Metabolomic Profiling in Lung Cancer: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Study Selection Criteria
2.3. Data Extraction
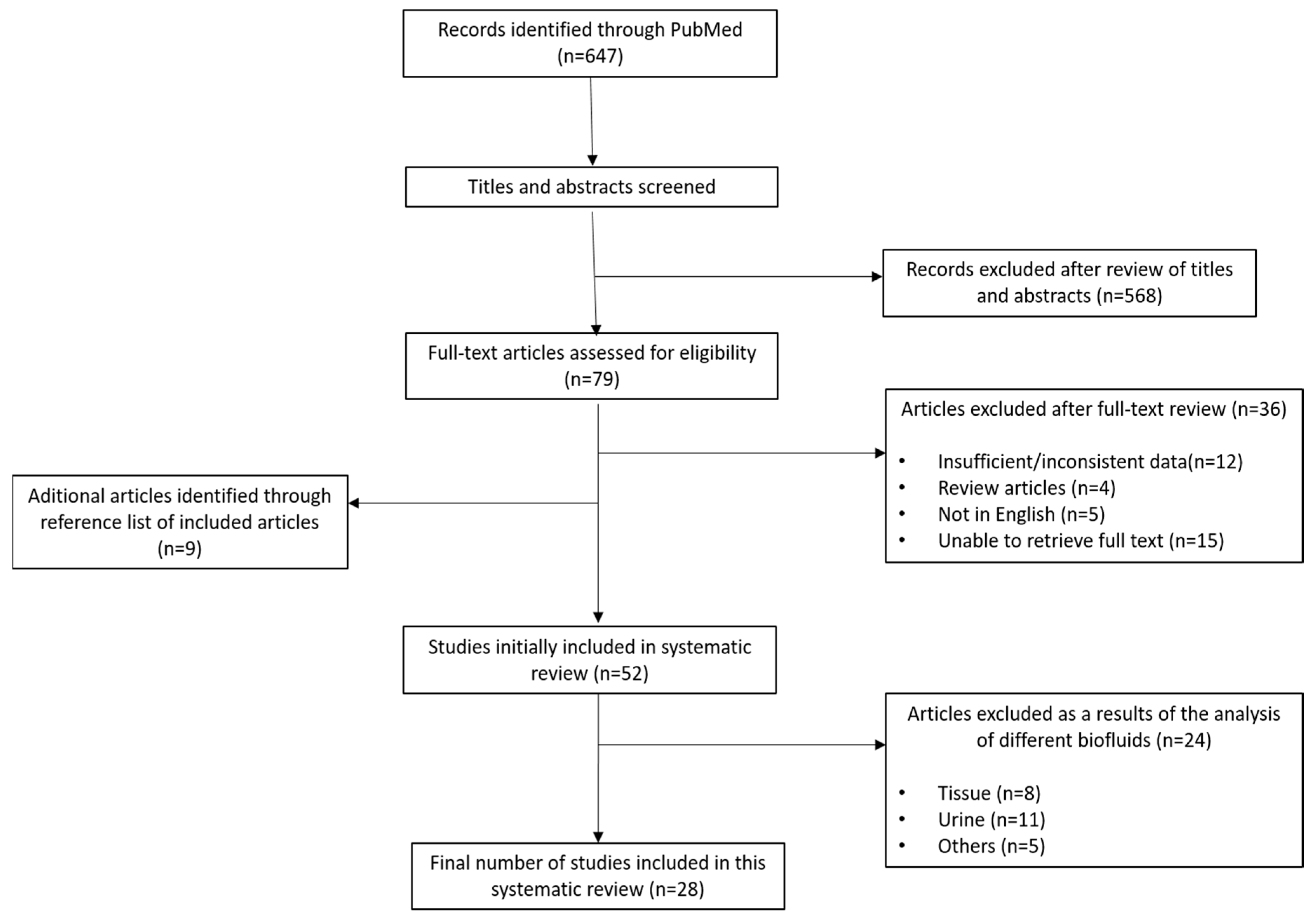
3. Results
3.1. Eligible Studies
3.2. Study Characteristics
4. Discussion
4.1. Amino Acids
4.1.1. Carnitine and Cadaverine
4.1.2. Methionine
4.1.3. Tryptophan
4.1.4. Proline
4.1.5. Glutamine
4.1.6. Valine and Glycine
4.2. Proteins
4.3. Lipids
4.4. Glucose and Its Metabolites
4.5. Smoking-Related Metabolites: Nicotine and Cotinine
4.6. N–Acetylneuraminic Acid (NANA)
4.7. Folate and Vitamin B6
4.8. Published Results Including Groups/Panels of Discriminative Metabolites
4.9. Metabolites and the Response to Treatment
4.10. Limitations of this Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Section/Topic | # | Checklist Item | Reported on Section |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | Title |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | Abstract |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | Introduction |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes and study design (PICOS). | Introduction |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | X |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | Section 2.2 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | Section 2.1 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | Appendix B Search 2 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | Section 2.2 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | Section 2.3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | Section 2.3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | X |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | Section 2.2 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | X |
Appendix B
References
- Haznadar, M.; Cai, Q.; Krausz, K.W.; Bowman, E.D.; Margono, E.; Noro, R.; Thompson, M.D.; Mathe, E.; Munro, H.M.; Steinwandel, M.D.; et al. Urinary Metabolite Risk Biomarkers of Lung Cancer: A Prospective Cohort Study. Cancer Epidemiol. Biomark. Prev. 2016, 25, 978–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; on behalf of the ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv1–iv21. [Google Scholar] [CrossRef]
- Escuín, J.S.D.C. New Immunotherapy and Lung Cancer. Arch. Bronconeumol. 2017, 53, 682–687. [Google Scholar] [CrossRef]
- Rihawi, K.; Gelsomino, F.; Sperandi, F.; Melotti, B.; Fiorentino, M.; Casolari, L.; Ardizzoni, A. Pembrolizumab in the treatment of metastatic non-small cell lung cancer: A review of current evidence. Ther. Adv. Respir. Dis. 2017, 11, 353–373. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, S.; Taylor, S.L.; Barupal, D.K.; Taguchi, A.; Wohlgemuth, G.; Wikoff, W.R.; Yoneda, K.Y.; Gandara, D.R.; Hanash, S.M.; Kim, K.; et al. Systemic Metabolomic Changes in Blood Samples of Lung Cancer Patients Identified by Gas Chromatography Time-of-Flight Mass Spectrometry. Metabolites 2015, 5, 192–210. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 17 March 2021).
- Xie, Y.; Menga, W.X.; Li, R.Z.; Wanga, Y.W.; Qianb, X.; Chanb, C.; Yub, Z.F.; Fana, X.X.; Pana, H.D.; Xie, C.; et al. Early lung cancer diagnostic biomarker discovery by machine learning. Transl. Oncol. 2021, 14, 100907. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Wang, X.-F.; Beukemann, M.; Zhang, Q.; Seeley, M.; Mohney, R.; Holt, T.; Pappan, K.L. Metabolite Profiles of the Serum of Patients with Non–Small Cell Carcinoma. J. Thorac. Oncol. 2016, 11, 72–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-J.; Hayward, C.; Fong, P.-Y.; Dominguez, M.; Hunsucker, S.W.; Lee, L.W.; McLean, M.; Law, S.; Butler, H.; Schirm, M.; et al. A Blood-Based Proteomic Classifier for the Molecular Characterization of Pulmonary Nodules. Sci. Transl. Med. 2013, 5, 207ra142. [Google Scholar] [CrossRef] [Green Version]
- Chuang, S.-C.; Fanidi, A.; Ueland, P.M.; Relton, C.; Midttun, Ø.; Vollset, S.E.; Gunter, M.J.; Seckl, M.J.; Travis, R.C.; Wareham, N.; et al. Circulating Biomarkers of Tryptophan and the Kynurenine Pathway and Lung Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2014, 23, 461–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puchades-Carrasco, L.; Lewintre, E.J.; Pérez-Rambla, C.; Garcia-Garcia, F.; Lucas-Dominguez, R.; Calabuig, S.; Blasco, A.; Dopazo, J.; Camps, C.; Pineda-Lucena, A. Serum metabolomic profiling facilitates the non-invasive identification of metabolic biomarkers associated with the onset and progression of non-small cell lung cancer. Oncotarget 2016, 7, 12904–12916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klupczynska, A.; Dereziński, P.; Garrett, T.J.; Rubio, V.; Dyszkiewicz, W.; Kasprzyk, M.; Kokot, Z.J. Study of early stage non-small-cell lung cancer using Orbitrap-based global serum metabolomics. J. Cancer Res. Clin. Oncol. 2017, 143, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zheng, J.; Ahmed, R.; Huang, G.; Reid, J.; Mandal, R.; Maksymuik, A.; Sitar, D.S.; Tappia, P.S.; Ramjiawan, B.; et al. A High-Performing Plasma Metabolite Panel for Early-Stage Lung Cancer Detection. Cancers 2020, 12, 622. [Google Scholar] [CrossRef] [Green Version]
- Miyagi, Y.; Higashiyama, M.; Gochi, A.; Akaike, M.; Ishikawa, T.; Miura, T.; Saruki, N.; Bando, E.; Kimura, H.; Imamura, F.; et al. Plasma Free Amino Acid Profiling of Five Types of Cancer Patients and Its Application for Early Detection. PLoS ONE 2011, 6, e24143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terlizzi, M.; Colarusso, C.; De Rosa, I.; De Rosa, N.; Somma, P.; Curcio, C.; Sanduzzi, A.Z.; Micheli, P.; Molino, A.; Saccomanno, A.; et al. Circulating and tumor-associated caspase-4: A novel diagnostic and prognostic biomarker for non-small cell lung cancer. Oncotarget 2018, 9, 19356–19367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shingyoji, M.; Iizasa, T.; Higashiyama, M.; Imamura, F.; Saruki, N.; Imaizumi, A.; Yamamoto, H.; Daimon, T.; Tochikubo, O.; Mitsushima, T.; et al. The significance and robustness of a plasma free amino acid (PFAA) profile-based multiplex function for detecting lung cancer. BMC Cancer 2013, 13, 77. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Chen, H.; Ai, J.; Zhu, Y.; Li, Y.; Borgia, J.A.; Yang, J.-S.; Zhang, J.; Jiang, B.; Gu, W.; et al. Global lipidomics identified plasma lipids as novel biomarkers for early detection of lung cancer. Oncotarget 2017, 8, 107899–107906. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Husemoen, L.L.N.; Thuesen, B.; Pisinger, C.; Jørgensen, T.; Roswall, N.; Larsen, S.C.; Linneberg, A. Prospective Population-Based Study of the Association between Serum 25-Hydroxyvitamin-D Levels and the Incidence of Specific Types of Cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1220–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, J.; Xu, L.; Li, W.; Wu, L. Simultaneous determination of thirteen kinds of amino acid and eight kinds of acylcarnitine in human serum by LC-MS/MS and its application to measure the serum concentration of lung cancer patients. Biomed. Chromatogr. 2016, 30, 1796–1806. [Google Scholar] [CrossRef]
- Ni, J.; Xu, L.; Li, W.; Zheng, C.; Wu, L. Targeted metabolomics for serum amino acids and acylcarnitines in patients with lung cancer. Exp. Ther. Med. 2019, 18, 188–198. [Google Scholar] [CrossRef] [Green Version]
- LaRose, T.L.; Guida, F.; Fanidi, A.; Langhammer, A.; Kveem, K.; Stevens, V.L.; Jacobs, E.J.; Smith-Warner, S.A.; Giovannucci, E.; Albanes, D.; et al. Circulating cotinine concentrations and lung cancer risk in the Lung Cancer Cohort Consortium (LC3). Int. J. Epidemiol. 2018, 47, 1760–1771. [Google Scholar] [CrossRef]
- Pietzke, M.; Arroyo, S.F.; Sumpton, D.; Mackay, G.M.; Martin-Castillo, B.; Camps, J.; Joven, J.; Menendez, J.A.; Vazquez, A. Stratification of cancer and diabetes based on circulating levels of formate and glucose. Cancer Metab. 2019, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klupczynska, A.; Plewa, S.; Kasprzyk, M.; Dyszkiewicz, W.; Kokot, Z.; Matysiak, J. Serum lipidome screening in patients with stage I non-small cell lung cancer. Clin. Exp. Med. 2019, 19, 505–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Zhao, W.; Wang, L.; Guo, F.; Song, D.; Zhang, Q.; Zhang, D.; Fan, Y.; Wang, J. Integration of metabolomic and transcriptomic profiles to identify biomarkers in serum of lung cancer. J. Cell. Biochem. 2019, 120, 11981–11989. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Kim, K.; DeFelice, B.C.; Taylor, S.; Gandara, D.R.; Yoneda, K.Y.; Cooke, D.T.; Fiehn, O.; Kelly, K.; Miyamoto, S. Investigation of Metabolomic Blood Biomarkers for Detection of Adenocarcinoma Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1716–1723. [Google Scholar] [CrossRef] [Green Version]
- Singhal, S.; Rolfo, C.; Maksymiuk, A.W.; Tappia, P.S.; Sitar, D.S.; Russo, A.; Akhtar, P.S.; Khatun, N.; Rahnuma, P.; Rashiduzzaman, A.; et al. Liquid Biopsy in Lung Cancer Screening: The Contribution of Metabolomics. Results of A Pilot Study. Cancers 2019, 11, 1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cui, J.; Gu, J.; He, H.; Li, B.; Li, W. Plasma 25-hydroxyvitamin D deficiency is associated with the risk of non-small cell lung cancer in a Chinese population. Cancer Biomark. 2015, 15, 663–668. [Google Scholar] [CrossRef]
- Fanidi, A.; Muller, D.; Yuan, J.-M.; Stevens, V.L.; Weinstein, S.J.; Albanes, D.; Prentice, R.; Thomsen, C.A.; Pettinger, M. Circulating Folate, Vitamin B6, and Methionine in Relation to Lung Cancer Risk in the Lung Cancer Cohort Consortium (LC3). J. Natl. Cancer Inst. 2018, 110, 57–67. [Google Scholar] [CrossRef]
- Ros-Mazurczyk, M.; Jelonek, K.; Marczyk, M.; Binczyk, F.; Pietrowska, M.; Polanska, J.; Dziadziuszko, R.; Jassem, J.; Rzyman, W.; Widlak, P. Serum lipid profile discriminates patients with early lung cancer from healthy controls. Lung Cancer 2017, 112, 69–74. [Google Scholar] [CrossRef]
- Maosheng, H.; Ye, Y.; Chang, D.W.; Huang, M.; Heymach, J.V.; Roth, J.A.; Wu, X.; Zhao, H. Circulating metabolite profiles to predict overall survival in advanced non-small cell lung cancer patients receiving first-line chemotherapy. Lung Cancer 2017, 114, 70–78. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Z.; Liu, X.; Duan, J.; Feng, G.; Yin, Y.; Gu, J.; Chen, Z.; Gao, S.; Bai, H.; et al. Prediction of Chemotherapeutic Efficacy in Non–Small Cell Lung Cancer by Serum Metabolomic Profiling. Clin. Cancer Res. 2018, 24, 2100–2109. [Google Scholar] [CrossRef] [Green Version]
- Hao, D.; Sengupta, A.; Ding, K.; Ubeydullah, E.; Krishnaiah, S.; Leighl, N.B.; Shepherd, F.A.; Seymour, L.; Weljie, A. Metabolites as Prognostic Markers for Metastatic Non-Small Cell Lung Cancer (NSCLC) Patients Treated with First-Line Platinum-Doublet Chemotherapy. Cancers 2020, 12, 1926. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Sarfaraz, M.O.; Farshidfar, F.; Bebb, D.G.; Lee, C.Y.; Card, C.M.; David, M.; Weljie, A.M. Temporal characterization of serum metabolite signatures in lung cancer patients undergoing treatment. Metabolomics 2016, 12, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghini, V.; Laera, L.; Fantechi, B.; Del Monte, F.; Benelli, M.; McCartney, A.; Leonardo, T.; Luchinat, C.; Pozzessere, D. Metabolomics to Assess Response to Immune Checkpoint Inhibitors in Patients with Non-Small-Cell Lung Cancer. Cancers 2020, 12, 3574. [Google Scholar] [CrossRef]
- Kosmides, A.K.; Kamisoglu, K.; Calvano, S.E.; Corbett, S.A.; Androulakis, I.P. Metabolomic Fingerprinting: Challenges and Opportunities. Crit. Rev. Biomed. Eng. 2013, 41, 205–221. [Google Scholar] [CrossRef]
- Wishart, D.S. Computational approaches to metabolomics. In Bioinformatics Methods in Clinical Research Methods in Molecular Biology; Matthiesen, R., Ed.; Humana Press: New York, NY, USA, 2010; pp. 283–313. [Google Scholar]
- Stevens, V.L.; Hoover, E.; Wang, Y.; Zanetti, K.A. Pre-Analytical Factors that Affect Metabolite Stability in Human Urine, Plasma, and Serum: A Review. Metabolites 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringer, K.A.; McKay, R.; Karnovsky, A.; Quémerais, B.; Lacy, P. Metabolomics and Its Application to Acute Lung Diseases. Front. Immunol. 2016, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Beuchel, C.; Becker, S.; Dittrich, J.; Kirsten, H.; Toenjes, A.; Stumvoll, M.; Loeffler, M.; Thiele, H.; Beutner, F.; Thiery, J.; et al. Clinical and lifestyle related factors influencing whole blood metabolite levels—A comparative analysis of three large cohorts. Mol. Metab. 2019, 29, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Playdon, M.C.; Sampson, J.N.; Cross, A.J.; Sinha, R.; Guertin, K.; Moy, K.A.; Rothman, N.; Irwin, M.L.; Mayne, S.T.; Stolzenberg-Solomon, R.; et al. Comparing metabolite profiles of habitual diet in serum and urine. Am. J. Clin. Nutr. 2016, 104, 776–789. [Google Scholar] [CrossRef] [Green Version]
- Kami, K.; Fujimori, T.; Sato, H.; Sato, M.; Yamamoto, H.; Ohashi, Y.; Sugiyama, N.; Ishihama, Y.; Onozuka, H.; Ochiai, A.; et al. Metabolomic profiling of lung and prostate tumor tissues by capillary electrophoresis time-of-flight mass spectrometry. Metabolomics 2012, 9, 444–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.B.; Ang, L.; Yau, W.-P.; Seow, W.J. Association between Metabolites and the Risk of Lung Cancer: A Systematic Literature Review and Meta-Analysis of Observational Studies. Metabolites 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Heng, R.B.; Lim, E.; Lovejoy, D.B.; Bessede, A.; Gluch, L.; Guillemin, G.J. Understanding the role of the kynurenine pathway in human breast cancer immunobiology. Oncotarget 2015, 7, 6506–6520. [Google Scholar] [CrossRef] [Green Version]
- Phang, J.M. Proline Metabolism in Cell Regulation and Cancer Biology: Recent Advances and Hypotheses. Antioxid. Redox Signal. 2019, 30, 635–649. [Google Scholar] [CrossRef] [Green Version]
- Elia, I.; Broekaert, D.; Christen, S.; Boon, R.; Radaelli, E.; Orth, M.; Verfaillie, C.; Grünewald, T.G.P.; Fendt, S.-M. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat. Commun. 2017, 8, 15267. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hancock, C.N.; Fischer, J.; Harman, M.; Phang, J.M. Proline biosynthesis augments tumor cell growth and aerobic glycolysis: Involvement of pyridine nucleotides. Sci. Rep. 2015, 5, 17206. [Google Scholar] [CrossRef] [Green Version]
- Sahu, N.; Cruz, D.D.; Gao, M.; Sandoval, W.; Haverty, P.M.; Liu, J.; Stephan, J.-P.; Haley, B.; Classon, M.; Hatzivassiliou, G.; et al. Proline Starvation Induces Unresolved ER Stress and Hinders mTORC1-Dependent Tumorigenesis. Cell Metab. 2016, 24, 753–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berker, Y.; Vandergrift, L.A.; Wagner, I.; Su, L.; Kurth, J.; Schuler, A.; Dinges, S.S.; Habbel, P.; Nowak, J.; Mark, E.; et al. Magnetic Resonance Spectroscopy-based Metabolomic Biomarkers for Typing, Staging, and Survival Estimation of Early-Stage Human Lung Cancer. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Xu, Y.; Fitch, W.L.; Zheng, M.; Merritt, R.E.; Shrager, J.B.; Zhang, W.; Dill, D.L.; Peltz, G.; Hoang, C.D. Liquid chromatography/mass spectrometry methods for measuring dipeptide abundance in non-small-cell lung cancer. Rapid Commun. Mass Spectrom. 2013, 27, 2091–2098. [Google Scholar] [CrossRef] [Green Version]
- Terlizzi, M.; Molino, A.; Colarusso, C.; Pasquale, S.; Ilaria, D.R.; Jacopo, T.; Scala, G.; Salvi, R.; Pinto, A.; Sorrentino, R. Altered lung tissue lipidomic profile in caspase-4 positive nonsmall cell lung cancer (NSCLC) patients. Oncotarget 2020, 11, 3515–3525. [Google Scholar]
- Wikoff, W.R.; Grapov, D.; Fahrmann, J.F.; DeFelice, B.; Rom, W.; Pass, H.; Kim, K.; Nguyen, U.; Taylor, S.; Gandara, D.R.; et al. Metabolomic Markers of Altered Nucleotide Metabolism in Early Stage Adenocarcinoma. Cancer Prev. Res. 2015, 8, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Du, W.; Wu, M. Regulation of the pentose phosphate pathway in cancer. Protein Cell 2014, 5, 592–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. In Handbook of Experimental Pharmacology; Springer: Berlin, Germany, 2009. [Google Scholar]
- Yuan, J.-M.; Gao, Y.-T.; Murphy, S.E.; Carmella, S.G.; Wang, R.; Zhong, Y.; Moy, K.A.; Davis, A.B.; Tao, L.; Chen, M.; et al. Urinary Levels of Cigarette Smoke Constituent Metabolites Are Prospectively Associated with Lung Cancer Development in Smokers. Cancer Res. 2011, 71, 6749–6757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.-M.; Gao, Y.-T.; Wang, R.; Chen, M.; Carmella, S.G.; Hecht, S. Urinary levels of volatile organic carcinogen and toxicant biomarkers in relation to lung cancer development in smokers. Carcinogenesis 2012, 33, 804–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.-M.; Nelson, H.H.; Carmella, S.G.; Wang, R.; Kuriger-Laber, J.; Jin, A.; Adams-Haduch, J.; Hecht, S.S.; Koh, W.-P.; Murphy, S.E. CYP2A6 genetic polymorphisms and biomarkers of tobacco smoke constituents in relation to risk of lung cancer in the Singapore Chinese Health Study. Carcinogenesis 2017, 38, 411–418. [Google Scholar] [CrossRef]
- Yuan, J.-M.; Butler, L.M.; Gao, Y.-T.; Murphy, S.E.; Carmella, S.G.; Wang, R.; Nelson, H.; Hecht, S. Urinary metabolites of a polycyclic aromatic hydrocarbon and volatile organic compounds in relation to lung cancer development in lifelong never smokers in the Shanghai Cohort Study. Carcinogenesis 2014, 35, 339–345. [Google Scholar] [CrossRef]
- Hwang, S.; Ryu, H.-J.; Kang, S.J.; Yun, E.H.; Lim, M.K.; Kim, H.T.; Lee, J.S.; Lee, D.-H. Levels of Tobacco-specific Metabolites among Non-smoking Lung Cancer Cases at Diagnosis: Case-control Findings. Asian Pac. J. Cancer Prev. 2013, 14, 6591–6593. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, E.; de la Monte, S. Tobacco nitrosamines as culprits in disease: Mechanisms reviewed. J. Physiol. Biochem. 2016, 72, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S.; Chen, M.; Yagi, H.; Jerina, D.M.; Carmella, S.G. r-1,t-2,3,c-4-Tetrahydroxy-1,2,3,4-tetrahydrophenanthrene in human urine: A potential biomarker for assessing polycyclic aromatic hydrocarbon metabolic activation. Cancer Epidemiol. Biomark. Prev. 2003, 12, 1501. [Google Scholar]
- Callejón-Leblic, B.; García-Barrera, T.; Pereira-Vega, A.; Gómez-Ariza, J.L. Metabolomic study of serum, urine and bronchoalveolar lavage fluid based on gas chromatography mass spectrometry to delve into the pathology of lung cancer. J. Pharm. Biomed. Anal. 2019, 163, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.M.; Barros, A.S.; Goodfellow, B.J.; Carreira, I.M.; Gomes, A.; Sousa, V.; Bernardo, J.; Carvalho, L.; Gil, A.M.; Duarte, I.F. NMR metabolomics of human lung tumors reveals distinct metabolic signatures for adenocarcinoma and squamous cell carcinoma. Carcinogenesis 2015, 36, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guertin, K.A.; Loftfield, E.; Boca, S.M.; Sampson, J.N.; Moore, S.C.; Xiaoqin, X.; Huang, W.-Y.; Xiong, X.; Freedman, N.D.; Cross, A.J.; et al. Serum biomarkers of habitual coffee consumption may provide insight into the mechanism underlying the association between coffee consumption and colorectal cancer. Am. J. Clin. Nutr. 2015, 101, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Pozner, J.; Papatestas, A.E.; Fagerstrom, R.; Schwartz, I.; Saevitz, J.; Feinberg, M.; Aufses, A.H. Association of tumor differentiation with caffeine and coffee intake in women with breast cancer. Surgery 1986, 100, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, J.N.; Busby, E.C.; Tibbetts, R.; Roos, P.; Taya, Y.; Karnitz, L.M.; Abraham, R.T. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999, 59, 4375–4382. [Google Scholar] [PubMed]
- Hao, D.; Sengupta, A.; Ding, K.; Leighl, N.; Shepherd, F.; Seymour, L.; Weljie, A. P2.01-055 Examining Metabolomics as a Prognostic Marker in Metastatic Non–Small Cell Lung Cancer Patients Undergoing First-Line Chemotherapy. J. Thorac. Oncol. 2017, 12, S2090. [Google Scholar] [CrossRef]
- Moreno, P.; Jiménez-Jiménez, C.; Garrido-Rodriguez, M.; Calderón-Santiago, M.; Molina, S.; Lara-Chica, M.; Priego-Capote, F.; Salvatierra, Á.; Munoz, E.; Calzado, M.A. Metabolomic profiling of human lung tumor tissues—nucleotide metabolism as a candidate for therapeutic interventions and biomarkers. Mol. Oncol. 2018, 12, 1778–1796. [Google Scholar] [CrossRef] [Green Version]
| Authors, Year | Selection (0–4) | Comparability (0–2) | Exposure (0–3) | Risk of Bias (0–9) |
|---|---|---|---|---|
| Xie et al., 2021 [10] | 4 | 2 | 2 | 8 |
| Mazzone et al., 2015 [11] | 4 | 2 | 2 | 8 |
| Li et al., 2013 [12] | 4 | 2 | 2 | 8 |
| Chuang et al., 2014 [13] | 3 | 2 | 2 | 7 |
| Puchades et al., 2016 [14] | 4 | 2 | 2 | 8 |
| Klupczynska et al., 2017 [15] | 3 | 2 | 2 | 7 |
| Zhang et al., 2020 [16] | 4 | 2 | 2 | 8 |
| Miyagi et al., 2011 [17] | 3 | 2 | 3 | 8 |
| Terlizzi et al., 2018 [18] | 3 | 2 | 2 | 7 |
| Shingyogi et al., 2013 [19] | 3 | 0 | 2 | 5 |
| Yu et al., 2017 [20] | 4 | 2 | 2 | 8 |
| Skaaby et al., 2014 [21] | 3 | 2 | 3 | 8 |
| Ni et al., 2016 [22] | 3 | 2 | 2 | 7 |
| Ni et al., 2019 [23] | 3 | 2 | 2 | 7 |
| Larose et al., 2018 [24] | 2 | 2 | 2 | 6 |
| Pietzke et al., 2019 [25] | 2 | 0 | 2 | 4 |
| Klupczynska et al., 2019 [26] | 2 | 2 | 2 | 6 |
| Sun et al., 2018 [27] | 4 | 2 | 1 | 7 |
| Faharmann et al., 2015 [28] | 4 | 2 | 2 | 8 |
| Singhal et al., 2019 [29] | 3 | 2 | 1 | 6 |
| Wang et al., 2015 [30] | 3 | 2 | 2 | 7 |
| Fanidi et al., 2018 [31] | 3 | 2 | 2 | 7 |
| Ros-Mazurczyk et al., 2017 [32] | 4 | 2 | 1 | 7 |
| Maosheng et al., 2017 [33] | 3 | 2 | 2 | 7 |
| Tian et al., 2018 [34] | 3 | 2 | 2 | 7 |
| Hao et al., 2020 [35] | 2 | 1 | 2 | 5 |
| Hao et al., 2016 [36] | 3 | 1 | 2 | 6 |
| Ghini et al., 2020 [37] | 3 | 2 | 2 | 7 |
| Subject Groups (No. of Samples) | Analytical Technique | Objective of the Metabolomic Profile Analysis | Reference | ||||
|---|---|---|---|---|---|---|---|
| Healthy Controls | NSCLC Patients | Compare Cancer vs. Control | Distinguish Histological Types | Disease Staging | Other | ||
| Blood, Serum and/or Plasma | |||||||
| 43 | 110 | LC-MS | × | Biomarker | Xie et al., 2021 [10] | ||
| 190 | 94 | LC-MS, GC-MS | × | × | Mazzone et al., 2015 [11] | ||
| 71 | 72 | NS | × | Early diagnosis | Li et al., 2013 [12] | ||
| 893 | 1748 | LC-MS, GC-MS | × | Biomarker | Chuang et al., 2014 [13] | ||
| 114 | 182 | NMRs | × | × | Puchades et al., 2016 [14] | ||
| 25 | 50 | LC-MS | × | Biomarker | Klupczynska et al., 2017 [15] | ||
| 60 | 156 | LC-MS | × | × | Zhang et al., 2020 [16] | ||
| 200 | 996 | ESI-MS | × | Early diagnosis | Miyagi et al., 2011 [17] | ||
| 79 | 125 | ELISA | × | Overall survival, Biomarker | Terlizzi et al., 2018 [18] | ||
| 86 | 323 | ESI-MS | × | Early diagnosis | Shingyogi et al., 2013 [19] | ||
| 147 | 199 | ESI-MS | × | × | Yu et al., 2017 [20] | ||
| 10,485 | 126 | UPLC-MS, Immunoassay | × | Skaaby et al., 2014 [21] | |||
| 40 | 100 | LC-MS | × | Ni et al., 2016 [22] | |||
| 17 | 30 | LC-MS | × | Ni et al., 2019 [23] | |||
| 5364 | 5364 | LC-MS | × | Biomarker | Larose et al., 2018 [24] | ||
| 56 | 50 | LC-MS, GC-MS | × | Pietzke et al., 2019 [25] | |||
| 20 | 20 | MS | × | × | Klupczynska et al., 2019 [26] | ||
| 29 | 31 | GC-MS | × | Sun et al., 2018 [27] | |||
| 74 | 95 | GC-MS | × | Faharmann et al., 2015 [28] | |||
| 29 | 57 | LC-MS | × | Treatment monitoring tool | Singhal et al., 2019 [29] | ||
| 100 | 100 | LC-MS | × | × | Wang et al., 2015 [30] | ||
| 5364 | 5364 | LC-MS, GC-MS | × | Risk factors | Fanidi et al., 2018 [31] | ||
| 300 | 100 | LC-MS | × | Ros-Mazurczyk et al., 2017 [32] | |||
| 0 | 220 | LC-MS | Overall survival, Treatment efficacy | Maosheng et al., 2017 [33] | |||
| 0 | 354 | LC-MS | Overall survival, Treatment efficacy | Tian et al., 2018 [34] | |||
| 0 | 774 | LC-MS, UPLC-MS, NMRs | Treatment efficacy | Hao et al., 2020 [35] | |||
| 0 | 25 | NMRs, GC-MS | × | Prognosis | Hao et al., 2016 [36] | ||
| 0 | 50 | NMRs | Treatment efficacy | Ghini et al., 2020 [37] | |||
| Subject Groups | Place Where the Study Was Carried Out | Identified Metabolites | Measure of Association | Effect Size | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Mean | Gender Male (%) | Smoking Status | Amino Acids | Lipids | Others | |||||
| NS | NS | NS | China | Proline | AUC | 0.989 | Xie et al., 2021 [10] | |||
| l-kynurenine spermidine amino-hippuric acid | Sensitivity = 98.1% | |||||||||
| palmitoyll-carnitinetaurine | Specificity = 100.0% | |||||||||
| 67 | 52% | S or FS 97% | USA | 10 amino acids had higher values in lung patients and 12 had lower values | 44 different lipids had higher values in lung patients and 24 had lower values | Differences in 12 peptides, 4 carbohydrates, 5 nucleotides and 30 xenobiotics between healthy controls and lung cancer patients | Mazzone et al., 2015 [11] | |||
| 65 | 33% | S–14% N–6% | USA | 13-protein lung cancer classifier | Negative predictive value (NPV) of 90% | Li et al., 2013 [12] | ||||
| specificity of 44 ± 13% | ||||||||||
| 59 | 62% | S–59% N–11% | Europe | Tryptofan Kynurenine | OR | 0.88 (0.59–1.30) | Chuang et al., 2014 [13] | |||
| OR | 1.30 (0.92–1.84) | |||||||||
| 63 | 87% | S–44% N–7% | Spain | Specific increase in the serum concentrations of lysine (13.16%), valine (21.05%) and phenylalanine (52.10%) | P | 0.0025 | Puchades et al., 2016 [14] | |||
| P | 0.0000 | |||||||||
| P | 0.0000 | |||||||||
| 64 ± 6.9 | 64% | S–48% N–51% | Poland | Panel of 12 compounds, including some amino acids | Panel of 12 compounds, including acylcarnitine, organic acids | AUC | 0.836 (0.722–0.946) | Klupczynska et al., 2017 [15] | ||
| 42–79 | 38% | S–19% N–11% | China | β-hydroxybutyric acid, LysoPC 20:3, PC ae C40:6, citric acid, fumaric acid | AUC | >0.9 | Zhang et al., 2020 [16] | |||
| 65 ± 10 | 62.5% | S–42% N–30% | Japan | Profile of plasma free amino acids | AUC | 0.75 | Miyagi et al., 2011 [17] | |||
| 60 ± 10 | 66% | Italy | Higher levels of Caspase 4 in NSCLC | Sensitivity: 97.07–100% | Terlizzi et al., 2018 [18] | |||||
| specificity 88.1% | ||||||||||
| positive predictive value of 92.54% | ||||||||||
| accuracy of 95.19% | ||||||||||
| AUC of 0.971 | ||||||||||
| 67.8 ± 8.2 | 43% | S–34% N–21% | Japan | Profile of plasma free amino acids | AUC | 0.731–0.806 | Shingyogi et al., 2013 [19] | |||
| 67 ± 8 | 54% | All S or FS | China and USA | Four lipid markers (LPE(18:1), ePE(40:4), C(18:2)CE and SM(22:0)) | AUC | 82.3% | Yu et al., 2017 [20] | |||
| 18–71 | 48% | S–37% N–35% | Denmark | Vitamin D | HR | 0.98 (0.91–1.05) | Skaaby et al., 2014 [21] | |||
| 51–83 | 65% | China | Panel of 13 amino acids | Panel of 8 acylcarnitines | Ni et al., 2016 [22] | |||||
| 66.7 | 65% | S–23% N–47% | China | Glycine, valine, methionine, citrulline and arginine | p | 0.033 | Ni et al., 2019 [23] | |||
| 0.378 | ||||||||||
| 0.067 | ||||||||||
| 0.039 | ||||||||||
| 0.015 | ||||||||||
| 60 | 54% | S–47% N–25% | Europe, USA, China | Cotinine | OR | S: 1.39 (1.32–1.47) | Larose et al., 2018 [24] | |||
| FS: 1.17 (1.07–1.28) | ||||||||||
| N: 1.64 (1.10–2.30) | ||||||||||
| 66 ± 9 | 87.5% | S–50% N–50% | Europe | Formate levels higher in lung cancer patients | Pietzke et al., 2019 [25] | |||||
| 62 ± 5 | 55% | S–60% | Poland | Lysophosphatidylcholine aC26:0 | AUC | 0.87 (0.73–0.96) | Klupczynska et al., 2019 [26] | |||
| Lysophosphatidylcholine aC26:1 | AUC | 0.84 (0.68–0.95) | ||||||||
| Phosphatidylcholine aaC42:4 | AUC | 0.81 (0.65–0.93) | ||||||||
| Phosphatidylcholine aaC34:4 | AUC | 0.82 (0.65–0.94) | ||||||||
| 54.1 ± 9.9 | 67.7% | S–71% | China | Erythritol, indole-3-lactate, adenosine-5-phosphate, paracetamol, threitol | AUC | 0.9 | Sun et al., 2018 [27] | |||
| 65.9 ± 9.7 | 62% | USA | Aspartate Glutamate | Sensitivity: 67.5% | Faharmann et al., 2015 [28] | |||||
| specificity 95.4% | ||||||||||
| Sensitivity: 70.9% | ||||||||||
| specificity 74.4% | ||||||||||
| 52 | 53% | USA and Canada | Valine | LysoPhosphatidylcholine acyl C18:2 | AUC | 0.97 (0.875–1.0) | Singhal et al., 2019 [29] | |||
| decadienyl-L-carnitine | ||||||||||
| phosphatidylcholine | ||||||||||
| acyl-alkyl C36:0 | ||||||||||
| phosphatidylcholine diacyl C30:2 | ||||||||||
| spermine | ||||||||||
| iacetylspermine | ||||||||||
| 57.1 ± 8.6 | 52% | S–48% | China | 25(OH)D deficiency → related to higher risk of NSCLC | P | 0.03 | Wang et al., 2015 [30] | |||
| N–32% | ||||||||||
| 60 | 54% | S–47% | Singapore | Vitamin B6 and folate elevated → decreased risk | OR | 0.88 (0.78–1) | Fanidi et al., 2018 [31] | |||
| N–25% | 0.86 (0.74–0.99) | |||||||||
| Poland | Increased levels in lung cancer patients: phosphatidylcholines, diacylophospholipids and sphingomyelins; decreased levels of lysophosphatidylcholines | AUC | 0.88 | Ros-Mazurczyk et al., 2017 [32] | ||||||
| 60.2 | 56.3% | S–44% | USA | Caffeine | P | <0.05 | Maosheng et al., 2017 [33] | |||
| paraxanthine | ||||||||||
| stachydrine | ||||||||||
| N–16% | methyl glucopyranoside (αβ) | |||||||||
| 37–84 | 86% | N–55% | China | Hypotaurine | AUC | 0.912 | Tian et al., 2018 [34] | |||
| uridine | ||||||||||
| dodecanoylcarnitine | ||||||||||
| choline | ||||||||||
| dimethylglycine | ||||||||||
| niacinamide | ||||||||||
| FS–45% | L-palmitoylcarnitine → longer PFS | |||||||||
| Canada | Elevated blood 2-hydroxybutyrate, glycine, sphingomyelin and formate were positively associated with better OS | Hao et al., 2020 [35] | ||||||||
| 64 | 60% | Canada | Hydroxylamine, tridecan-1-ol | P | <0.05 | Hao et al., 2016 [36] | ||||
| octadecan-1-ol → better survival Tagatose | ||||||||||
| hydroxylamine | ||||||||||
| glucopyranose | ||||||||||
| 54% | S–34% | Italy | Alanine and pyruvate → responders were characterized by lower serum levels | threonine → progression | P | <0.05 | Ghini et al., 2020 [37] | |||
| N–5% | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madama, D.; Martins, R.; Pires, A.S.; Botelho, M.F.; Alves, M.G.; Abrantes, A.M.; Cordeiro, C.R. Metabolomic Profiling in Lung Cancer: A Systematic Review. Metabolites 2021, 11, 630. https://doi.org/10.3390/metabo11090630
Madama D, Martins R, Pires AS, Botelho MF, Alves MG, Abrantes AM, Cordeiro CR. Metabolomic Profiling in Lung Cancer: A Systematic Review. Metabolites. 2021; 11(9):630. https://doi.org/10.3390/metabo11090630
Chicago/Turabian StyleMadama, Daniela, Rosana Martins, Ana S. Pires, Maria F. Botelho, Marco G. Alves, Ana M. Abrantes, and Carlos R. Cordeiro. 2021. "Metabolomic Profiling in Lung Cancer: A Systematic Review" Metabolites 11, no. 9: 630. https://doi.org/10.3390/metabo11090630
APA StyleMadama, D., Martins, R., Pires, A. S., Botelho, M. F., Alves, M. G., Abrantes, A. M., & Cordeiro, C. R. (2021). Metabolomic Profiling in Lung Cancer: A Systematic Review. Metabolites, 11(9), 630. https://doi.org/10.3390/metabo11090630










