Differential Glycosylation Levels in Saliva from Patients with Lung or Breast Cancer: A Preliminary Assessment for Early Diagnostic Purposes
Abstract
:1. Introduction
2. Results and Discussion
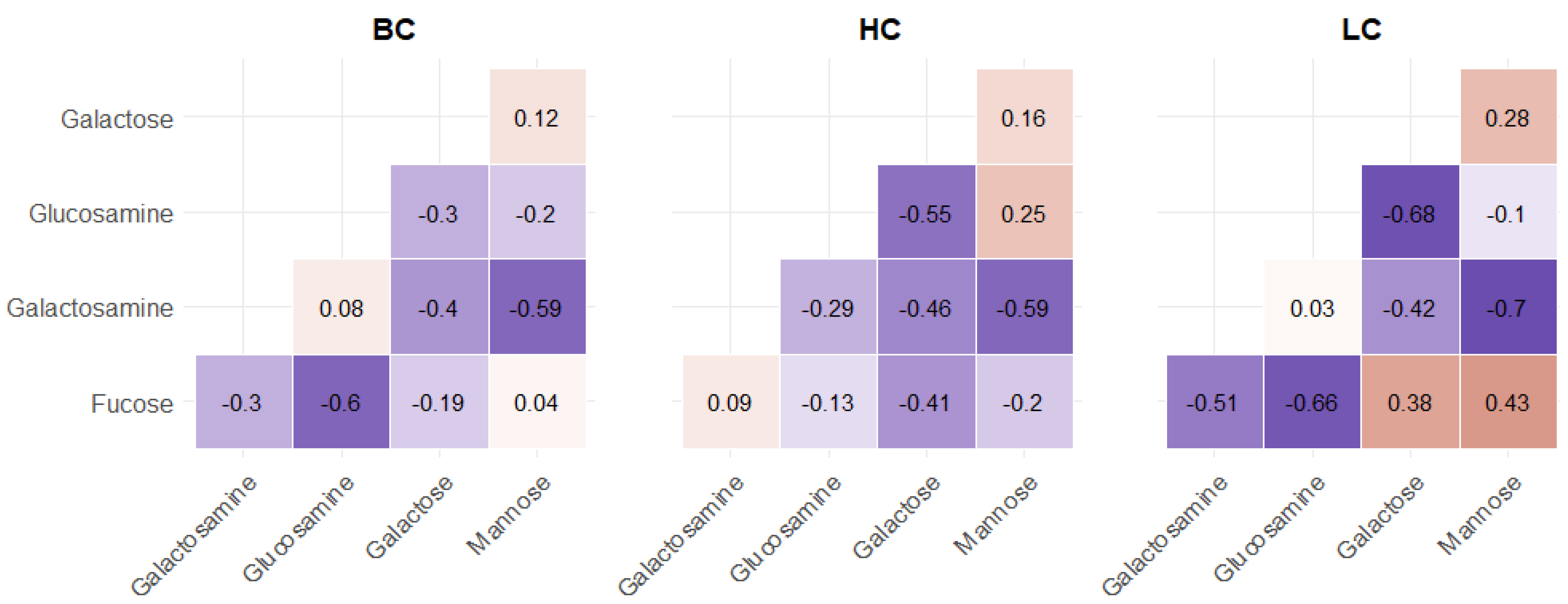
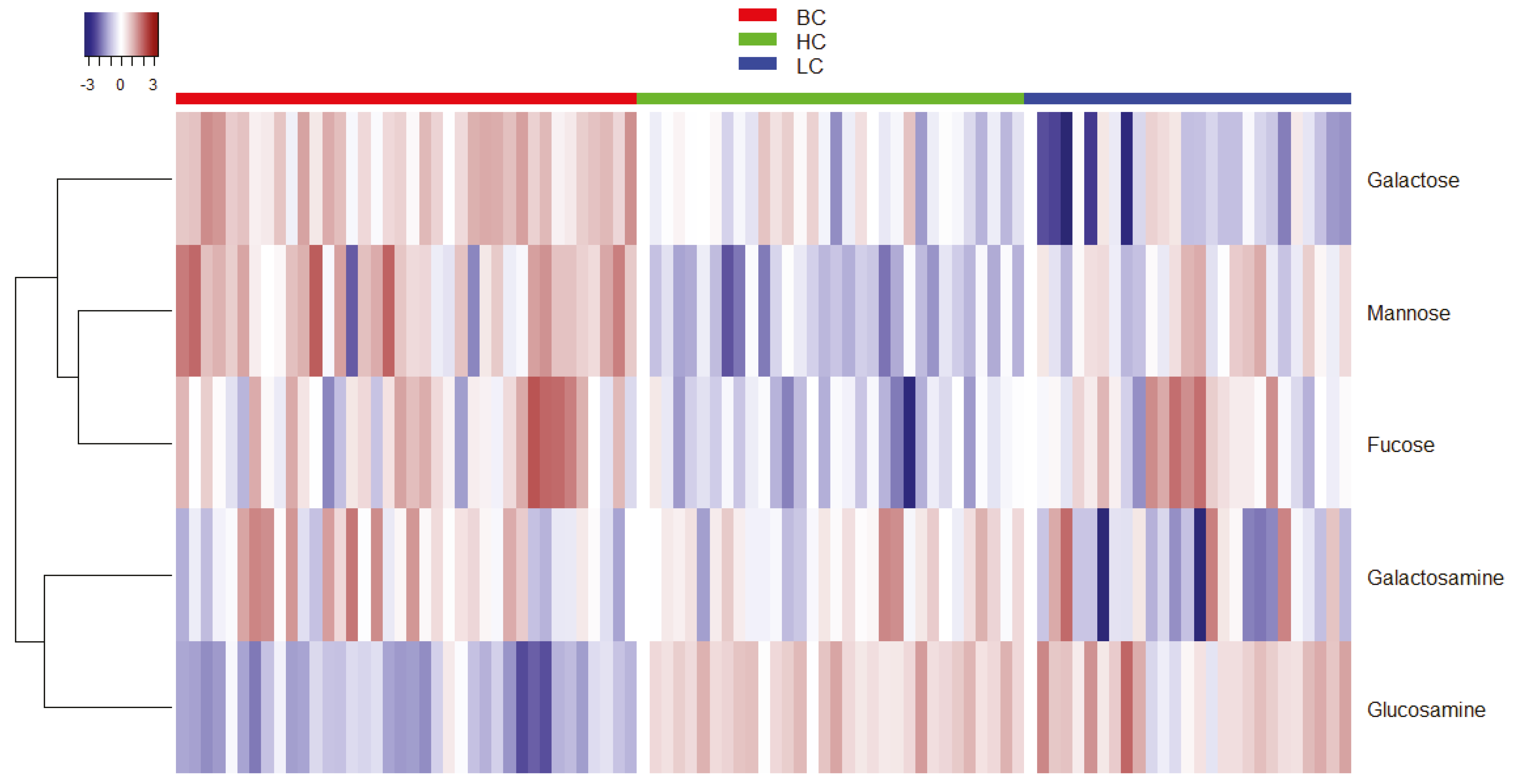
2.1. Statistical Analysis
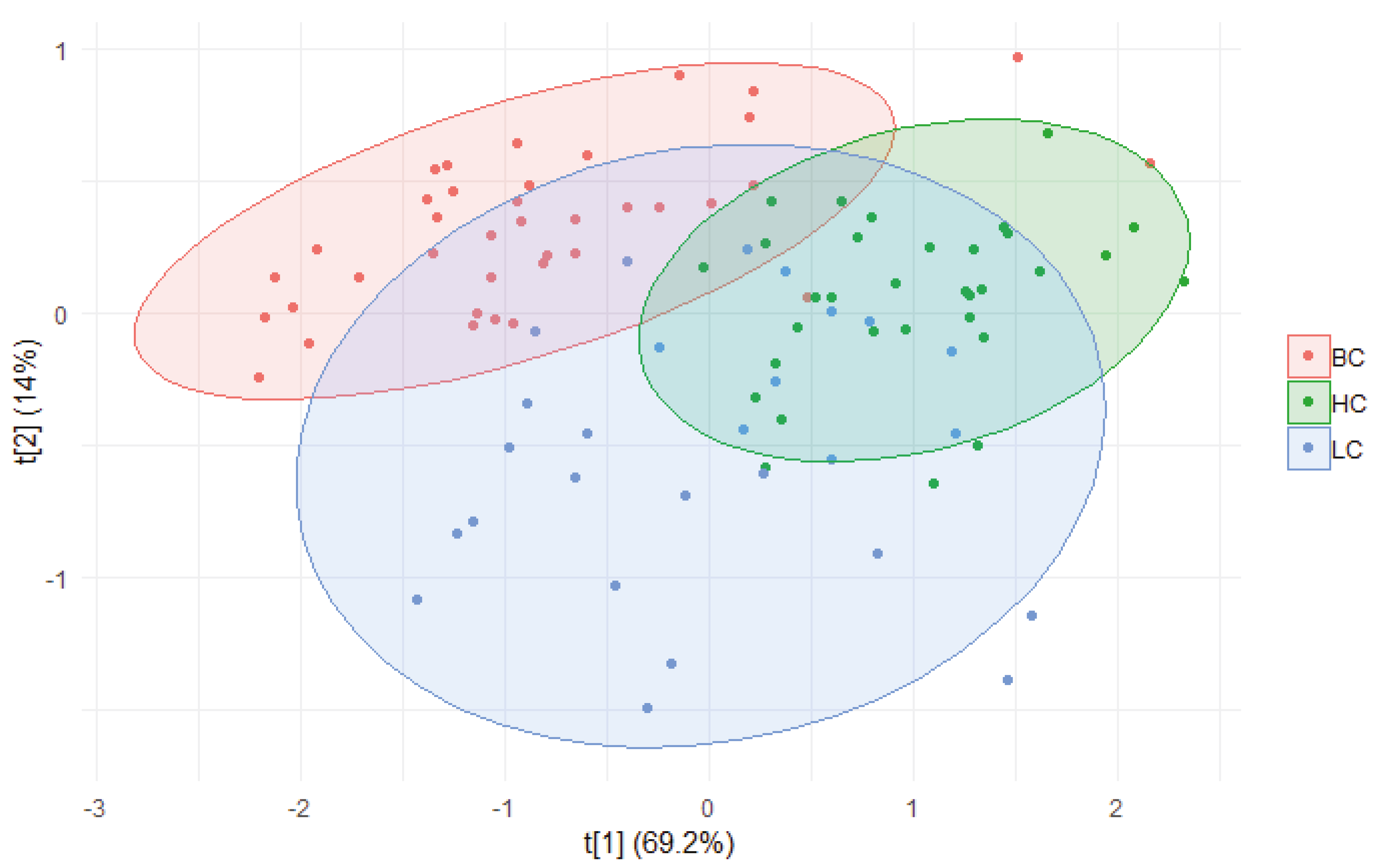
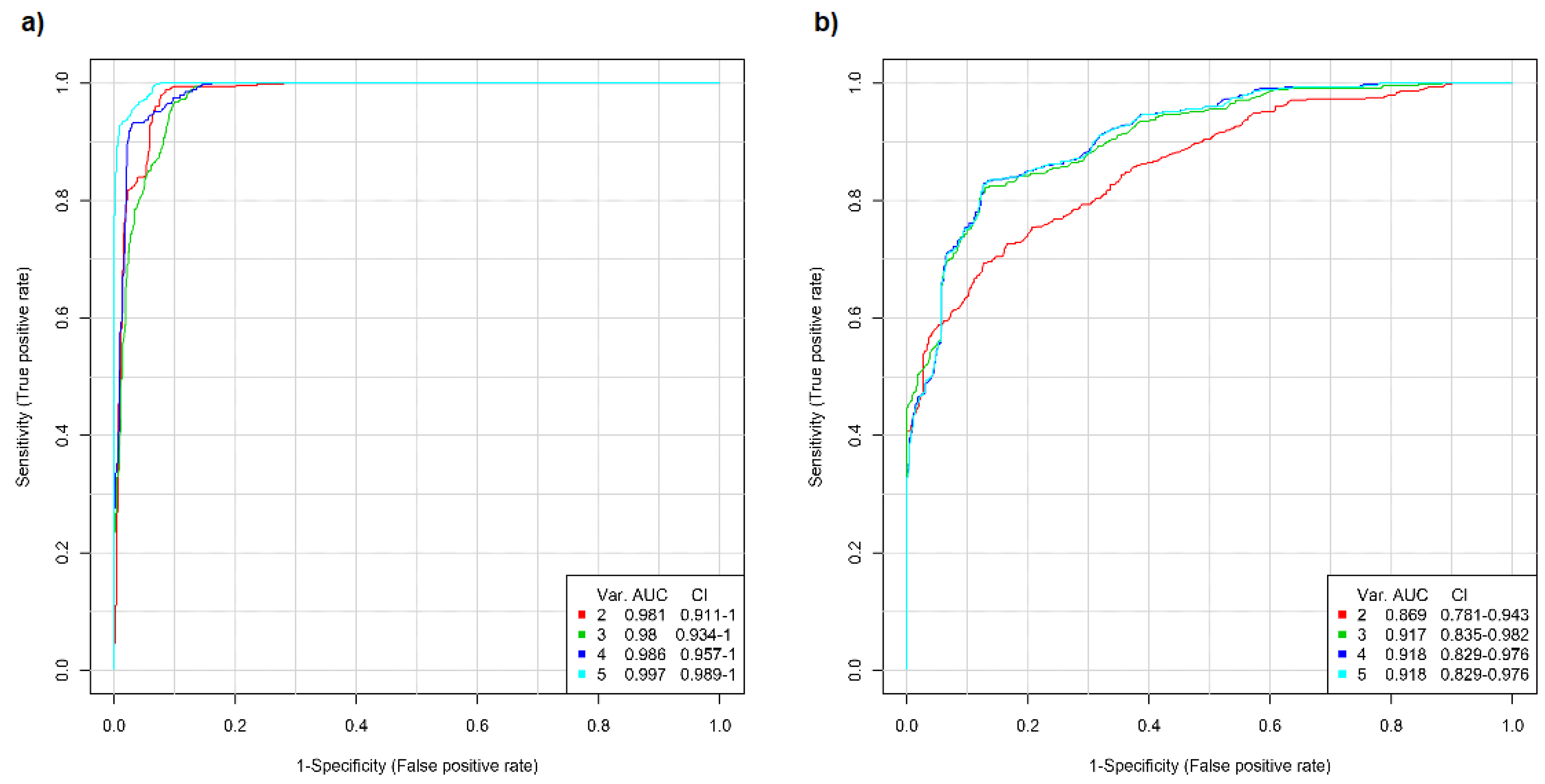
2.2. ROC Analysis
3. Materials and Methods
3.1. Cohort Recruitment and Sample Preparation
3.1.1. Patients’ Recruitment
3.1.2. Inclusion and Exclusion Criteria
3.1.3. Saliva Samples Collection
3.2. Hydrolysis Procedure
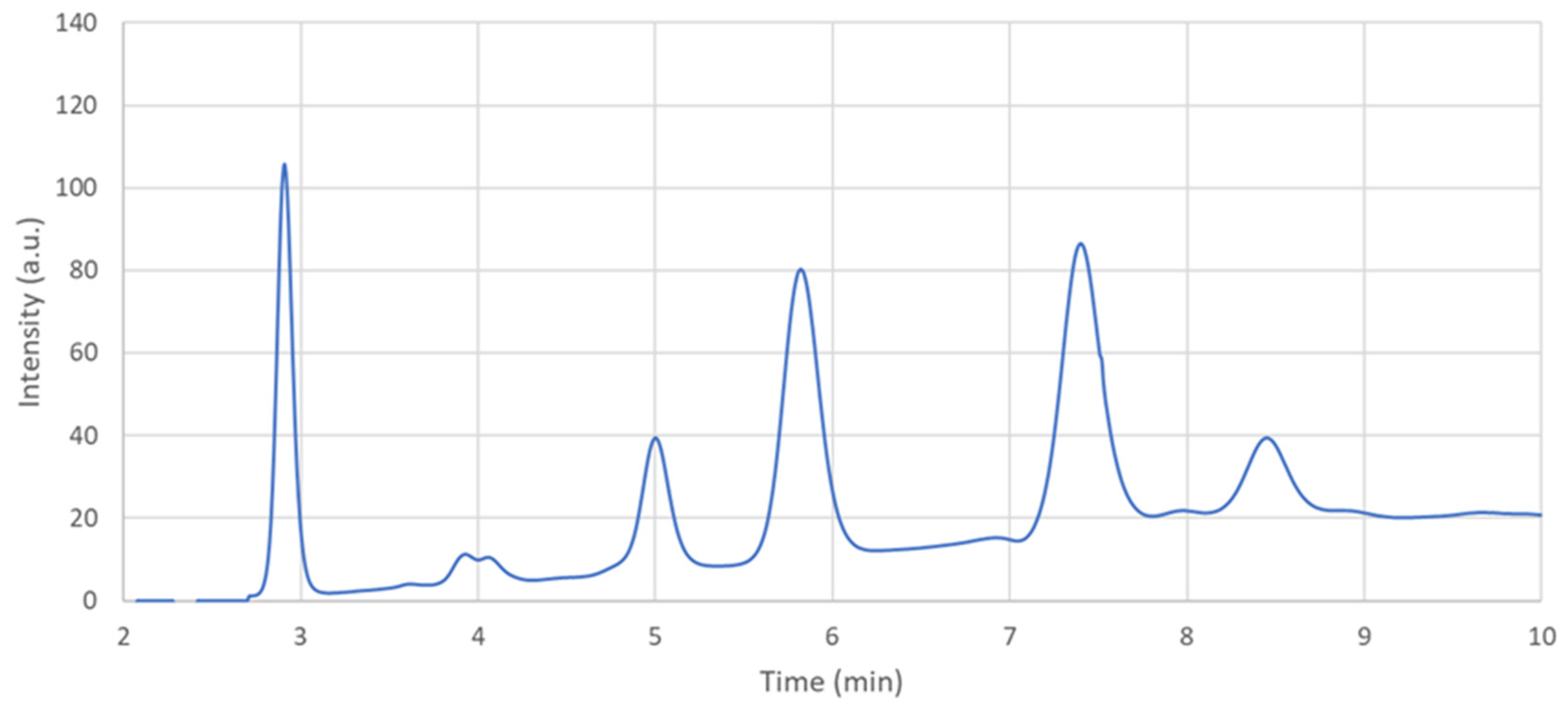
3.3. HPAEC-PAD Analysis
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Hoang, G.; Udupa, S.; Le, A. Application of metabolomics technologies toward cancer prognosis and therapy. Int. Rev. Cell. Mol. Biol. 2019, 347, 191–223. [Google Scholar] [CrossRef]
- Li, B.; He, X.; Jia, W.; Li, H. Novel applications of metabolomics in personalized medicine: A mini-review. Molecules 2017, 22, 1173. [Google Scholar] [CrossRef]
- Everett, J.R. From metabonomics to pharmacometabonomics: The role of metabolic profiling in personalized medicine. Front. Pharmacol. 2016, 7, 297. [Google Scholar] [CrossRef]
- Giudetti, A.M.; De Domenico, S.; Ragusa, A.; Lunetti, P.; Gaballo, A.; Franck, J.; Simeone, P.; Nicolardi, G.; De Nuccio, F.; Santino, A.; et al. A specific lipid metabolic profile is associated with the epithelial mesenchymal transition program. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2019, 1864, 344–357. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, S.; Ragusa, A.; Marisi, G.; De Domenico, S.; Casadei Gardini, A.; Bonafè, M.; Giudetti, A.M. Aberrant metabolism in hepatocellular carcinoma provides diagnostic and therapeutic opportunities. Oxidative Med. Cell. Longev. 2018, 2018, 7512159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Coco, L.; Vergara, D.; De Matteis, S.; Mensà, E.; Sabbatinelli, J.; Prattichizzo, F.; Bonfigli, A.R.; Storci, G.; Bravaccini, S.; Pirini, F.; et al. NMR-Based metabolomic approach tracks potential serum biomarkers of disease progression in patients with type 2 diabetes mellitus. J. Clin. Med. 2019, 8, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015. [Google Scholar]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef] [Green Version]
- Ashkani, J.; Naidoo, K.J. Glycosyltransferase gene expression profiles classify cancer types and propose prognostic subtypes. Sci. Rep. 2016, 6, 26451. [Google Scholar] [CrossRef] [Green Version]
- Häuselmann, I.; Borsig, L. Altered tumor-cell glycosylation promotes metastasis. Front. Oncol. 2014, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Drake, P.M.; Cho, W.; Li, B.; Prakobphol, A.; Johansen, E.; Anderson, N.L.; Regnier, F.E.; Gibson, B.W.; Fisher, S.J. Sweetening the pot: Adding glycosylation to the biomarker discovery equation. Clin. Chem. 2010, 56, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Saldova, R.; Struwe, W.B.; Wynne, K.; Elia, G.; Duffy, M.J.; Rudd, P.M. Exploring the glycosylation of serum CA125. Int. J. Mol. Sci. 2013, 14, 15636–15654. [Google Scholar] [CrossRef] [Green Version]
- Scott, E.; Munkley, J. Glycans as biomarkers in prostate cancer. Int. J. Mol. Sci. 2019, 20, 1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Sugano, A.; Nakamura, M.; Kaneko, M.; Ota, S.; Hiwatari, K.; Enomoto, A.; Soga, T.; et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci. Rep. 2016, 6, 31520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.; Ngo, L.Q.; Promsudthi, A.; Surarit, R. Salivary oxidative stress biomarkers in chronic periodontitis and acute coronary syndrome. Clin. Oral Investig. 2017, 21, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rehman, S.; Khurshid, Z.; Hussain Niazi, F.; Naseem, M.; Al Waddani, H.; Sahibzada, H.A.; Sannam Khan, R. Role of salivary biomarkers in detection of cardiovascular diseases (CVD). Proteomes 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Gebri, E.; Kiss, A.; Tóth, F.; Hortobágyi, T. Salivary osteopontin as a potential biomarker for oral mucositis. Metabolites 2021, 11, 208. [Google Scholar] [CrossRef]
- Cameron, S.J.; Lewis, K.E.; Beckmann, M.; Allison, G.G.; Ghosal, R.; Lewis, P.D.; Mur, L.A. The metabolomic detection of lung cancer biomarkers in sputum. Lung Cancer 2016, 94, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The human saliva metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Rai, N.P.; Anekar, J.; Shivaraja Shankara, Y.M.; Divakar, D.D.; Al Kheraif, A.A.; Ramakrishnaiah, R.; Sebastian, R.; Raj, A.C.; Al-Hazmi, A.; Mustafa, S.M. Comparison of serum fucose levels in leukoplakia and oral cancer patients. Asian Pac. J. Cancer Prev. 2015, 16, 7497–7500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.; Telang, S.; Raval, G.; Shah, P.; Patel, P.S. Serum fucosylation changes in oral cancer and oral precancerous conditions: Alpha-L-fucosidase as a marker. Cancer 2008, 113, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Sugimoto, M.; Edamatsu, K.; Sugano, A.; Kitabatake, K.; Iino, M. Discrimination of oral squamous cell carcinoma from oral lichen planus by salivary metabolomics. Oral. Dis. 2020, 26, 35–42. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.; Qiao, Y.; Yang, J.; Shu, J.; Zhang, J.; Zhang, Z.; He, J.; Li, Z. Salivary glycopatterns as potential biomarkers for screening of early-stage breast cancer. EBioMedicine 2018, 28, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva diagnostics-Current views and directions. Exp. Biol. Med. Maywood 2017, 242, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Khurshid, Z.; Zafar, M.S.; Khan, R.S.; Najeeb, S.; Slowey, P.D.; Rehman, I.U. Role of salivary biomarkers in oral cancer detection. Adv. Clin. Chem. 2018, 86, 23–70. [Google Scholar] [CrossRef] [PubMed]
- Marti-Alamo, S.; Mancheno-Franch, A.; Marzal-Gamarra, C.; Carlos-Fabuel, L. Saliva as a diagnostic fluid. Literature review. J. Clin. Exp. Dent. 2012, 4, e237–e243. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.A.; Mussavira, S.; Bindhu, O.S. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kaczor-Urbanowicz, K.E.; Wong, D.T. Salivary biomarkers in cancer detection. Med. Oncol. 2017, 34, 7. [Google Scholar] [CrossRef] [Green Version]
- Corradini, C.; Cavazza, A.; Bignardi, C. High-Performance anion-exchange chromatography coupled with pulsed electrochemical detection as a powerful tool to evaluate carbohydrates of food interest: Principles and applications. Int. J. Carbohydr. Chem. 2012, 2012, 487564. [Google Scholar] [CrossRef] [Green Version]
- Cataldi, T.R.I.; Campa, C.; De Benedetto, G.E. Carbohydrate analysis by high-performance anion-exchange chromatography with pulsed amperometric detection: The potential is still growing. Fresenius J. Anal. Chem. 2000, 368, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Lenucci, M.S.; Leucci, M.R.; Piro, G.; Dalessandro, G. Variability in the content of soluble sugars and cell wall polysaccharides in red-ripe cherry and high-pigment tomato cultivars. J. Sci. Food Agric. 2008, 88, 1837–1844. [Google Scholar] [CrossRef]
- Leucci, M.R.; Lenucci, M.S.; Piro, G.; Dalessandro, G. Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance. J. Plant Physiol. 2008, 165, 1168–1180. [Google Scholar] [CrossRef]
- Lenucci, M.S.; Durante, M.; Montefusco, A.; Dalessandro, G.; Piro, G. Possible use of the carbohydrates present in tomato pomace and in byproducts of the supercritical carbon dioxide lycopene extraction process as biomass for bioethanol production. J. Agric. Food Chem. 2013, 61, 3683–3692. [Google Scholar] [CrossRef] [PubMed]
- Talà, A.; Lenucci, M.S.; Gaballo, A.; Durante, M.; Tredici, S.M.; Debowles, D.A.; Pizzolante, G.; Marcuccio, C.; Carata, E.; Piro, G.; et al. Sphingomonas cynarae sp. nov., a proteobacterium that produces an unusual type of sphingan. Int. J. Syst. Evol. Microbiol. 2013, 63, 72–79. [Google Scholar] [CrossRef]
- Ramazzotti, M.; Stefanini, I.; Di Paola, M.; De Filippo, C.; Rizzetto, L.; Berná, L.; Dapporto, L.; Rivero, D.; Tocci, N.; Weil, T.; et al. Population genomics reveals evolution and variation of Saccharomyces cerevisiae in the human and insects gut. Environ. Microbiol. 2019, 21, 50–71. [Google Scholar] [CrossRef] [Green Version]
- Freney, M.; Irth, H.; Lindberg, H.; Alkner, U.; Greiff, L.; Persson, C.G.A.; Andersson, M.; Marko-Varga, G. Rapid screening of airway secretions for fucose by parallel ligand-exchange chromatography with post-column derivatization and fluorescence detection. Chromatographia 2001, 54, 439–445. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Taylor, S.L.; Stroble, C.; Nguyen, U.T.; Parker, E.A.; Song, T.; Lebrilla, C.B.; Rom, W.N.; Pass, H.; Kim, K.; et al. Differential N-glycosylation patterns in lung adenocarcinoma tissue. J. Proteome Res. 2015, 14, 4538–4549. [Google Scholar] [CrossRef]
- Togayachi, A.; Iwaki, J.; Kaji, H.; Matsuzaki, H.; Kuno, A.; Hirao, Y.; Nomura, M.; Noguchi, M.; Ikehara, Y.; Narimatsu, H. Glycobiomarker, fucosylated short-form secretogranin III levels are increased in serum of patients with small cell lung carcinoma. J. Proteome Res. 2017, 16, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Lin, Y.; Zhan, M.; Jiang, F. An integromic signature for lung cancer early detection. Oncotarget 2018, 9, 24684–24692. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, J.; Ma, T.; Guo, Y.; Yu, Y.; Cui, J. The function of fucosylation in progression of lung cancer. Front. Oncol. 2018, 8, 565. [Google Scholar] [CrossRef]
- Listinsky, J.J.; Siegal, G.P.; Listinsky, C.M. The emerging importance of α-L-fucose in human breast cancer: A review. Am. J. Transl. Res. 2011, 3, 292–322. [Google Scholar] [PubMed]
- Deja, S.; Porebska, I.; Kowal, A.; Zabek, A.; Barg, W.; Pawelczyk, K.; Stanimirova, I.; Daszykowski, M.; Korzeniewska, A.; Jankowska, R.; et al. Metabolomics provide new insights on lung cancer staging and discrimination from chronic obstructive pulmonary disease. J. Pharm. Biomed. Anal. 2014, 100, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Tao, T.; Zhang, Y.; Lu, H. A barcode mode based on glycosylation sites of membrane type mannose receptor as a new potential diagnostic marker for breast cancer. Talanta 2019, 191, 21–26. [Google Scholar] [CrossRef]
- Burchell, J.M.; Mungul, A.; Taylor-Papadimitriou, J. O-Linked glycosylation in the mammary gland: Changes that occur during malignancy. J. Mammary Gland Biol. Neoplasia 2001, 6, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Balog, C.I.; Stavenhagen, K.; Fung, W.L.; Koeleman, C.A.; McDonnell, L.A.; Verhoeven, A.; Mesker, W.E.; Tollenaar, R.A.; Deelder, A.M.; Wuhrer, M. N-Glycosylation of colorectal cancer tissues: A liquid chromatography and mass spectrometry-based investigation. Mol. Cell. Proteom. 2012, 11, 571–585. [Google Scholar] [CrossRef] [Green Version]
- Takakura, D.; Tada, M.; Kawasaki, N. Membrane glycoproteomics of fetal lung fibroblasts using LC/MS. Proteomics 2016, 16, 47–59. [Google Scholar] [CrossRef]
- Keeley, T.S.; Yang, S.; Lau, E. The Diverse Contributions of Fucose Linkages in Cancer. Cancers 2019, 11, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, C.F.; Wu, M.Y.; Lin, Y.C.; Kannagi, R.; Yang, R.B. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 2017, 19, 111. [Google Scholar] [CrossRef] [Green Version]
- Oswald, D.M.; Sim, E.S.; Baker, C.; Farhan, O.; Debanne, S.M.; Morris, N.J.; Rodriguez, B.G.; Jones, M.B.; Cobb, B.A. Plasma glycomics predict cardiovascular disease in patients with ART-controlled HIV infections. FASEB J. 2019, 33, 1852–1859. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Cai, S.; Ma, X.; Zeng, Q.; Qin, H.; Han, W.; Peng, X.; Wang, H. Discrimination of histopathologic types of childhood peripheral neuroblastic tumors based on clinical and biological factors. Sci. Rep. 2018, 8, 10924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Yao, F.; Yang, F.; Zhang, F.; Xu, Z.; Shi, J.; Kuno, A.; Zhao, H.; Zhang, Y. Glycomic signatures of plasma IgG improve preoperative prediction of the invasiveness of small lung nodules. Molecules 2020, 25, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, R.; Zhao, J.; Qin, W.; Zhang, Z.; Zhao, R.; Han, J.; Yang, Y.; Li, L.; Wang, X.; Ren, S.; et al. Discovery of non-invasive glycan biomarkers for detection and surveillance of gastric cancer. J. Cancer 2017, 8, 1908–1916. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Chong, J.; Yamamoto, M.; Xia, J. MetaboAnalystR 2.0: From raw spectra to biological insights. Metabolites 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]


| BC (n = 38) | HC (n = 34) | LC (n = 30) | |
|---|---|---|---|
| Gender 1 (n, %) | 0, 0% | 16, 42% | 22, 58% |
| Age (years) | 54.2 ±13.0 | 46.2 ± 10.8 | 69.8 ± 10.3 |
| BMI (kg/m2) | 26.6 ± 5.0 | 25.0 ± 3.1 | 25.2 ± 4.3 |
| Cancer Type | Clinical Information 1 | Yes | No | N.A. |
|---|---|---|---|---|
| BC | Surgery (n, %) | 18, 47% | 12, 32% | 6, 16% |
| First diagnosis (n, %) | 5, 13% | 30, 79% | 1, 3% | |
| LC | Surgery (n, %) | 7, 23% | 20, 67% | 3, 10% |
| First diagnosis (n, %) | 2, 7% | 25, 83% | 3, 10% |
| Fucose | Galactosamine | Galactose | Glucosamine | Mannose | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | HC | LC | BC | HC | LC | BC | HC | LC | BC | HC | LC | BC | HC | LC | |
| Mean | 5.07 | 4.37 | 5.70 | 5.32 | 6.99 | 5.63 | 7.79 | 7.23 | 5.73 | 10.30 | 19.47 | 17.81 | 1.69 | 0.56 | 1.11 |
| Median | 4.65 | 4.16 | 4.58 | 5.00 | 6.93 | 5.21 | 7.21 | 6.80 | 4.30 | 9.36 | 18.97 | 16.97 | 1.45 | 0.55 | 1.02 |
| sd 1 | 3.09 | 1.81 | 2.90 | 2.65 | 3.66 | 3.49 | 3.56 | 3.03 | 3.48 | 4.07 | 6.94 | 5.83 | 1.15 | 0.19 | 0.53 |
| se 2 | 0.50 | 0.32 | 0.56 | 0.43 | 0.65 | 0.67 | 0.58 | 0.54 | 0.67 | 0.66 | 1.23 | 1.12 | 0.19 | 0.03 | 0.10 |
| Group 1 | Group 2 | p-Value | Adjusted p-Value | Significance Level | |
|---|---|---|---|---|---|
| Fucose | BC | HC | 3.35 × 10−7 | 1.00 × 10−6 | **** |
| BC | LC | 0.77783 | 0.78 | ns | |
| HC | LC | 8.94 × 10−6 | 1.80 × 10−5 | **** | |
| Galactosamine | BC | HC | 0.873575 | 0.87 | ns |
| BC | LC | 0.018677 | 0.056 | * | |
| HC | LC | 0.019561 | 0.056 | * | |
| Glucosamine | BC | HC | 1.21 × 10−23 | 3.60 × 10−23 | **** |
| BC | LC | 1.68 × 10−15 | 3.40 × 10−15 | **** | |
| HC | LC | 0.773069 | 0.77 | ns | |
| Galactose | BC | HC | 1.49 × 10−8 | 4.50 × 10−8 | **** |
| BC | LC | 1.87 × 10−8 | 4.50 × 10−8 | **** | |
| HC | LC | 0.002665 | 0.0027 | ** | |
| Mannose | BC | HC | 6.47 × 10−13 | 1.90 × 10−12 | **** |
| BC | LC | 0.002107 | 0.0021 | ** | |
| HC | LC | 9.65 × 10−7 | 1.90 × 10−6 | **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragusa, A.; Romano, P.; Lenucci, M.S.; Civino, E.; Vergara, D.; Pitotti, E.; Neglia, C.; Distante, A.; Romano, G.D.; Di Renzo, N.; et al. Differential Glycosylation Levels in Saliva from Patients with Lung or Breast Cancer: A Preliminary Assessment for Early Diagnostic Purposes. Metabolites 2021, 11, 566. https://doi.org/10.3390/metabo11090566
Ragusa A, Romano P, Lenucci MS, Civino E, Vergara D, Pitotti E, Neglia C, Distante A, Romano GD, Di Renzo N, et al. Differential Glycosylation Levels in Saliva from Patients with Lung or Breast Cancer: A Preliminary Assessment for Early Diagnostic Purposes. Metabolites. 2021; 11(9):566. https://doi.org/10.3390/metabo11090566
Chicago/Turabian StyleRagusa, Andrea, Pietrina Romano, Marcello Salvatore Lenucci, Emanuela Civino, Daniele Vergara, Elena Pitotti, Cosimo Neglia, Alessandro Distante, Giampiero Diego Romano, Nicola Di Renzo, and et al. 2021. "Differential Glycosylation Levels in Saliva from Patients with Lung or Breast Cancer: A Preliminary Assessment for Early Diagnostic Purposes" Metabolites 11, no. 9: 566. https://doi.org/10.3390/metabo11090566
APA StyleRagusa, A., Romano, P., Lenucci, M. S., Civino, E., Vergara, D., Pitotti, E., Neglia, C., Distante, A., Romano, G. D., Di Renzo, N., Surico, G., Piscitelli, P., & Maffia, M. (2021). Differential Glycosylation Levels in Saliva from Patients with Lung or Breast Cancer: A Preliminary Assessment for Early Diagnostic Purposes. Metabolites, 11(9), 566. https://doi.org/10.3390/metabo11090566











