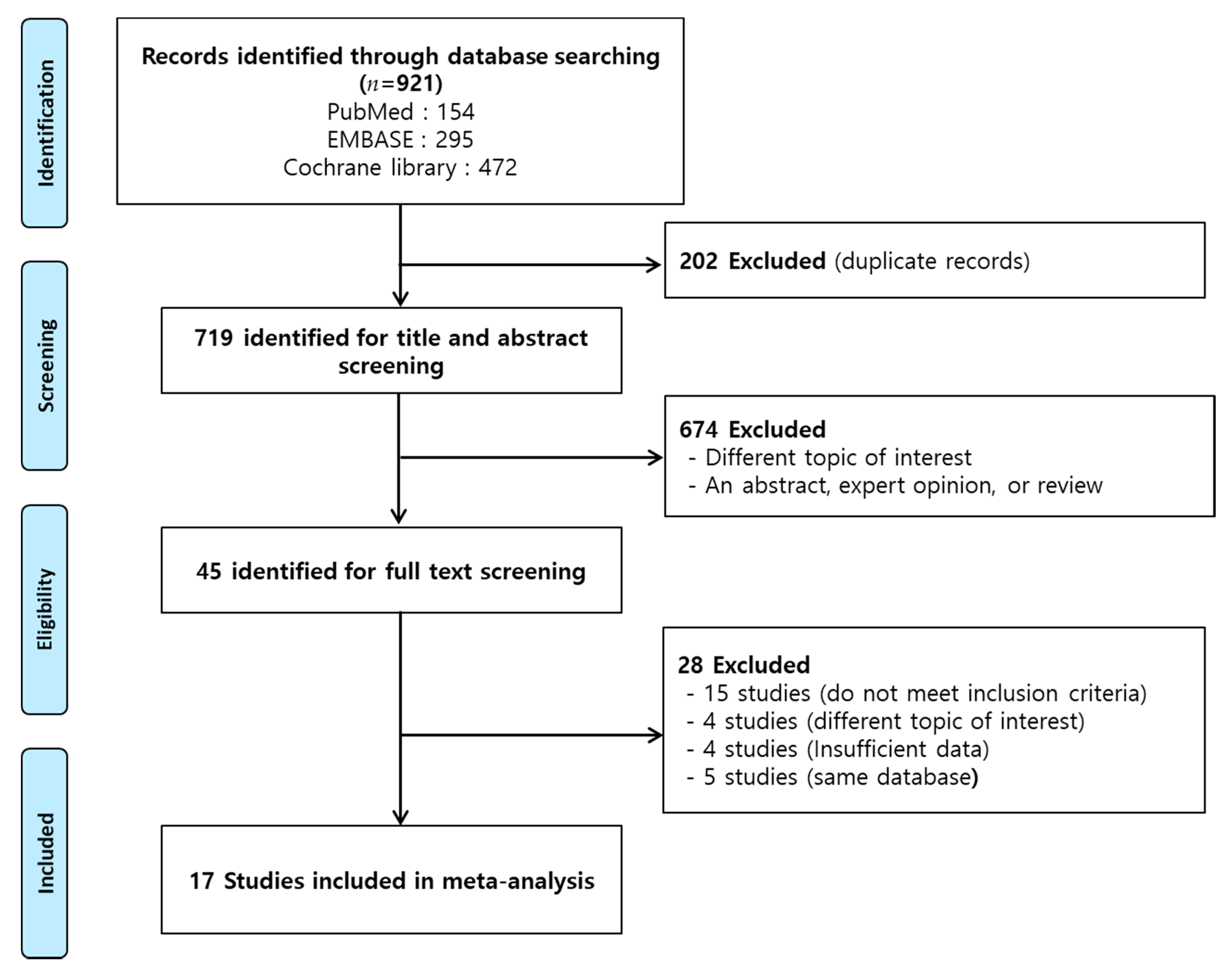
4.1. Search Strategy
4.2. Study Selection
- Population: patients with diabetes mellitus (DM) who used insulin injection.
- Intervention: once- or twice-daily injection of IDegAsp for 4 weeks or more.
- Comparators: patients with a conventional premixed insulin or basal insulin in RCTs or non-RCTs, or patients using a conventional premixed insulin or basal insulin before IDegAsp intervention in studies with a one-group pretest–posttest design.
- Outcomes: data on changes in HbA1C, FPG, self-measured mean glucose, or PPG and number of confirmed hypoglycemia events.
- Study design: clinical trials using IDegAsp.
- In vivo or in vitro experiments, only abstracts, and non-original articles, including expert opinions or reviews.
- Studies on type 1 DM.
- Studies involving participants who used multiple-dose insulin injection therapy or glucagon-like peptide-1 (GLP-1) agonist.
- Studies involving participants with diseases, such as cancer, that affected the outcomes.
4.3. Data Extraction
4.4. Quality Assessment
4.5. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellido, V.; Suarez, L.; Rodriguez, M.G.; Sanchez, C.; Dieguez, M.; Riestra, M.; Casal, F.; Delgado, E.; Menendez, E.; Umpierrez, G.E. Comparison of Basal-Bolus and Premixed Insulin Regimens in Hospitalized Patients with Type 2 Diabetes. Diabetes Care 2015, 38, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Badlani, S.; Ford, W.T.; David, J.Y.; Brogan, G.X.; Pollack, C.V.; Volturo, G.A.J.C.E.; Reports, H.M. Evidence for basal–bolus insulin versus slide scale insulin. Curr. Emerg. Hosp. Med. Rep. 2014, 2, 26–34. [Google Scholar] [CrossRef][Green Version]
- Wu, T.; Betty, B.; Downie, M.; Khanolkar, M.; Kilov, G.; Orr-Walker, B.; Senator, G.; Fulcher, G. Practical Guidance on the Use of Premix Insulin Analogs in Initiating, Intensifying, or Switching Insulin Regimens in Type 2 Diabetes. Diabetes Ther. 2015, 6, 273–287. [Google Scholar] [CrossRef]
- Elizarova, S.; Galstyan, G.; Wolffenbuttel, B.H. Role of premixed insulin analogues in the treatment of patients with type 2 diabetes mellitus: A narrative review. J. Diabetes 2014, 6, 100–110. [Google Scholar] [CrossRef]
- Christiansen, J.S.; Home, P.; Kumar, A. IDegAsp (insulin degludec + insulin aspart) for the management of type 2 diabetes: Current status. Expert Rev. Endocrinol. Metab. 2016, 11, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Haahr, H.; Fita, E.G.; Heise, T. A Review of Insulin Degludec/Insulin Aspart: Pharmacokinetic and Pharmacodynamic Properties and Their Implications in Clinical Use. Clin. Pharmacokinet. 2017, 56, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Glastras, S.J.; Cohen, N.; Dover, T.; Kilov, G.; MacIsaac, R.J.; McGill, M.; Fulcher, G.R. The Clinical Role of Insulin Degludec/Insulin Aspart in Type 2 Diabetes: An Empirical Perspective from Experience in Australia. J. Clin. Med. 2020, 9, 1091. [Google Scholar] [CrossRef]
- Fulcher, G.; Mehta, R.; Fita, E.G.; Ekelund, M.; Bain, S.C. Efficacy and Safety of IDegAsp versus BIAsp 30, Both Twice Daily, in Elderly Patients with Type 2 Diabetes: Post Hoc Analysis of Two Phase 3 Randomized Controlled BOOST Trials. Diabetes Ther. 2018, 10, 107–118. [Google Scholar] [CrossRef]
- Bode, B.W.; Iotova, V.; Kovarenko, M.; Laffel, L.M.; Rao, P.V.; Deenadayalan, S.; Ekelund, M.; Larsen, S.F.; Danne, T. Efficacy and Safety of Fast-Acting Insulin Aspart Compared with Insulin Aspart, Both in Combination with Insulin Degludec, in Children and Adolescents with Type 1 Diabetes: The onset 7 Trial. Diabetes Care 2019, 42, 1255–1262. [Google Scholar] [CrossRef]
- Shimoda, S.; Sakamoto, W.; Hokamura, A.; Matsuo, Y.; Sekigami, T.; Ichimori, S.; Iwashita, S.; Ishii, N.; Otsu, K.; Yoshimura, R.; et al. Comparison of the efficacy and safety of once-daily insulin degludec/insulin aspart (IDegAsp) and long-acting second-generation basal insulin (insulin degludec and insulin glargine 300 units/mL) in insulin-naïve Japanese adults with type 2 diabetes: A pilot, randomized, controlled study. Endocr. J. 2019, 66, 745–752. [Google Scholar] [CrossRef]
- Semlitsch, T.; Engler, J.; Siebenhofer, A.; Jeitler, K.; Berghold, A.; Horvath, K. (Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 11, CD005613. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.L.; Chaykin, L.B.; Jabbour, S.; Sheikh-Ali, M.; Hansen, C.T.; Nielsen, T.S.; Norwood, P. Insulin Degludec 200 Units/mL Is Associated with Lower Injection Frequency and Improved Patient-Reported Outcomes Compared with Insulin Glargine 100 Units/mL in Patients with Type 2 Diabetes Requiring High-Dose Insulin. Clin. Diabetes 2017, 35, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, S.; Çelik, M.; Vural, A.; Aydın, B.; Özçelik, M.; Gozu, H. Outcomes of transition from premixed and intensive insulin therapies to insulin aspart/degludec co-formulation in type 2 diabetes mellitus: A real-world experience. Arch. Med. Sci. 2021, 17, 1–8. [Google Scholar] [CrossRef]
- Fujimoto, K.; Iwakura, T.; Aburaya, M.; Matsuoka, N. Twice-daily insulin degludec/insulin aspart effectively improved morning and evening glucose levels and quality of life in patients previously treated with premixed insulin: An observational study. Diabetol. Metab. Syndr. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, J.; Hong, T.; Liu, M.; Miao, H.; Peng, Y.; Wang, C.; Xu, X.; Yang, T.; Nielsen, A.M.; et al. Efficacy and safety of insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Chinese adults with type 2 diabetes: A phase III, open-label, 2:1 randomized, treat-to-target trial. Diabetes Obes. Metab. 2019, 21, 1652–1660. [Google Scholar] [CrossRef]
- Onishi, Y.; Yamada, K.; Zacho, J.; Ekelund, J.; Iwamoto, Y. Insulin degludec/insulin aspart vs biphasic insulin aspart 30 twice daily in Japanese patients with type 2 diabetes: A randomized controlled trial. J. Diabetes Investig. 2016, 8, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Chow, F.; Choi, D.S.; Taneda, S.; Hirao, K.; Park, Y.; Andersen, T.H.; Gall, M.-A.; Christiansen, J.S. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre-/self-mixed insulin: A 26-week, randomised, treat-to-target trial. Diabetes Res. Clin. Pr. 2015, 107, 139–147. [Google Scholar] [CrossRef]
- Franek, E.; Haluzik, M.; Varžić, S.C.; Sargin, M.; Macura, S.; Zacho, J.; Christiansen, J.S. Twice-daily insulin degludec/insulin aspart provides superior fasting plasma glucose control and a reduced rate of hypoglycaemia compared with biphasic insulin aspart 30 in insulin-naïve adults with Type 2 diabetes. Diabet. Med. 2015, 33, 497–505. [Google Scholar] [CrossRef]
- Fulcher, G.R.; Christiansen, J.S.; Bantwal, G.; Polaszewska-Muszynska, M.; Mersebach, H.; Andersen, T.H.; Niskanen, L.K. Comparison of Insulin Degludec/Insulin Aspart and Biphasic Insulin Aspart 30 in Uncontrolled, Insulin-Treated Type 2 Diabetes: A Phase 3a, Randomized, Treat-to-Target Trial. Diabetes Care 2014, 37, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, L.; Leiter, L.A.; Franek, E.; Weng, J.; Damci, T.; Munoz-Torres, M.; Donnet, J.-P.; Endahl, L.; Skjøth, T.V.; Vaag, A. Comparison of a soluble co-formulation of insulin degludec/insulin aspart vs biphasic insulin aspart 30 in type 2 diabetes: A randomised trial. Eur. J. Endocrinol. 2012, 167, 287. [Google Scholar] [CrossRef] [PubMed]
- Na Jang, H.; Yang, Y.S.; Lee, S.O.; Oh, T.J.; Koo, B.K.; Jung, H.S. Favorable Glycemic Control with Once-Daily Insulin Degludec/Insulin Aspart after Changing from Basal Insulin in Adults with Type 2 Diabetes. Endocrinol. Metab. 2019, 34, 382–389. [Google Scholar] [CrossRef]
- Cho, K.Y.; Nakamura, A.; Oba-Yamamoto, C.; Tsuchida, K.; Yanagiya, S.; Manda, N.; Kurihara, Y.; Aoki, S.; Atsumi, T.; Miyoshi, H. Switching to Once-Daily Insulin Degludec/Insulin Aspart from Basal Insulin Improves Postprandial Glycemia in Patients with Type 2 Diabetes Mellitus: Randomized Controlled Trial. Diabetes Metab. J. 2020, 43, 532–541. [Google Scholar] [CrossRef]
- Philis-Tsimikas, A.; Astamirova, K.; Gupta, Y.; Haggag, A.; Roula, D.; Bak, B.; Fita, E.; Nielsen, A.; Demir, T. Similar glycaemic control with less nocturnal hypoglycaemia in a 38-week trial comparing the IDegAsp co-formulation with insulin glargine U100 and insulin aspart in basal insulin-treated subjects with type 2 diabetes mellitus. Diabetes Res. Clin. Pr. 2019, 147, 157–165. [Google Scholar] [CrossRef]
- Nagai, Y.; Nishine, A.; Hashimoto, E.; Nakayama, T.; Sasaki, Y.; Murakami, M.; Ishii, S.; Kato, H.; Tanaka, Y. Efficacy and safety of switching from basal insulin to once-daily insulin degludec/insulin aspart in Japanese patients with inadequately controlled type 2 diabetes: A 4-week, randomized, open-label, treat-to-target study. J. Diabetes Investig. 2017, 9, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Franek, E.; Wise, J.; Niemeyer, M.; Mersebach, H.; Simó, R. Efficacy and Safety of Once-Daily Insulin Degludec/Insulin Aspart versus Insulin Glargine (U100) for 52 Weeks in Insulin-Naïve Patients with Type 2 Diabetes: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0163350. [Google Scholar] [CrossRef]
- Kumar, S.; Jang, H.C.; Demirağ, N.G.; Skjøth, T.V.; Endahl, L.; Bode, B. Efficacy and safety of once-daily insulin degludec/insulin aspart compared with once-daily insulin glargine in participants with Type 2 diabetes: A randomized, treat-to-target study. Diabet. Med. 2016, 34, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Ono, Y.; Rabøl, R.; Endahl, L.; Nakamura, S. Superior glycaemic control with once-daily insulin degludec/insulin aspart versus insulin glargine in Japanese adults with type 2 diabetes inadequately controlled with oral drugs: A randomized, controlled phase 3 trial. Diabetes Obes. Metab. 2013, 15, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Tack, C.J.; Cuddihy, R.; Davidson, J.; Gouet, D.; Liebl, A.; Romero, E.; Mersebach, H.; Dykiel, P.; Jorde, R. A New-Generation Ultra-Long-Acting Basal Insulin with a Bolus Boost Compared with Insulin Glargine in Insulin-Naive People with Type 2 Diabetes: A randomized, controlled trial. Diabetes Care 2011, 34, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Latif, Z.A.; Comlekci, A.; Galvez, G.G.; Malik, R.; Pathan, F.; Kumar, A. Pragmatic use of insulin degludec/insulin aspart co-formulation: A multinational consensus statement. Indian J. Endocrinol. Metab. 2016, 20, 542–545. [Google Scholar] [CrossRef]
- Woo, V.; Berard, L.; Roscoe, R. Understanding the Clinical Profile of Insulin Degludec, the Latest Basal Insulin Approved for Use in Canada: A Narrative Review. Diabetes Ther. 2020, 11, 2539–2553. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, N.A.; Wagstaff, A.J. Insulin Aspart. Drugs 2004, 64, 1957–1974. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; da Rocha Fernandes, J.D.; Yamamoto, Y.; Langer, J.; Faurby, M. A Japanese Study Assessing Glycemic Control with Use of IDegAsp Co-formulation in Patients with Type 2 Diabetes in Clinical Practice: The JAGUAR Study. Adv. Ther. 2021, 38, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Eledrisi, M.; Suleiman, N.N.; Salameh, O.; Hamad, M.K.; Rabadi, O.; Mohamed, A.; Al Adawi, R.; Salam, A. Twice-daily insulin glargine for patients with uncontrolled type 2 diabetes mellitus. J. Clin. Transl. Endocrinol. 2018, 15, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, H.E.; Jacques, R.; Gardner, K.J.; Amiel, S.A.; Mansell, P. Twice- rather than once-daily basal insulin is associated with better glycaemic control in Type 1 diabetes mellitus 12 months after skills-based structured education in insulin self-management. Diabet. Med. 2015, 32, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, S.N.; Reynolds, L.R. Insulin degludec, the new generation basal insulin or just another basal insulin? Clin. Med. Insights Endocrinol. Diabetes 2012, 5, S9494. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, X.; Huang, J. Efficacy and Safety of Insulin Degludec versus Insulin Glargine: A Systematic Review and Meta-Analysis of Fifteen Clinical Trials. Int. J. Endocrinol. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
| Study [Ref] | Study Design | Characteristics of Participants | Study Duration | No. of Participants | Age (Years), Mean ± SD or Median (IQR) | Main Outcomes, Mean Difference ± SD or Mean Difference (95%CI) | Insulin Type and Dose, Mean ± SD or Median (IQR) | Reports of Hypoglycemia, Events or Estimated Treatment Ratio (%) |
|---|---|---|---|---|---|---|---|---|
| Clinical trials comparing IGegAsp with the conventional premixed insulin (eight studies) | ||||||||
| Ozcelik et al., 2021 [13] | Premixed insulin for at least 3 months, then replaced insulin by IDegAsp for 12 weeks | Type 2 diabetes, Age ≥ 18, Patients on premixed in addition to oral antidiabetic drugs | 12 weeks | 55 | 67.0 (62–69) * | FPG: −72.9 ± 79.3 mg/dL PPG: −84.8 ± 86.6 mg/dL HbA1c: −1.90 ± 1.96% | I: IDegAsp; 38(36–40) U/day C: BIAsp30 or insulin lispro 25/75; 48 (40–55) U/day | Overall (event/week) I: 0.03 ± 0.11 C: 1.5 ± 0.85 |
| Fujimoto et al., 2018 [14] | Premixed insulin for 2 months, then followed by IDegAsp for the next 2 months | Type 2 diabetes, Patients on premixed insulin therapy twice daily for more than 6 months. | 4 months | 22 | 68.0 ± 9.9 | MBG: −0.46 (−1.05, 0.14) mg/dL HbA1c: −0.26 (−0.85, 0.33)% | I: IDegAsp; Morning 8.05 ± 0.78 U/day, Evening 7.77 ± 0.72 U/day C: BIAsp or biphasic human insulin; Morning 7.91 ± 0.70 U/day, Evening 8.23 ± 0.76 | 0 event per month in both group |
| Yang et al., 2019 [15] | RCT I: twice daily IDegAsp C: twice daily BIAsp30 | Type 2 diabetes, Age ≥ 18, Patients on once- or twice- daily premixed insulin or basal insulin ± metformin | 26 weeks | I:360 C:181 | I: 59.6 ± 9.0 C: 58.8 ± 9.4 | FPG: −1.42 (−1.74, −1.10) mmol/L HbA1c: non-inferior | I: IDegAsp; 0.78 ± 0.35 U/kg/day C: BIAsp30; 0.95 ± 0.35 U/kg/day | Nocturnal: 47% Total: 43% |
| Onishi et al., 2017 [16] | RCT I: twice daily IDegAsp C: twice daily BIAsp30 | Type 2 diabetes, Age ≥ 20, Patients on twice-daily on same basal insulin or premixed insulin | 6 weeks | I: 33 C: 32 | I: 64.3 ± 8.4 C: 64.7 ± 11.2 | FPG: −1.6 (−2.4, −0.8) mmol/L PPG: 1.0 (−0.1, 2.2) mmol/L Mean PG: −0.4 (−1.3, 0.5) mmol/L | I: IDegAsp; Morning 11.4–12.7 U/day, Evening 10.5–10.7 U/day C: BIAsp30; Morning 12.3–13.3 U/day, Evening 9.9–11.8 U/day | Nocturnal: 51% Total: 47% |
| Kaneko et al., 2015 [17] | RCT I: twice daily IDegAsp C: twice daily BIAsp30 | Type 2 diabetes, Age ≥ 18 or 20, Patients on once- or twice- daily premixed insulin or basal insulin ± metformin | 26 weeks | I: 282 C: 142 | I: 59.1 ± 10.2 C: 61.2 ± 9.5 | FPG: −1.06 (−1.43, −0.70) mmol/L Mean PG: −0.19 (−0.54, 0.16) HbA1c: 0.05 (−0.10, 0.20)% | I: IDegAsp; Morning 34 U/day, Evening 21 U/day C: BIAsp30; Morning 38 U/day, Evening 30 U/day | Nocturnal: NS Total: NS |
| Franek et al., 2015 [18] | RCT I: twice daily IDegAsp C: twice daily BIAsp30 | Type 2 diabetes, Age ≥ 18, insulin naïve patients | 26 weeks | I: 197 C: 197 | I: 59.0 ± 9.5 C: 58.8 ± 8.4 | FPG: −1.0 (−1.4, −0.6) mmol/L Mean PG: NS HbA1c: 0.02 (−0.12, 0.17)% | I: IDegAsp; Morning 0.44 U/kg/day, Evening 0.35 U/kg/day C: BIAsp30; Morning 0.42 U/kg/day, Evening 0.40 kgU/day | Nocturnal: 73% Total: 32% |
| Flutcher et al., 2014 [19] | RCT I: twice daily IDegAsp C: twice daily BIAsp30 | Type 2 diabetes, Age ≥ 18 or 20, Patients on once- or twice- daily premixed insulin ± oral hypoglycemic agents | 26 weeks | I: 224 C: 223 | I: 58.7 ± 9.9 C: 58.8 ± 9.8 | FPG: −1.14 (−1.53, −0.76) mmol/L Mean PG: −0.4 (−0.75, −0.05) mmol/L HbA1c: −0.03 (−0.18, 0.13)% | I: IDegAsp; Morning 38 U/day, Evening 52 U/day C: BIAsp30; Morning 44 U/day, Evening 54 U/day | Nocturnal: 74% Total: 54% |
| Nikanen et al., 2012 [20] | RCT I: twice daily IDegAsp C: twice daily BIAsp30 | Type 2 diabetes, 75 ≥ Age ≥ 18, insulin naïve patients or patients with insulin treatment within 14 days in the 3 months prior to trial | 16 weeks | I: 61 C: 62 | I: 58.7 ± 8.5 C: 59.7 ± 8.0 | FPG: −0.99 (−1.68, −0.29) mmol/L Mean PG: NS HbA1c: −0.02 (−0.27, 0.24)% | I: IDegAsp; 0.57 ± 0.23 U/kg/day C: BIAsp30; 0.66 ± 0.30 U/kg/day | Nocturnal: 74% Total: 67% |
| Clinical trials comparing IDegAsp with Basal insulin (nine studies) | ||||||||
| Jang et al., 2019 [21] | Basal insulin for at least 4 months, then replaced insulin by IDegAsp | Type 2 diabetes | 3 months basal insulin, then 3 months IDegAsp | 80 | 67 ± 9.8 | HbA1c: −0.4% | I: IDegAsp; 0.39 ± 015 U/kg/day C: IGlr-100 or IGlr-300 or insulin detemir or IDeg; 0.36 ± 0.14 U/kg/day | NR |
| Cho et al., 2020 [22] | RCT I: once daily IDegAsp C: once daily basal insulin (IDeg or IGlr) | Type 2 diabetes, 80 ≥ Age ≥ 20 | 12 weeks | I: 30 C: 29 | I: 64.8 ± 1.8 C: 63.3 ± 1.9 | FPG: NS PPG: −0.9 mmol/L HbA1c: NS | I: IDegAsp; 13.7 ± 8.9 U/day C: IGlr; 13.7 ± 6.9 U/day | NR |
| Shimonda et al., 2019 [10] | RCT I: once daily IDegAsp C: once daily basal insulin (IDeg or IGla-300) | Type 2 diabetes, Age ≥ 20 | 12 weeks | I: 26 C: 26 | I: 57.0 (48.0–68.0) C: 49.0 (45.0–60.0) | HbA1c: NS | I: IDegAsp; 0.154 (0.143–0.198) U/kg/day C: IDeg; 0.145 (0.128–0.158), IGlr-300; 0.189 (0.160–0.220) U/kg/day | NR |
| Tsimikas et al., 2019 [23] | RCT I: once daily IDegAsp C: once daily basal insulin (IGla-100) + IAsp) | Type 2 diabetes, Age ≥ 18 | 26 weeks | I: 267 C: 265 | I: 58.2 ± 8.9 C: 59.2 ± 9.1 | FPG: −2.3 ±2.9 mmol/L HbA1c: −1.1 ± 0.9% Mean PG: −0.6 ± 2.6 mmol/L | I: IDegAsp; 83.4 ± 51.3 U/day C: IGlr-100; 89.3 ± 43.1 U/day | Nocturnal (events/100 person-years of exposure) I: 47.9, C:92.9 Overall (events/100 person-years of exposure) I: 258.5, C: 296.1 |
| Nagai et al., 2017 [24] | RCT I: once daily IDegAsp C: once daily basal insulin | Type 2 diabetes, Age ≥ 20 or 20, Patients on once-daily basal insulin plus oral hypoglycemic agents | 4 weeks | I: 12 C: 11 | I: 66 ± 13 C: 68 ± 8 | Mean PG: −28 (−47, −8) mg/dL HbA1c: NS | I: IDegAsp; 16.9 ± 6.8 U/day C: IGlr or IDeg; 23.0 ± 7.8 U/day | NS |
| Kumar et al., 2016 [25] | RCT I: once daily IDegAsp C: once daily basal insulin (IGla) | Type 2 diabetes, Age ≥ 18, insulin naïve patients | 52 weeks | I: 266 C: 263 | I: 57.4 ± 9.0 C: 56.4 ± 9.2 | FPG: NS PPG: −0.34 (−0.64, −0.04) mmol/L HbA1c: −0.08 (−0.26, 0.09)% | I: IDegAsp; 66 U/day C: IGlr-100; 59 U/day | Nocturnal: 75% Total: 186% |
| Kumar et al., 2017 [26] | RCT I: once daily IDegAsp C: once daily basal insulin (IGlar) | Type 2 diabetes, Age ≥ 18, Patients on once-daily basal insulin plus oral hypoglycemic agents | 26 weeks | I: 196 C: 205 | I: 57.8 ± 9.5 C: 58.4 ± 10.1 | FPG: NS Mean PG: 0.55 (0.23, 0.88) mmol/L, HbA1c: −0.03 (−0.20, 0.14)% | I: IDegAsp; 60 U/day C: IGlr; 60 U/day | NS |
| Onishi et al., 2013 [27] | RCTI : once daily IDegAsp C: once daily basal insulin (IGlar) | Type 2 diabetes, Age ≥ 20, insulin naïve patients | 26 weeks | I: 147 C: 149 | I: 60.0 ± 10.0 C: 61.0 ± 9.6 | FPG: NS Mean PG: −3.2 (−4.1, −2.3) mmol/L HbA1c: −0.28 (−0.46, −0.10)% | I: IDegAsp; 28 U/day C: IGlr-100; 29 U/day | NS |
| Heise et al., 2011 [28] | RCT I: once daily IDegAsp C: once daily basal insulin (IGlar) | Type 2 diabetes, 75 ≥ Age ≥ 18, insulin naïve patients | 16 weeks | I: 100 C: 100 | I: 58.7 ± 8.8 C: 58.4 ± 8.4 | FPG: −0.13 (−1.03, 0.77) mmol/L Mean PG: −1.34 (−2.45, −0.23) mmol/L HbA1c: −0.11 (−0.41, 0.19) | I: IDegAsp; 0.38 ± 0.16 U/kg/day C: IGlr-100; 0.45 ± 0.20 U/kg/day | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.; Chung, H.-S.; Kim, Y.-J.; Yu, J.-M.; Jeong, W.-J.; Park, J.; Oh, C.-M. Efficacy and Safety of Insulin Degludec/Insulin Aspart Compared with a Conventional Premixed Insulin or Basal Insulin: A Meta-Analysis. Metabolites 2021, 11, 639. https://doi.org/10.3390/metabo11090639
Moon S, Chung H-S, Kim Y-J, Yu J-M, Jeong W-J, Park J, Oh C-M. Efficacy and Safety of Insulin Degludec/Insulin Aspart Compared with a Conventional Premixed Insulin or Basal Insulin: A Meta-Analysis. Metabolites. 2021; 11(9):639. https://doi.org/10.3390/metabo11090639
Chicago/Turabian StyleMoon, Shinje, Hye-Soo Chung, Yoon-Jung Kim, Jae-Myung Yu, Woo-Ju Jeong, Jiwon Park, and Chang-Myung Oh. 2021. "Efficacy and Safety of Insulin Degludec/Insulin Aspart Compared with a Conventional Premixed Insulin or Basal Insulin: A Meta-Analysis" Metabolites 11, no. 9: 639. https://doi.org/10.3390/metabo11090639
APA StyleMoon, S., Chung, H.-S., Kim, Y.-J., Yu, J.-M., Jeong, W.-J., Park, J., & Oh, C.-M. (2021). Efficacy and Safety of Insulin Degludec/Insulin Aspart Compared with a Conventional Premixed Insulin or Basal Insulin: A Meta-Analysis. Metabolites, 11(9), 639. https://doi.org/10.3390/metabo11090639





