Novel Serum and Urinary Metabolites Associated with Diabetic Retinopathy in Three Asian Cohorts
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Assessment of Diabetes and Presence and Severity of DR
2.3. Assessment of Covariates
2.4. Sample Collection, Storage, and Handling
2.5. Metabolite Quantification
2.6. Statistical Analyses
3. Results
3.1. Study Population
3.2. Results of NMR Detection of Metabolites in Serum/Plasma
3.2.1. Association of Serum/Plasma Metabolites with Any DR
3.2.2. Association of Serum/Plasma Metabolites with Moderate/above DR
3.2.3. Association of Serum/Plasma Metabolites with VTDR
3.3. Results of NMR Detection of Metabolites in Urinary Samples
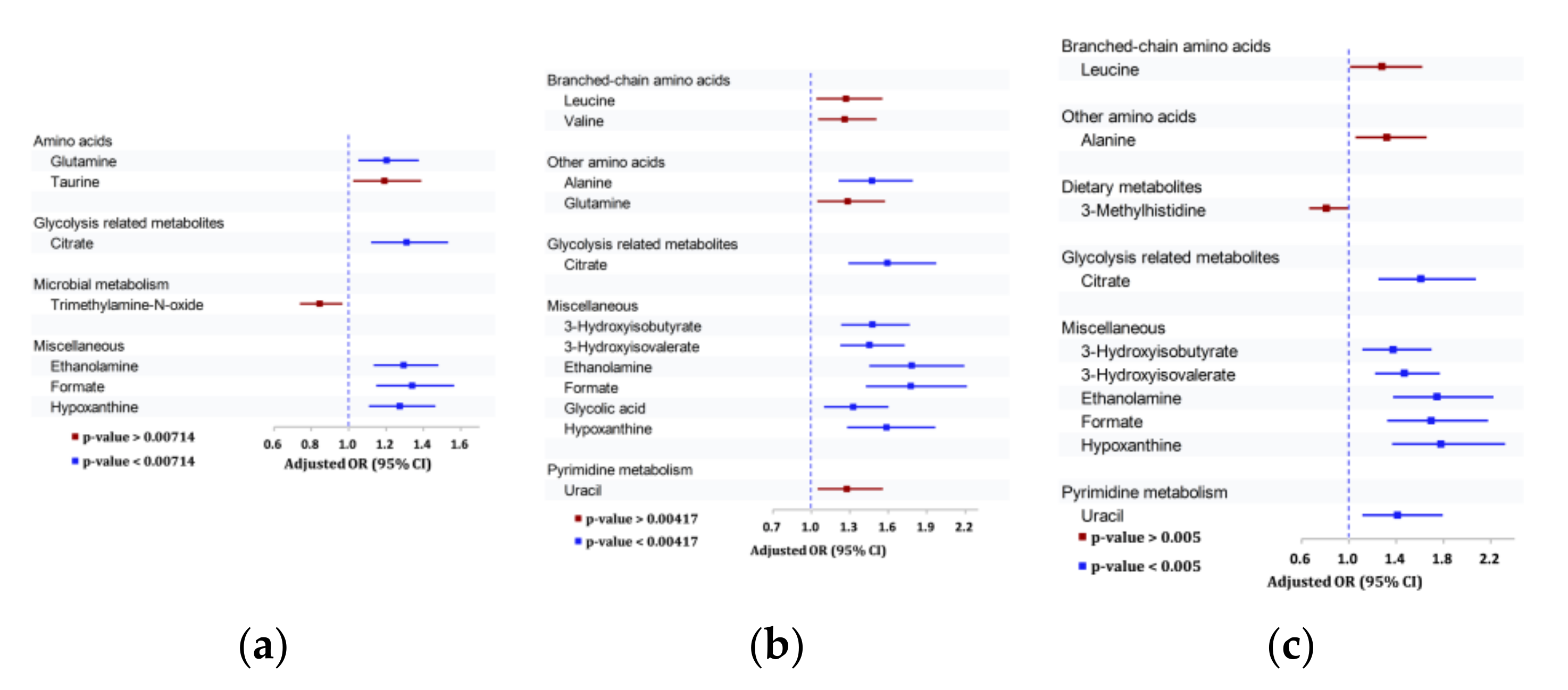
3.3.1. Association of Urinary Metabolites with Any DR
3.3.2. Association of Urinary Metabolites with Moderate/Severe DR outcomes
3.3.3. Association of Urinary Metabolites with VTDR
3.4. Serum/Plasma or Urinary Metabolites Associated with All Three Outcomes
3.5. Correlation between Serum/Plasma and Urinary Metabolites
3.6. Receiver Operating Characteristic (ROC) Curve Analysis Comparing Traditional Risk Factors with the Addition of Serum and Urinary Metabolite Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed. Available online: https://www.diabetesatlas.org/upload/resources/2019/IDF_Atlas_9th_Edition_2019.pdf (accessed on 9 September 2021).
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic Retinopathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parhofer, K.G. Interaction between Glucose and Lipid Metabolism: More than Diabetic Dyslipidemia. Diabetes Metab. J. 2015, 39, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Temelkova-Kurktschiev, T.; Siegert, G.; Bergmann, S.; Henkel, E.; Koehler, C.; Jaross, W.; Hanefeld, M. Subclinical inflammation is strongly related to insulin resistance but not to impaired insulin secretion in a high risk population for diabetes. Metabolism 2002, 51, 743–749. [Google Scholar] [CrossRef]
- Barba, I.; Garcia-Ramirez, M.; Hernandez, C.; Alonso, M.A.; Masmiquel, L.; Garcia-Dorado, D.; Simo, R. Metabolic fingerprints of proliferative diabetic retinopathy: An 1H-NMR-based metabonomic approach using vitreous humor. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4416–4421. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, C.Y.; Choi, H.; Ikram, M.K.; Sabanayagam, C.; Tan, G.S.; Tian, D.; Zhang, L.; Venkatesan, G.; Tai, E.S.; et al. Plasma Metabonomic Profiling of Diabetic Retinopathy. Diabetes 2016, 65, 1099–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munipally, P.K.; Agraharm, S.G.; Valavala, V.K.; Gundae, S.; Turlapati, N.R. Evaluation of indoleamine 2,3-dioxygenase expression and kynurenine pathway metabolites levels in serum samples of diabetic retinopathy patients. Arch. Physiol. Biochem. 2011, 117, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Paris, L.P.; Johnson, C.H.; Aguilar, E.; Usui, Y.; Cho, K.; Hoang, L.T.; Feitelberg, D.; Benton, H.P.; Westenskow, P.D.; Kurihara, T.; et al. Global metabolomics reveals metabolic dysregulation in ischemic retinopathy. Metabolomics 2016, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Sumarriva, K.; Uppal, K.; Ma, C.; Herren, D.J.; Wang, Y.; Chocron, I.M.; Warden, C.; Mitchell, S.L.; Burgess, L.G.; Goodale, M.P.; et al. Arginine and Carnitine Metabolites Are Altered in Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 2019, 60, 3119–3126. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.R.; Yang, F.Y.; Lu, J.; Zhang, H.R.; Sun, R.; Zhou, J.B.; Yang, J.K. Plasma metabolomic profiling of proliferative diabetic retinopathy. Nutr. Metab. (Lond.) 2019, 16, 37. [Google Scholar] [CrossRef] [Green Version]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Wurtz, P.; Kangas, A.J.; Soininen, P.; Lawlor, D.A.; Davey Smith, G.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 2017, 186, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Badeau, R.M.; Honka, M.J.; Lautamaki, R.; Stewart, M.; Kangas, A.J.; Soininen, P.; Ala-Korpela, M.; Nuutila, P. Systemic metabolic markers and myocardial glucose uptake in type 2 diabetic and coronary artery disease patients treated for 16 weeks with rosiglitazone, a PPARgamma agonist. Ann. Med. 2014, 46, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, R.; Jeganathan, V.S.; Zheng, Y.; Raju, P.; Cheung, N.; Tai, E.S.; Wang, J.J.; Lamoureux, E.; Mitchell, P.; Young, T.L.; et al. Methodology of the Singapore Indian Chinese Cohort (SICC) eye study: Quantifying ethnic variations in the epidemiology of eye diseases in Asians. Ophthalmic Epidemiol. 2009, 16, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Majithia, S.; Tham, Y.C.; Chee, M.L.; Nusinovici, S.; Teo, C.L.; Chee, M.L.; Thakur, S.; Da Soh, Z.; Kumari, N.; Lamoureaux, E.; et al. Cohort profile: The Singapore Epidemiology of Eye Diseases study (SEED). Int. J. Epidemiol. 2021, 50, 41–52. [Google Scholar] [CrossRef]
- Tan, G.S.; Gan, A.; Sabanayagam, C.; Tham, Y.C.; Neelam, K.; Mitchell, P.; Wang, J.J.; Lamoureux, E.L.; Cheng, C.Y.; Wong, T.Y. Ethnic Differences in the Prevalence and Risk Factors of Diabetic Retinopathy: The Singapore Epidemiology of Eye Diseases Study. Ophthalmology 2018, 125, 529–536. [Google Scholar] [CrossRef]
- Pinto, J.; Domingues, M.R.; Galhano, E.; Pita, C.; do Céu Almeida, M.; Carreira, I.M.; Gil, A.M. Human plasma stability during handling and storage: Impact on NMR metabolomics. Analyst 2014, 139, 1168–1177. [Google Scholar] [CrossRef]
- Jobard, E.; Tredan, O.; Postoly, D.; Andre, F.; Martin, A.L.; Elena-Herrmann, B.; Boyault, S. A Systematic Evaluation of Blood Serum and Plasma Pre-Analytics for Metabolomics Cohort Studies. Int. J. Mol. Sci. 2016, 17, 2035. [Google Scholar] [CrossRef] [Green Version]
- Bervoets, L.; Louis, E.; Reekmans, G.; Mesotten, L.; Thomeer, M.; Adriaensens, P.; Linsen, L. Influence of preanalytical sampling conditions on the 1H NMR metabolic profile of human blood plasma and introduction of the Standard PREanalytical Code used in biobanking. Metabolomics 2015, 11, 1197–1207. [Google Scholar] [CrossRef]
- Haid, M.; Muschet, C.; Wahl, S.; Römisch-Margl, W.; Prehn, C.; Möller, G.; Adamski, J. Long-Term Stability of Human Plasma Metabolites during Storage at −80 °C. J. Proteome Res. 2017, 17, 203–211. [Google Scholar] [CrossRef]
- Remer, T.; Montenegro-Bethancourt, G.; Shi, L. Long-term urine biobanking: Storage stability of clinical chemical parameters under moderate freezing conditions without use of preservatives. Clin. Biochem. 2014, 47, 307–311. [Google Scholar] [CrossRef]
- Hebels, D.G.; Georgiadis, P.; Keun, H.C.; Athersuch, T.J.; Vineis, P.; Vermeulen, R.; Portengen, L.; Bergdahl, I.A.; Hallmans, G.; Palli, D.; et al. Performance in omics analyses of blood samples in long-term storage: Opportunities for the exploitation of existing biobanks in environmental health research. Environ. Health Perspect. 2013, 121, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.H.; Li, J.; Feng, X.F.; Sun, X.Y.; Li, J.; Yang, X.; Fang, Z.Z. Plasma phenylalanine and tyrosine and their interactions with diabetic nephropathy for risk of diabetic retinopathy in type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e000877. [Google Scholar] [CrossRef]
- Aung, M.H.; Park, H.N.; Han, M.K.; Obertone, T.S.; Abey, J.; Aseem, F.; Thule, P.M.; Iuvone, P.M.; Pardue, M.T. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J. Neurosci. 2014, 34, 726–736. [Google Scholar] [CrossRef]
- Kim, M.K.; Aung, M.H.; Mees, L.; Olson, D.E.; Pozdeyev, N.; Iuvone, P.M.; Thule, P.M.; Pardue, M.T. Dopamine Deficiency Mediates Early Rod-Driven Inner Retinal Dysfunction in Diabetic Mice. Investig. Ophthalmol. Vis. Sci. 2018, 59, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Rankin, N.; Li, Q.; Mark, P.B.; Wurtz, P.; Ala-Korpela, M.; Marre, M.; Poulter, N.; Hamet, P.; Chalmers, J.; et al. Circulating amino acids and the risk of macrovascular, microvascular and mortality outcomes in individuals with type 2 diabetes: Results from the ADVANCE trial. Diabetologia 2018, 61, 1581–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, A.I.; Stevens, R.J.; Manley, S.E.; Bilous, R.W.; Cull, C.A.; Holman, R.R.; Ukpds, G. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003, 63, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Klein, B.E.; Moss, S.E.; Davis, M.D.; DeMets, D.L. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch. Ophthalmol. 1984, 102, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M. Management of dyslipidemia in adults with diabetes. Diabetes Care 2003, 26 (Suppl. 1), S83–S86. [Google Scholar] [CrossRef]
- The Microalbuminuria Collaborative Study Group. Predictors of the development of microalbuminuria in patients with Type 1 diabetes mellitus: A seven-year prospective study. Diabet. Med. 1999, 16, 918–925. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.W.; Zhang, Y.; Li, S.; Xu, R.X.; Sun, J.; Zhu, C.G.; Wu, N.Q.; Gao, Y.; Guo, Y.L.; et al. Analysis of Lipoprotein Subfractions in 920 Patients With and Without Type 2 Diabetes. Heart Lung Circ. 2017, 26, 211–218. [Google Scholar] [CrossRef]
- Alhazmi, A.; Stojanovski, E.; Garg, M.L.; McEvoy, M. Fasting whole blood fatty acid profile and risk of type 2 diabetes in adults: A nested case control study. PLoS ONE 2014, 9, e97001. [Google Scholar] [CrossRef] [Green Version]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kroger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Lin, J.S.; Aris, I.M.; Yang, G.; Chen, W.Q.; Li, L.J. Circulating Saturated Fatty Acids and Incident Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lankinen, M.A.; Stancakova, A.; Uusitupa, M.; Agren, J.; Pihlajamaki, J.; Kuusisto, J.; Schwab, U.; Laakso, M. Plasma fatty acids as predictors of glycaemia and type 2 diabetes. Diabetologia 2015, 58, 2533–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemaitre, R.N.; Fretts, A.M.; Sitlani, C.M.; Biggs, M.L.; Mukamal, K.; King, I.B.; Song, X.; Djousse, L.; Siscovick, D.S.; McKnight, B.; et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2015, 101, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Wu, J.H.; Wang, Q.; Lemaitre, R.N.; Mukamal, K.J.; Djousse, L.; King, I.B.; Song, X.; Biggs, M.L.; Delaney, J.A.; et al. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2015, 101, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.T.; Schalinske, K.L. Homocysteine metabolism and its relation to health and disease. Biofactors 2010, 36, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarre, S.G.; Morrow, G.; Macmillan, L.; Brosnan, M.E.; Brosnan, J.T. Formate: An essential metabolite, a biomarker, or more? Clin. Chem. Lab. Med. 2013, 51, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Sager, L.N.; Pruszynski, J.; Wesson, D.E. Acid retention in chronic kidney disease is inversely related to GFR. Am. J. Physiol. Renal. Physiol. 2018, 314, F985–F991. [Google Scholar] [CrossRef]
- Abate, N.; Chandalia, M.; Cabo-Chan, A.V., Jr.; Moe, O.W.; Sakhaee, K. The metabolic syndrome and uric acid nephrolithiasis: Novel features of renal manifestation of insulin resistance. Kidney Int. 2004, 65, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goraya, N.; Simoni, J.; Sager, L.N.; Madias, N.E.; Wesson, D.E. Urine citrate excretion as a marker of acid retention in patients with chronic kidney disease without overt metabolic acidosis. Kidney Int. 2019, 95, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Meola, M.; D’Alessandro, C.; Bernabini, G.; Pasquali, E.; Carpi, A.; Barsotti, G. Insulin resistance and low urinary citrate excretion in calcium stone formers. Biomed. Pharmacother. 2007, 61, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Karl, B.; Mathew, A.V.; Gangoiti, J.A.; Wassel, C.L.; Saito, R.; Pu, M.; Sharma, S.; You, Y.H.; Wang, L.; et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 2013, 24, 1901–1912. [Google Scholar] [CrossRef] [PubMed]

| Variables | Any DR | Moderate/above DR | VTDR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No n = 2211 | Yes n = 666 | p-Value | No n = 2549 | Yes n = 328 | p-Value | No n = 2660 | Yes n = 217 | p-Value | |
| Age, years | 61.7 (10.2) | 61.1 (9.1) | 0.128 | 61.6 (10.1) | 61.7 (8.5) | 0.759 | 61.6 (10) | 61.7 (8.3) | 0.790 |
| Gender, n (%) | 0.207 | 0.076 | 0.050 | ||||||
| Female | 1077 (75.8) | 343 (24.2) | 1243 (87.5) | 177 (12.5) | 1299 (91.5) | 121 (8.5) | |||
| Male | 1134 (77.8) | 323 (22.2) | 1306 (89.6) | 151 (10.4) | 1361 (93.4) | 96 (6.6) | |||
| Ethnicity, n (%) | <0.001 | 0.372 | 0.561 | ||||||
| Chinese | 460 (79.2) | 121 (20.8) | 516 (88.8) | 65 (11.2) | 542 (93.3) | 39 (6.7) | |||
| Malay | 806 (80) | 202 (20) | 882 (87.5) | 126 (12.5) | 934 (92.7) | 74 (7.3) | |||
| Indian | 945 (73.4) | 343 (26.6) | 1151 (89.4) | 137 (10.6) | 1184 (91.9) | 104 (8.1) | |||
| Diabetes duration, y | 5.6 (7.9) | 13.1 (10) | <0.001 | 6.4 (8.3) | 15.1 (10.1) | <0.001 | 6.6 (8.4) | 16.6 (10.2) | <0.001 |
| HbA1c, % | 7.5 (1.6) | 8.4 (1.9) | <0.001 | 7.6 (1.6) | 8.8 (2) | <0.001 | 7.6 (1.6) | 8.7 (1.9) | <0.001 |
| SBP, mm Hg | 144.3 (21.1) | 149.1 (23.7) | <0.001 | 144.2 (21.1) | 154.4 (24.8) | <0.001 | 144.6 (21.2) | 154.6 (26.5) | <0.001 |
| Total Cholesterol, mmol/L | 5.2 (1.2) | 5.1 (1.4) | 0.048 | 5.2 (1.2) | 5.2 (1.5) | 0.771 | 5.1 (1.2) | 5.3 (1.6) | 0.116 |
| LDL Cholesterol, mmol/L | 3.2 (1) | 3 (1.1) | <0.001 | 3.2 (1) | 3.1 (1.1) | 0.088 | 3.2 (1) | 3.2 (1.2) | 0.720 |
| HDL Cholesterol, mmol/L | 1.1 (0.3) | 1.2 (0.4) | 0.559 | 1.1 (0.3) | 1.2 (0.4) | 0.271 | 1.1 (0.3) | 1.2 (0.4) | 0.505 |
| Serum/Plasma/Urinary Metabolites | Any DR | Moderate/above DR | VTDR |
|---|---|---|---|
| Serum/plasma metabolites (Per SD increase) | |||
| Lipoprotein subclasses | |||
| Cholesterol esters to total lipids ratio in chylomicrons and extremely large VLDL | - | 1.42 (1.20–1.68) | 1.47 (1.20–1.79) |
| Cholesterol esters to total lipids ratio in chylomicrons and large VLDL | - | 1.36 (1.17–1.58) | - |
| Cholesterol esters to total lipids ratio in chylomicrons and very large VLDL | - | 1.54 (1.28–1.85) | - |
| Cholesterol esters to total lipids ratio in IDL | 0.79 (0.71–0.89) | 0.68 (0.59–0.79) | 0.76 (0.64–0.90) |
| Phospholipids in small LDL | - | 0.74 (0.64–0.87) | - |
| Total cholesterol to total lipids ratio in chylomicrons and extremely large VLDL | - | 1.45 (1.23–1.71) | 1.45 (1.20–1.76) |
| Total cholesterol to total lipids ratio in IDL | 0.82 (0.73–0.92) | 0.72 (0.62–0.83) | - |
| Total cholesterol to total lipids ratio in very large HDL | - | 0.75 (0.64–0.88) | - |
| Triglycerides to total lipids in chylomicrons and extremely large VLDL | - | - | 0.68 (0.55–0.83) |
| Triglycerides in IDL | - | - | 1.36 (1.14–1.61) |
| Glycerides and phospholipids | |||
| Sphingomyelins | - | 0.77 (0.67–0.89) | - |
| Apolipoproteins | |||
| Apolipoprotein A-I | - | 0.76 (0.65–0.88) | - |
| Fatty acid by saturation % | |||
| Ratio of SFA to TFA- FA saturation % | - | 0.76 (0.65–0.88) | 0.71 (0.60–0.85) |
| Amino acids | |||
| Tyrosine—Aromatic amino acid | 0.72 (0.65–0.81) | 0.69 (0.60–0.80) | 0.70 (0.59–0.83) |
| Ketone bodies | |||
| 3-hydroxybutyrate—Ketone bodies | 1.26 (1.13–1.41) | - | 1.34 (1.13–1.60) |
| Fluid balance | |||
| Creatinine | 1.44 (1.26–1.63) | 1.70 (1.45–2.01) | 1.96 (1.63–2.37) |
| Urinary metabolites (Per SD decrease) | |||
| Alanine—Amino acid | - | 1.47 (1.21–1.79) | - |
| Glutamine—Amino acid | 1.20 (1.05–1.38) | - | - |
| Citrate—Glycolysis | 1.31 (1.12–1.53) | 1.59 (1.29–1.97) | 1.61 (1.25–2.08) |
| 3-Hydroxyisobutyrate | - | 1.48 (1.23–1.77) | 1.38 (1.11–1.70) |
| 3-Hydroxyisovalerate | - | 1.45 (1.23–1.73) | 1.47 (1.22–1.77) |
| Ethanolamine—Dietary | 1.30 (1.13–1.48) | 1.78 (1.45–2.19) | 1.75 (1.38–2.22) |
| Formate—Microbial | 1.34 (1.15–1.57) | 1.78 (1.43–2.21) | 1.70 (1.32–2.18) |
| Glycolic Acid | - | 1.33 (1.10–1.60) | - |
| Hypoxanthine | 1.27 (1.11–1.46) | 1.59 (1.28–1.97) | 1.78 (1.37–2.32) |
| Uracil-Pyrimidine metabolism | - | - | 1.41 (1.11–1.79) |
| Author, Year | Study Population | Sample Type | Metabolites Platform | Results |
|---|---|---|---|---|
| Sumarriva et al. 2019 [11] | 83 DR patients and 90 Diabetic control patients | Plasma | Untargeted High-resolution MS with liquid chromatography | In DR, arginine and citrulline-related pathways are dysregulated. In PDR vs. NPDR patients, fatty acid metabolism is changed. |
| Zhu et al. 2019 [12] | 21 PDR patients, 21 controls with diabetes of >10 years but no DR | Serum | LC-MS | Impairment of metabolism of pyrimidines, arginine, and proline was associated with PDR. Fumaric acid, uridine, acetic acid, and cytidine were identified as potential PDR biomarkers. |
| Paris et al. 2016 [10] | 9 PDR patients and 11 non-diabetic control subjects | Vitreous humor | Targeted MS (RPLC-MS and HILIC-MS) | In patients with PDR, there were increases in proline, arginine, ornithine, and citrulline. |
| Munipally et al. 2011 [9] | 22 NPDR patients, 24 PDR patients, and 35 control subjects | Serum | HPLC | Increased kynurenine, kynurenic acid, and 3-hydroxykynurenine in NPDR and PDR—suggest a probable association of IDO and tryptophan metabolites with DR |
| Barba et al. 2010 [7] | 22 patients with T1DM with PDR and 22 non-diabetic patients with a macula hole | Vitreous humor | 1H NMR | Increased lactose and glucose and decreased galactitol and ascorbic acid in PDR patients. |
| Chen et al. 2016 [8] | 40 DR patients and 40 Diabetic control patients; with further validation on an independent set of 40 DR patients, 40 Diabetic control patients, and 40 controls without diabetes | Plasma | Gas chromatography-MS | In DR, there was enrichment of the pentose phosphate pathway and galactose metabolism pathway. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quek, D.Q.Y.; He, F.; Sultana, R.; Banu, R.; Chee, M.L.; Nusinovici, S.; Thakur, S.; Qian, C.; Cheng, C.-Y.; Wong, T.Y.; et al. Novel Serum and Urinary Metabolites Associated with Diabetic Retinopathy in Three Asian Cohorts. Metabolites 2021, 11, 614. https://doi.org/10.3390/metabo11090614
Quek DQY, He F, Sultana R, Banu R, Chee ML, Nusinovici S, Thakur S, Qian C, Cheng C-Y, Wong TY, et al. Novel Serum and Urinary Metabolites Associated with Diabetic Retinopathy in Three Asian Cohorts. Metabolites. 2021; 11(9):614. https://doi.org/10.3390/metabo11090614
Chicago/Turabian StyleQuek, Debra Q. Y., Feng He, Rehena Sultana, Riswana Banu, Miao Li Chee, Simon Nusinovici, Sahil Thakur, Chaoxu Qian, Ching-Yu Cheng, Tien Y. Wong, and et al. 2021. "Novel Serum and Urinary Metabolites Associated with Diabetic Retinopathy in Three Asian Cohorts" Metabolites 11, no. 9: 614. https://doi.org/10.3390/metabo11090614
APA StyleQuek, D. Q. Y., He, F., Sultana, R., Banu, R., Chee, M. L., Nusinovici, S., Thakur, S., Qian, C., Cheng, C.-Y., Wong, T. Y., & Sabanayagam, C. (2021). Novel Serum and Urinary Metabolites Associated with Diabetic Retinopathy in Three Asian Cohorts. Metabolites, 11(9), 614. https://doi.org/10.3390/metabo11090614






