PRM-MS Quantitative Analysis of Isomeric N-Glycopeptides Derived from Human Serum Haptoglobin of Patients with Cirrhosis and Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results and Discission
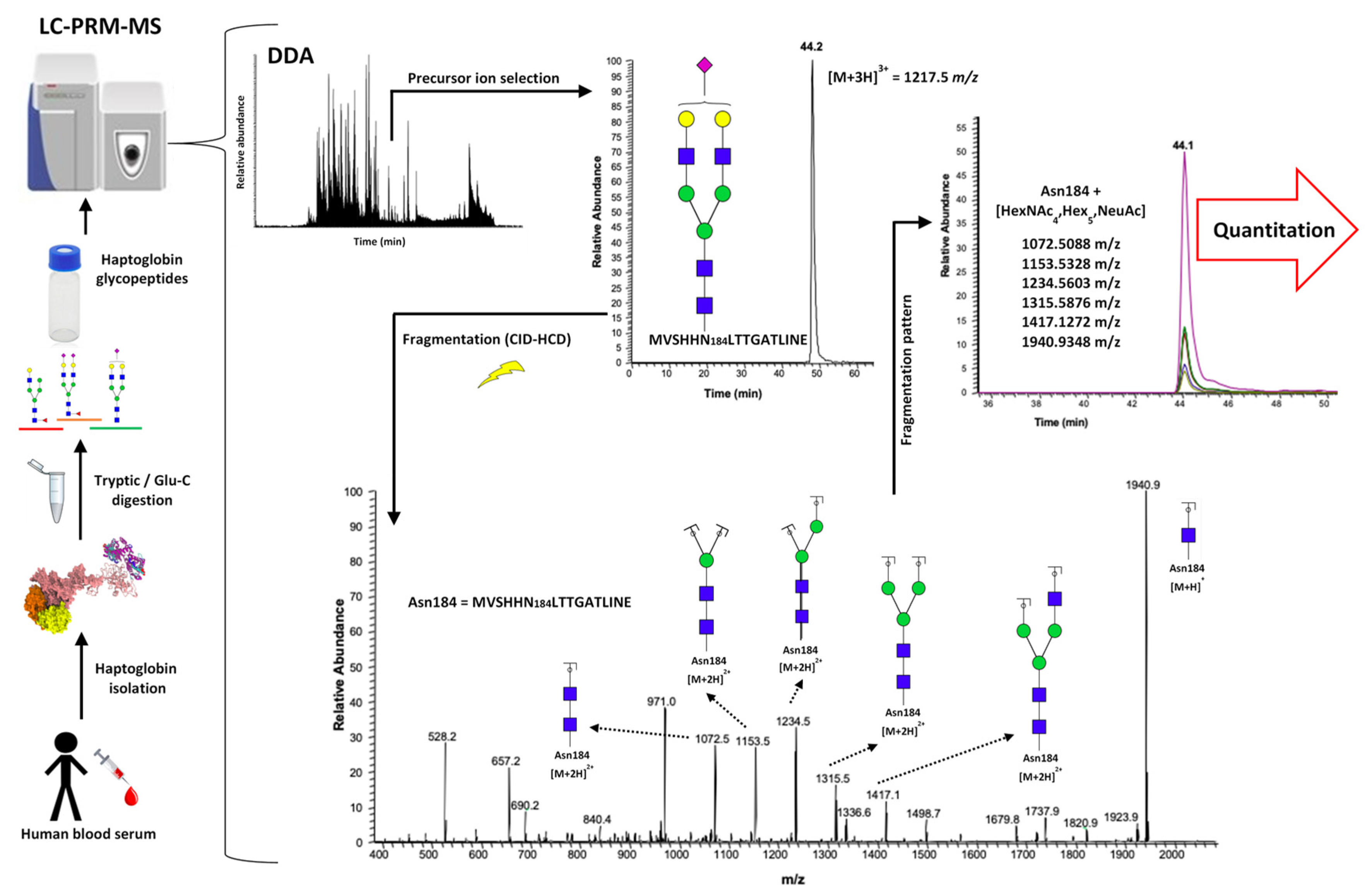
2.1. LC-PRM-MS Strategy
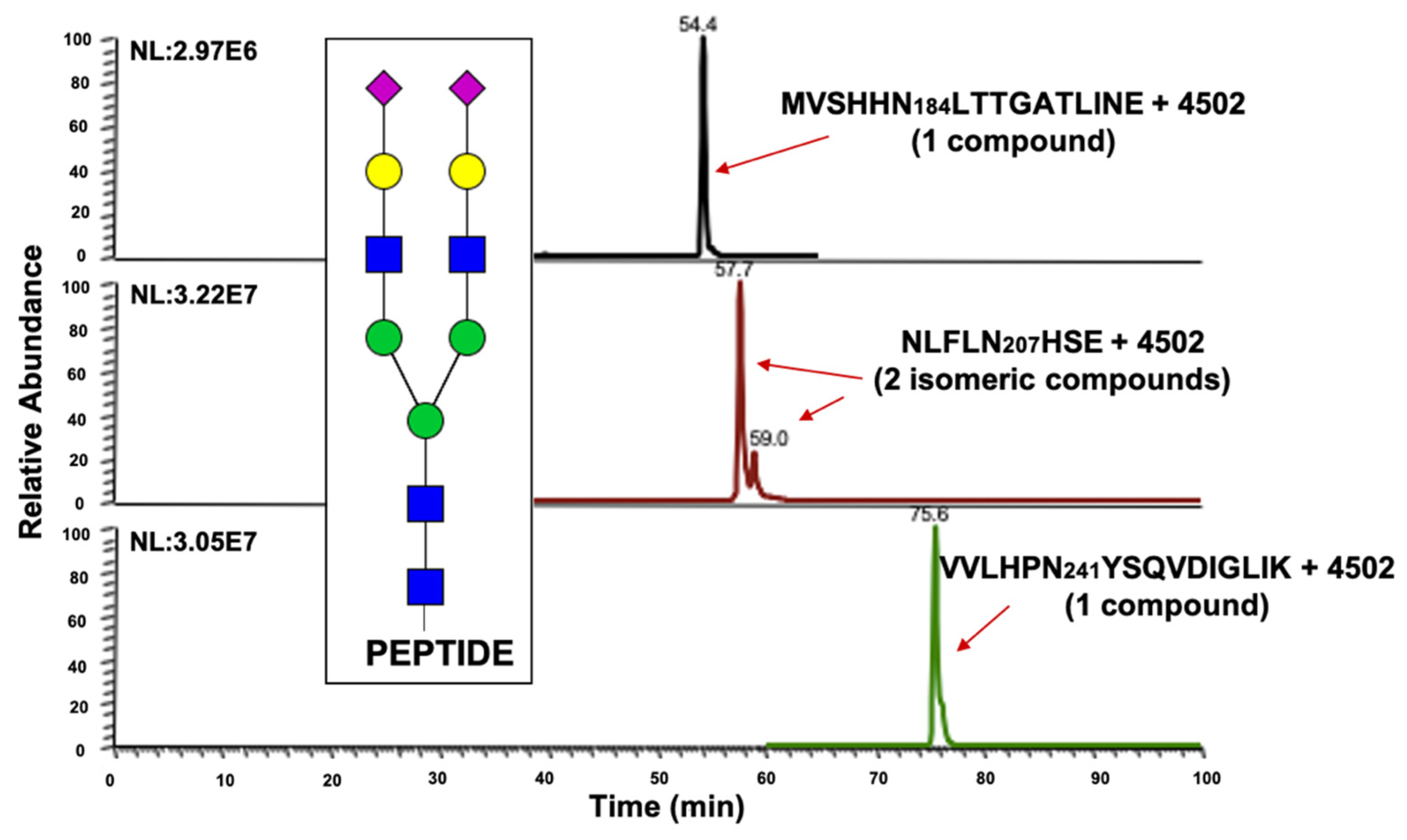
2.2. Haptoglobin Microheterogeneity
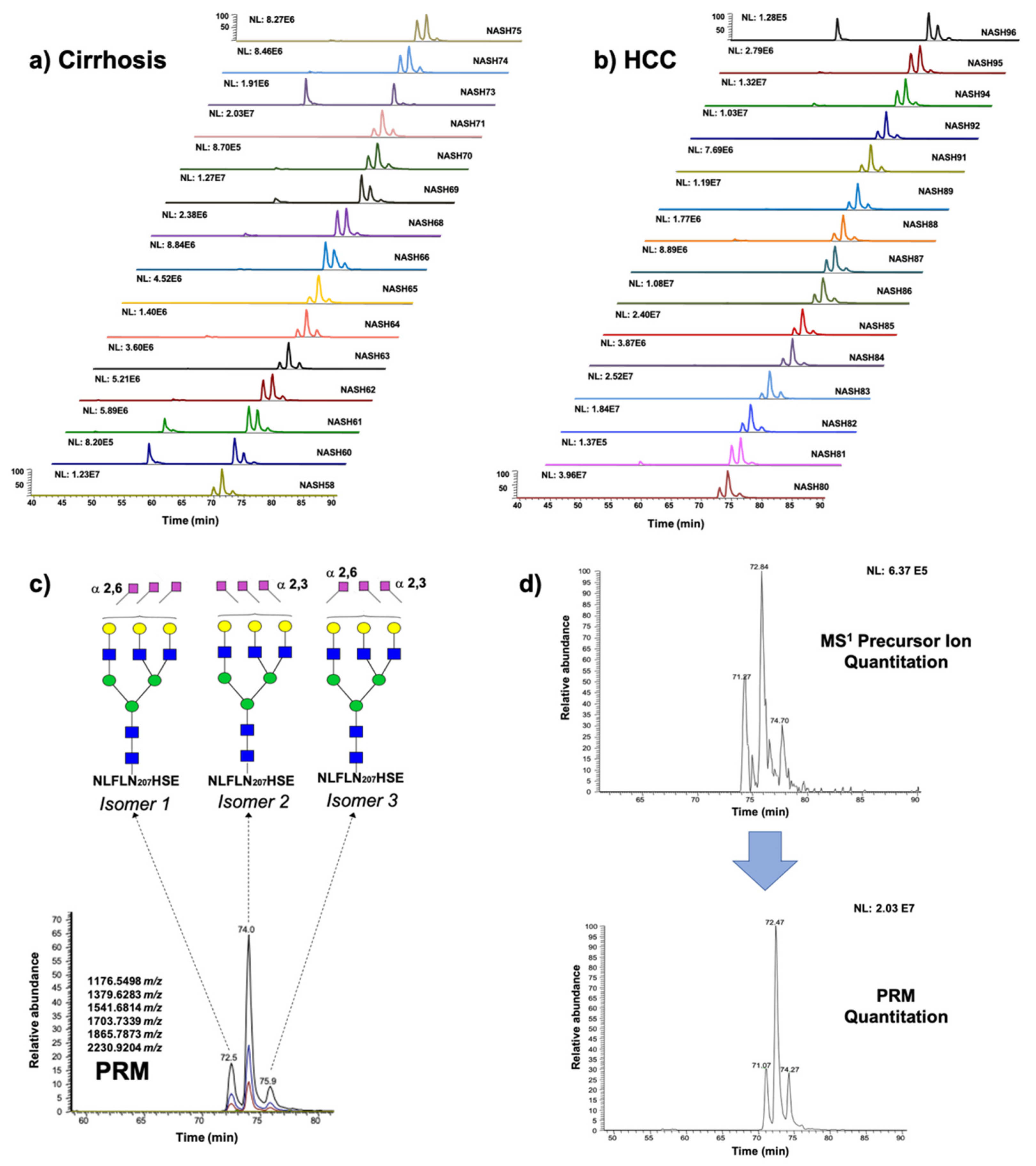
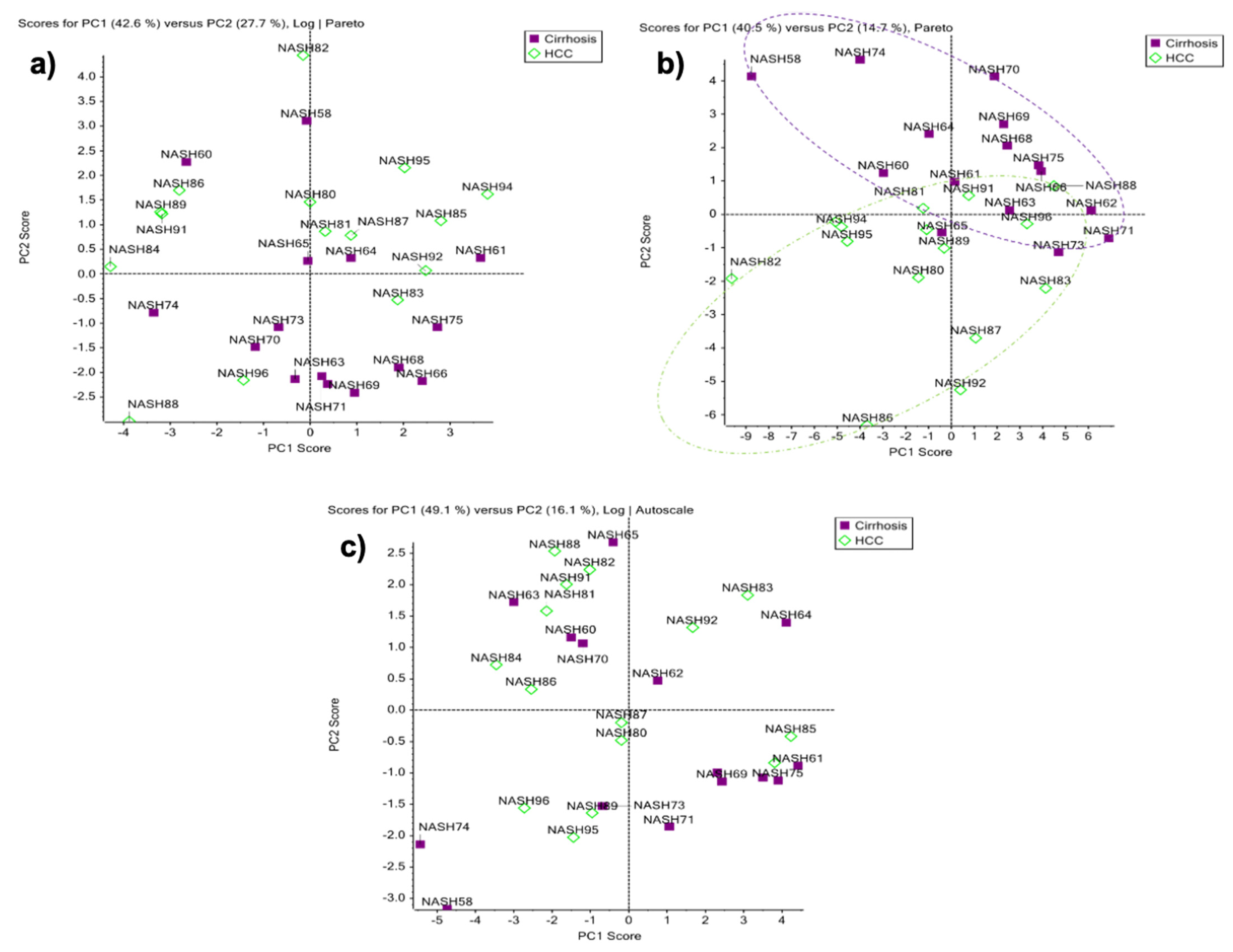
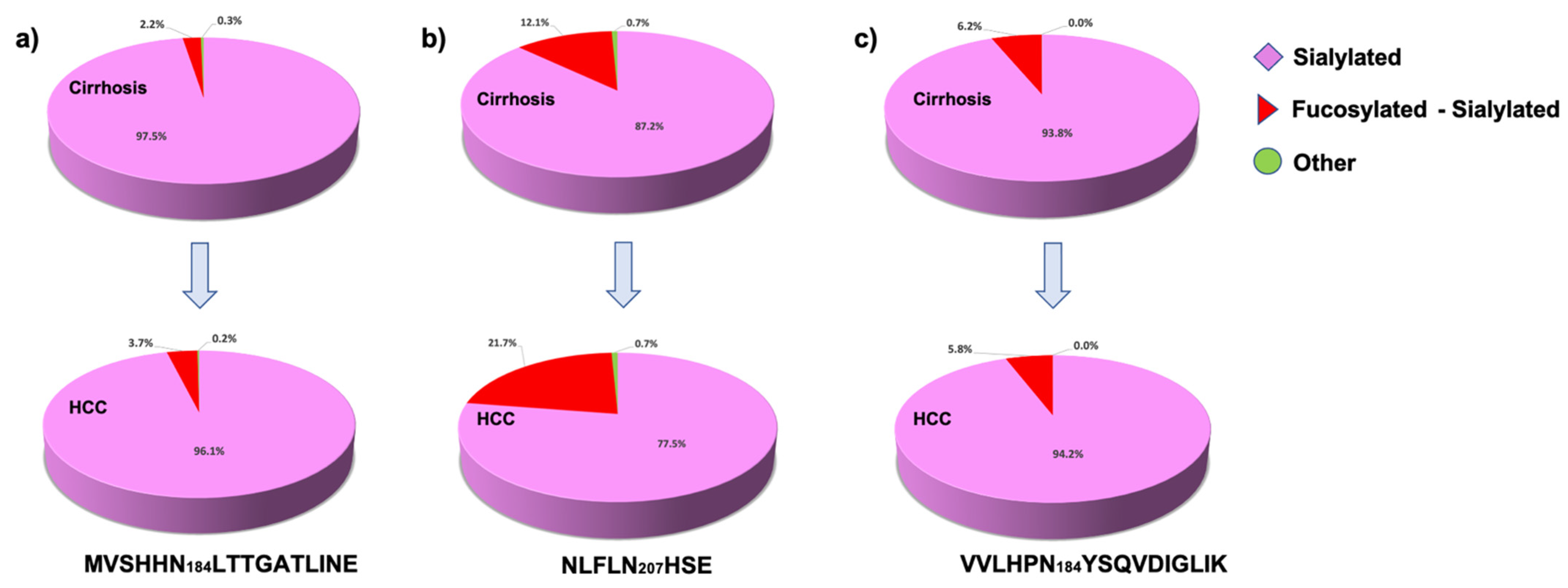
2.3. Differentially Relative Abundances of Haptoglobin N-Glycopeptides in Cirrhosis and HCC
3. Materials and Methods
3.1. Materials
3.2. Purification of Haptoglobin from Human Serum
3.3. Tryptic and Glu-C Haptoglobin Digestion
3.4. LC-PRM-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Zhou, S.; Zhu, J.; Lubman, D.M.; Mechref, Y. LC-MS/MS isomeric profiling of permethylated N-glycans derived from serum haptoglobin of hepatocellular carcinoma (HCC) and cirrhotic patients. Electrophoresis 2017, 38, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, G.W.; Jeong, J.E.; Ji, E.S.; An, H.J.; Kim, J.Y.; Yoo, J.S. Parallel reaction monitoring with multiplex immunoprecipitation of N-glycoproteins in human serum for detection of hepatocellular carcinoma. Anal. Bioanal. Chem. 2019, 411, 3009–3019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Warner, E.; Parikh, N.D.; Lubman, D.M. Glycoproteomic markers of hepatocellular carcinoma-mass spectrometry based approaches. Mass Spectrom. Rev. 2019, 38, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Li, A.A.; Perumpail, B.J.; Gadiparthi, C.; Kim, W.; Cholankeril, G.; Glenn, J.S.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Changing Trends in Etiology-Based and Ethnicity-Based Annual Mortality Rates of Cirrhosis and Hepatocellular Carcinoma in the United States. Hepatology 2019, 69, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Pillai, A.; Tiro, J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef]
- Cartier, V.; Aubé, C. Diagnosis of hepatocellular carcinoma. Diagn Interv. Imaging 2014, 95, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1701. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol 2019, 17, 748–755.e743. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Xie, S.H.; Hu, S.; Cheng, X.; Gao, T.; Zhang, C.; Song, Z. Age-specific sex difference in the incidence of hepatocellular carcinoma in the United States. Oncotarget 2017, 8, 68131–68137. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Chen, Z.; Zhang, J.; An, M.; Wu, J.; Yu, Q.; Skilton, S.J.; Bern, M.; Ilker Sen, K.; Li, L.; et al. Differential Quantitative Determination of Site-Specific Intact N-Glycopeptides in Serum Haptoglobin between Hepatocellular Carcinoma and Cirrhosis Using LC-EThcD-MS/MS. J. Proteome Res. 2019, 18, 359–371. [Google Scholar] [CrossRef]
- Comunale, M.A.; Wang, M.; Hafner, J.; Krakover, J.; Rodemich, L.; Kopenhaver, B.; Long, R.E.; Junaidi, O.; Bisceglie, A.M.; Block, T.M.; et al. Identification and development of fucosylated glycoproteins as biomarkers of primary hepatocellular carcinoma. J. Proteome Res. 2009, 8, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Pompach, P.; Ashline, D.J.; Brnakova, Z.; Benicky, J.; Sanda, M.; Goldman, R. Protein and site specificity of fucosylation in liver-secreted glycoproteins. J. Proteome Res. 2014, 13, 5561–5569. [Google Scholar] [CrossRef]
- Gutierrez Reyes, C.D.; Jiang, P.; Donohoo, K.; Atashi, M.; Mechref, Y.S. Glycomics and glycoproteomics: Approaches to address isomeric separation of glycans and glycopeptides. J. Sep. Sci. 2021, 44, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, C.; Yang, M.; Wei, M.; Huang, L.; Wang, Z. Glycoqueuing: Isomer-Specific Quantification for Sialylation-Focused Glycomics. Anal. Chem. 2019, 91, 10492–10500. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, B.; Chen, Z.; Urabe, G.; Glover, M.S.; Shi, X.; Guo, L.W.; Kent, K.C.; Li, L. Electron-Transfer/Higher-Energy Collision Dissociation (EThcD)-Enabled Intact Glycopeptide/Glycoproteome Characterization. J. Am. Soc. Mass Spectrom. 2017, 28, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhu, J.; Wang, M.; Yao, Z.P.; Lubman, D.M. Quantitative Analysis of α-1-Antitrypsin Glycosylation Isoforms in HCC Patients Using LC-HCD-PRM-MS. Anal. Chem. 2020, 92, 8201–8208. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; An, M.; So, P.K.; Wong, M.Y.; Lubman, D.M.; Yao, Z. The analysis of alpha-1-antitrypsin glycosylation with direct LC-MS/MS. Electrophoresis 2018, 39, 2351–2361. [Google Scholar] [CrossRef]
- Kurogochi, M.; Matsushista, T.; Amano, M.; Furukawa, J.; Shinohara, Y.; Aoshima, M.; Nishimura, S. Sialic acid-focused quantitative mouse serum glycoproteomics by multiple reaction monitoring assay. Mol. Cell Proteom. 2010, 9, 2354–2368. [Google Scholar] [CrossRef] [Green Version]
- Choo, M.S.; Wan, C.; Rudd, P.M.; Nguyen-Khuong, T. GlycopeptideGraphMS: Improved Glycopeptide Detection and Identification by Exploiting Graph Theoretical Patterns in Mass and Retention Time. Anal. Chem. 2019, 91, 7236–7244. [Google Scholar] [CrossRef]
- Xiao, H.; Hwang, J.E.; Wu, R. Mass spectrometric analysis of the N-glycoproteome in statin-treated liver cells with two lectin-independent chemical enrichment methods. Int. J. Mass Spectrom. 2018, 429, 66–75. [Google Scholar] [CrossRef]
- Mysling, S.; Palmisano, G.; Højrup, P.; Thaysen-Andersen, M. Utilizing Ion-Pairing Hydrophilic Interaction Chromatography Solid Phase Extraction for Efficient Glycopeptide Enrichment in Glycoproteomics. Anal. Chem. 2010, 82, 5598–5609. [Google Scholar] [CrossRef]
- Ronsein, G.E.; Pamir, N.; von Haller, P.D.; Kim, D.S.; Oda, M.N.; Jarvik, G.P.; Vaisar, T.; Heinecke, J.W. Parallel reaction monitoring (PRM) and selected reaction monitoring (SRM) exhibit comparable linearity, dynamic range and precision for targeted quantitative HDL proteomics. J. Proteom. 2015, 113, 388–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbatiello, S.E.; Mani, D.R.; Schilling, B.; Maclean, B.; Zimmerman, L.J.; Feng, X.; Cusack, M.P.; Sedransk, N.; Hall, S.C.; Addona, T.; et al. Design, implementation and multisite evaluation of a system suitability protocol for the quantitative assessment of instrument performance in liquid chromatography-multiple reaction monitoring-MS (LC-MRM-MS). Mol. Cell Proteom. 2013, 12, 2623–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, W.; Benicky, J.; Wei, R.; Goldman, R.; Sanda, M. Quantitative Analysis of Sex-Hormone-Binding Globulin Glycosylation in Liver Diseases by Liquid Chromatography-Mass Spectrometry Parallel Reaction Monitoring. J. Proteome Res. 2018, 17, 2755–2766. [Google Scholar] [CrossRef]
- Reiding, K.R.; Bondt, A.; Franc, V.; Heck, A.J.R. The benefits of hybrid fragmentation methods for glycoproteomics. TrAC Trends Anal. Chem. 2018, 108, 260–268. [Google Scholar] [CrossRef]
- West, C.A.; Wang, M.; Herrera, H.; Liang, H.; Black, A.; Angel, P.M.; Drake, R.R.; Mehta, A.S. N-Linked Glycan Branching and Fucosylation Are Increased Directly in Hcc Tissue As Determined through in Situ Glycan Imaging. J. Proteome Res. 2018, 17, 3454–3462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shang, S.; Li, W.; Qin, X.; Liu, Y. Insights on N-glycosylation of human haptoglobin and its association with cancers. Glycobiology 2016, 26, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Ji, E.S.; Lee, H.K.; Park, G.W.; Kim, K.H.; Kim, J.Y.; Yoo, J.S. Isomer separation of sialylated O- and N-linked glycopeptides using reversed-phase LC-MS/MS at high temperature. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1110–1111, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ang, I.L.; Poon, T.C.; Lai, P.B.; Chan, A.T.; Ngai, S.M.; Hui, A.Y.; Johnson, P.J.; Sung, J.J. Study of serum haptoglobin and its glycoforms in the diagnosis of hepatocellular carcinoma: A glycoproteomic approach. J. Proteome Res. 2006, 5, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Zhang, S.; Kang, X.; Li, S.; Qin, X.; Sun, C.; Lu, H.; Liu, Y. Protein expression and fucosylated glycans of the serum haptoglobin-{beta} subunit in hepatitis B virus-based liver diseases. Acta Biochim. Biophys. Sin. 2011, 43, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Lin, Z.; Wu, J.; Yin, H.; Dai, J.; Feng, Z.; Marrero, J.; Lubman, D.M. Analysis of serum haptoglobin fucosylation in hepatocellular carcinoma and liver cirrhosis of different etiologies. J. Proteome Res. 2014, 13, 2986–2997. [Google Scholar] [CrossRef] [PubMed]
- Pompach, P.; Brnakova, Z.; Sanda, M.; Wu, J.; Edwards, N.; Goldman, R. Site-specific glycoforms of haptoglobin in liver cirrhosis and hepatocellular carcinoma. Mol. Cell Proteom. 2013, 12, 1281–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, X.; Kang, X.; Sun, C.; Lu, H.; Yang, P.; Liu, Y. iTRAQ plus 18O: A new technique for target glycoprotein analysis. Talanta 2012, 91, 122–127. [Google Scholar] [CrossRef]
- Zhang, S.; Shu, H.; Luo, K.; Kang, X.; Zhang, Y.; Lu, H.; Liu, Y. N-linked glycan changes of serum haptoglobin β chain in liver disease patients. Mol. BioSyst. 2011, 7, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Asazawa, H.; Kamada, Y.; Takeda, Y.; Takamatsu, S.; Shinzaki, S.; Kim, Y.; Nezu, R.; Kuzushita, N.; Mita, E.; Kato, M.; et al. Serum fucosylated haptoglobin in chronic liver diseases as a potential biomarker of hepatocellular carcinoma development. Clin. Chem. Lab. Med. 2015, 53, 95–102. [Google Scholar] [CrossRef]
- Best, J.; Bechmann, L.P.; Sowa, J.-P.; Sydor, S.; Dechêne, A.; Pflanz, K.; Bedreli, S.; Schotten, C.; Geier, A.; Berg, T.; et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 728–735.e724. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Glycopeptide Backbone | Asn184 | Asn207 | Asn241 |
|---|---|---|---|
| N-glycosylation Microheterogeneity | --- | 3-4-0-1, 2 Isomers | --- |
| --- | 4-4-0-1, 3 Isomers | 4-4-0-1 | |
| --- | 4-5-0-0, 2 Isomers | 4-5-0-0 | |
| 4-5-0-0, 2 Isomers | 4-5-1-1, 2 Isomers | --- | |
| 4-5-1-1 | 4-5-1-2, 2 Isomers | 4-5-1-2, 2 Isomers | |
| 4-5-1-2 | 4-5-0-1 | 4-5-0-1 | |
| 4-5-0-1 | 4-5-0-2, 2 Isomers | 4-5-0-2 | |
| 4-5-0-2 | --- | --- | |
| 4-6-0-1 | 5-6-1-1, 2 Isomers | --- | |
| 5-6-1-1 | 5-6-1-2, 2 Isomers | 5-6-1-2 | |
| 5-6-1-2 | 5-6-1-3, 2 Isomers | --- | |
| 5-6-1-3 | 5-6-0-1, 2 Isomers | 5-6-0-1 | |
| 5-6-0-1 | 5-6-0-2, 2 Isomers | 5-6-0-2 | |
| 5-6-0-2, 2 Isomers | 5-6-0-3, 3 Isomers | 5-6-0-3, 3 Isomers | |
| --- | 6-7-1-1, 4 Isomers | --- | |
| --- | 6-7-1-2, 3 Isomers | --- | |
| --- | 6-7-1-3, 5 Isomers | --- | |
| --- | 6-7-0-1 | 6-7-0-1 | |
| --- | 6-7-0-2, 3 Isomers | 6-7-0-2 | |
| --- | --- | 6-7-0-3, 2 Isomers |
| Single-Glycopeptide Model, Differentiation of Cirrhosis and HCC | |||
| Glycopeptide | AUC | CI 1 (Low) | CI 1 (High) |
| AFP | 0.85 | 0.72 | 0.97 |
| Asn207 + 5-6-1-2 isomer 2 | 0.81 | 0.65 | 0.97 |
| Asn207 + 5-6-1-3 isomer 1 | 0.85 | 0.72 | 0.99 |
| Asn207 + 5-6-1-3 isomer 2 | 0.83 | 0.68 | 0.98 |
| Asn207 + 5-6-0-1 isomer 2 | 0.77 | 0.59 | 0.95 |
| Asn207 + 5-6-0-2 isomer 1 | 0.58 | 0.58 | 0.93 |
| Asn207 + 6-7-1-1 isomer 3 | 0.81 | 0.66 | 0.97 |
| Asn241 + 4-5-0-0 | 0.79 | 0.62 | 0.96 |
| AFP “early HCC” | 0.81 | 0.57 | 1.00 |
| Asn207 + 5-6-0-1 isomer 2 “early HCC” | 0.88 | 0.65 | 1.00 |
| Group-Glycopeptide Model, Differentiation of Cirrhosis and HCC | |||
| Group of Glycopeptides | AUC | CI (Low) | CI (High) |
| AFP + (Asn207 + 5-6-1-2 isomer 2) | 0.88 | 0.75 | 1.00 |
| AFP + (Asn207 + 5-6-1-3 isomer 1) | 0.94 | 0.87 | 1.00 |
| AFP + (Asn207 + 5-6-1-3 isomer 2) | 0.93 | 0.85 | 0.99 |
| AFP + (Asn207 + 5-6-1-2 isomer 1) + (Asn207 + 5-6-1-3 isomers 1 and 2) | 0.98 | 0.93 | 1.00 |
| (Asn207 + 5-6-1-2 isomer 2) + (Asn207 + 5-6-1-3 isomers 1 and 2) | 0.87 | 0.74 | 0.99 |
| Sialylated Fucosylated in Asn207 | 0.91 | 0.80 | 1.00 |
| Sialylated Fucosylated in Asn207 + Sialylated Fucosylated in Asn184 | 0.91 | 0.79 | 1.00 |
| Disease Diagnosis | Cirrhosis | HCC |
|---|---|---|
| Number | 15 1 | 15 2 |
| Etiology | NASH | NASH |
| Gender (M/F) | 3/12 | 7/8 |
| Age (mean ± SD) | 60 ± 9 | 63 ± 9 |
| AFP level 3 (median), ng/mL | 2.9 | 6.0 |
| ALT level 3 (mean ± SD) | 38 ± 20 | 47 ± 27 |
| AST level 3 (mean ± SD) | 44 ± 22 | 71 ± 50 |
| MELD 4 score (mean ± SD) | 8 ± 1.6 | 12.4 ± 6.7 |
| TNM stage, % (I *, II, III, IV) | NA | 47 */0/40/13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez Reyes, C.D.; Huang, Y.; Atashi, M.; Zhang, J.; Zhu, J.; Liu, S.; Parikh, N.D.; Singal, A.G.; Dai, J.; Lubman, D.M.; et al. PRM-MS Quantitative Analysis of Isomeric N-Glycopeptides Derived from Human Serum Haptoglobin of Patients with Cirrhosis and Hepatocellular Carcinoma. Metabolites 2021, 11, 563. https://doi.org/10.3390/metabo11080563
Gutierrez Reyes CD, Huang Y, Atashi M, Zhang J, Zhu J, Liu S, Parikh ND, Singal AG, Dai J, Lubman DM, et al. PRM-MS Quantitative Analysis of Isomeric N-Glycopeptides Derived from Human Serum Haptoglobin of Patients with Cirrhosis and Hepatocellular Carcinoma. Metabolites. 2021; 11(8):563. https://doi.org/10.3390/metabo11080563
Chicago/Turabian StyleGutierrez Reyes, Cristian D., Yifan Huang, Mojgan Atashi, Jie Zhang, Jianhui Zhu, Suyu Liu, Neehar D. Parikh, Amit G. Singal, Jianliang Dai, David M. Lubman, and et al. 2021. "PRM-MS Quantitative Analysis of Isomeric N-Glycopeptides Derived from Human Serum Haptoglobin of Patients with Cirrhosis and Hepatocellular Carcinoma" Metabolites 11, no. 8: 563. https://doi.org/10.3390/metabo11080563
APA StyleGutierrez Reyes, C. D., Huang, Y., Atashi, M., Zhang, J., Zhu, J., Liu, S., Parikh, N. D., Singal, A. G., Dai, J., Lubman, D. M., & Mechref, Y. (2021). PRM-MS Quantitative Analysis of Isomeric N-Glycopeptides Derived from Human Serum Haptoglobin of Patients with Cirrhosis and Hepatocellular Carcinoma. Metabolites, 11(8), 563. https://doi.org/10.3390/metabo11080563







