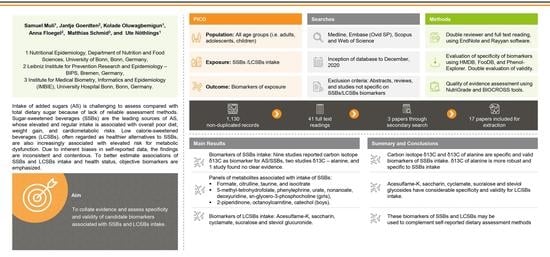
A Systematic Review of Metabolomic Biomarkers for the Intake of Sugar-Sweetened and Low-Calorie Sweetened Beverages
Abstract
1. Introduction
2. Materials and Methods
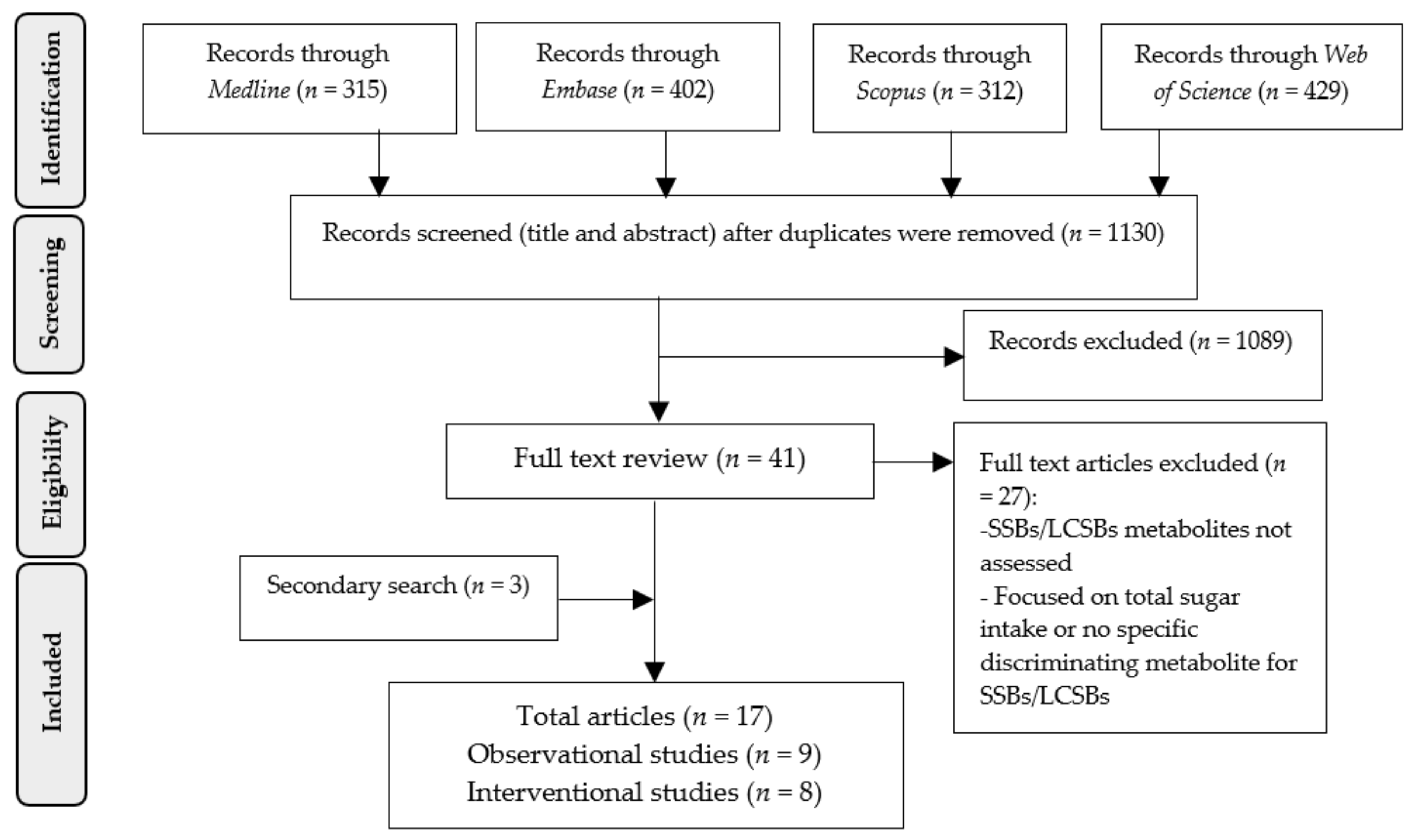
2.1. Literature Search
2.2. Evaluation of Specificity of Identified Biomarkers
2.3. Evaluating of Validity of Biomarkers
2.4. Evaluating Quality of Evidence
3. Results
3.1. Carbon Isotope Based Biomarkers for SSBs Intake
3.2. Other Candidate Biomarkers of SSBs Intake
3.3. Candidate Biomarkers of LCSBs Intake
3.4. Evaluation of Validity of Candidate Biomarkers
3.5. Risk of Bias and Quality of Study Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bowman, S.A. Added sugars: Definition and estimation in the USDA Food Patterns Equivalents Databases. J. Food Compos. Anal. 2017, 64, 64–67. [Google Scholar] [CrossRef]
- Guideline: Sugars Intake for Adults and Children. World Health Organization. Geneva. 2015. Available online: https://www.who.int/publications/i/item/9789241549028 (accessed on 25 June 2021).
- Ernst, J.B.; Arens-Azevedo, U.; Bitzer, B.; Bosy-Westphal, A.; de Zwaan, M.; Egert, S.; Fritsche, A.; Gerlach, S.; Hauner, H.; Heseker, H.; et al. Quantitative recommendation on sugar intake in Germany. Ernarhrungs Umsch. Int. 2019, 66, M78–M86. [Google Scholar]
- Perrar, I.; Schmitting, S.; Della Corte, K.W.; Buyken, A.E.; Alexy, U. Age and time trends in sugar intake among children and adolescents: Results from the DONALD study. Eur. J. Nutr. 2019, 59, 1043–1054. [Google Scholar] [CrossRef]
- Powell, E.S.; Smith-Taillie, L.P.; Popkin, B.M. Added sugars intake across the distribution of US children and adult consumers: 1977–2012. J. Acad. Nutr. Diet. 2016, 116, 1543–1545. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.C. Objective biomarkers for total added sugar intake—Are we on a wild goose chase? Adv. Nutr. 2020, 11, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Li, Q.; Zhao, Y.; Chen, Q.; Sun, X.; Liu, Y.; Li, H.; Wang, T.; Chen, X.; Zhou, Q.; et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020, 35, 655–671. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.-P.; Zheng, Y.; Li, Y.; Malik, V.; Pan, A.; Bhupathiraju, S.N.; Tobias, D.K.; Manson, J.E.; Willett, W.C.; Hu, F.B. Changes in Consumption of Sugary Beverages and Artificially Sweetened Beverages and Subsequent Risk of Type 2 Diabetes: Results from Three Large Prospective U.S. Cohorts of Women and Men. Diabetes Care 2019, 42, 2181–2189. [Google Scholar] [CrossRef]
- Dhingra, R.; Sullivan, L.; Jacques, P.F.; Wang, T.J.; Fox, C.S.; Meigs, J.B.; D’Agostino, R.B.; Gaziano, J.M.; Vasan, R.S. Soft Drink Consumption and Risk of Developing Cardiometabolic Risk Factors and the Metabolic Syndrome in Middle-Aged Adults in the Community. Circulation 2007, 116, 480–488. [Google Scholar] [CrossRef]
- de Koning, L.; Malik, V.S.; Kellogg, M.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Sweetened Beverage Consumption, Incident Coronary Heart Disease, and Biomarkers of Risk in Men. Circulation 2012, 125, 1735–1741. [Google Scholar] [CrossRef]
- Fung, T.T.; Malik, V.; Rexrode, K.; E Manson, J.; Willett, W.C.; Hu, F.B. Sweetened beverage consumption and risk of coronary heart disease in women. Am. J. Clin. Nutr. 2009, 89, 1037–1042. [Google Scholar] [CrossRef]
- Herter-Aeberli, I.; Gerber, P.; Hochuli, M.; Kohler, S.; Haile, S.; Gouni-Berthold, I.; Berthold, H.; A Spinas, G.; Berneis, K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-T.; Kao, Y.-H.; Sothern, M.S.; Seal, D.W.; Lee, C.-H.; Lin, H.-Y.; Chen, T.; Tseng, T.-S. The association between sugar-sweetened beverages intake, body mass index, and inflammation in US adults. Int. J. Public Health 2020, 65, 45–53. [Google Scholar] [CrossRef]
- Pang, M.D.; Goossens, G.H.; Blaak, E.E. The Impact of Artificial Sweeteners on Body Weight Control and Glucose Homeostasis. Front. Nutr. 2021, 7, 598340. [Google Scholar] [CrossRef]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. Can. Med. Assoc. J. 2017, 189, E929–E939. [Google Scholar] [CrossRef]
- Pase, M.P.; Himali, J.J.; Beiser, A.S.; Aparicio, H.J.; Satizabal, C.L.; Vasan, R.S.; Seshadri, S.; Jacques, P.F. Sugar- and Artificially Sweetened Beverages and the Risks of Incident Stroke and Dementia: A Prospective Cohort Study. Stroke 2017, 48, 1139–1146. [Google Scholar] [CrossRef]
- Dalenberg, J.R.; Patel, B.P.; Denis, R.; Veldhuizen, M.; Nakamura, Y.; Vinke, P.C.; Luquet, S.; Small, D.M. Short-Term Consumption of Sucralose with, but Not without, Carbohydrate Impairs Neural and Metabolic Sensitivity to Sugar in Humans. Cell Metab. 2020, 31, 493–502. [Google Scholar] [CrossRef]
- Chazelas, E.; Debras, C.; Srour, B.; Fezeu, L.K.; Julia, C.; Hercberg, S.; Deschasaux, M.; Touvier, M. Sugary drinks, artificially-sweetened beverages, and cardiovascular disease in the nutrinet-sante cohort. J. Am. Coll Cardiol. 2020, 76, 2175–2177. [Google Scholar] [CrossRef] [PubMed]
- Paeratakul, S.; Popkin, B.M.; Kohlmeier, L.; Hertz-Picciotto, I.; Guo, X.; Edwards, L. Measurement error in dietary data: Implications for the epidemiologic study of the diet–disease relationship. Eur. J. Clin. Nutr. 1998, 52, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Krebs-Smith, S.; Graubard, B.; Kahle, L.; Subar, A.; Cleveland, L.; Ballard-Barbash, R. Low energy reporters vs others: A comparison of reported food intakes. Eur. J. Clin. Nutr. 2000, 54, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Gemming, L.; Ni Mhurchu, C. Dietary under-reporting: What foods and which meals are typically under-reported? Eur. J. Clin. Nutr. 2016, 70, 640–641. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Bhupathiraju, S.N.; Hu, F.B. Use of Metabolomics in Improving Assessment of Dietary Intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef]
- Jahren, A.H.; Bostic, J.N.; Davy, B.M. The potential for a carbon stable isotope biomarker of dietary sugar intake. J. Anal. At. Spectrom. 2014, 29, 795–816. [Google Scholar] [CrossRef]
- Praticò, G.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Pedapati, S.H.; Afman, L.A.; Wishart, D.S.; et al. Guidelines for Biomarker of Food Intake Reviews (BFIRev): How to conduct an extensive literature search for biomarker of food intake discovery. Genes Nutr. 2018, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. On behalf of the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Dragsted, L.O.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Lacueva, C.A.; et al. Validation of biomarkers of food intake—critical assessment of candidate biomarkers. Genes Nutr. 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Knüppel, S.; Schwedhelm, C.; Hoffmann, G.; Missbach, B.; Stelmach-Mardas, M.; Dietrich, S.; Eichelmann, F.; Kontopantelis, E.; Iqbal, K.; et al. Perspective: NutriGrade: A Scoring System to Assess and Judge the Meta-Evidence of Randomized Controlled Trials and Cohort Studies in Nutrition Research. Adv. Nutr. 2016, 7, 994–1004. [Google Scholar] [CrossRef]
- Wirsching, J.; Graßmann, S.; Eichelmann, F.; Harms, L.M.; Schenk, M.; Barth, E.; Berndzen, A.; Olalekan, M.; Sarmini, L.; Zuberer, H.; et al. Development and reliability assessment of a new quality appraisal tool for cross-sectional studies using biomarker data (BIOCROSS). BMC Med. Res. Methodol. 2018, 18, 122. [Google Scholar] [CrossRef]
- Choy, K.; Nash, S.H.; Kristal, A.R.; Hopkins, S.; Boyer, B.B.; O’Brien, D.M. The Carbon Isotope Ratio of Alanine in Red Blood Cells Is a New Candidate Biomarker of Sugar-Sweetened Beverage Intake. J. Nutr. 2013, 143, 878–884. [Google Scholar] [CrossRef]
- Davy, B.M.; Jahren, A.H.; Hedrick, V.E.; Comber, D.L. Association of delta(1)(3)C in fingerstick blood with added-sugar and sugar-sweetened beverage intake. J. Am. Diet. Assoc. 2011, 111, 874–878. [Google Scholar] [CrossRef]
- Davy, B.M.; Jahren, A.H.; Hedrick, V.E.; You, W.; Zoellner, J.M. Influence of an intervention targeting a reduction in sugary beverage intake on the delta13C sugar intake biomarker in a predominantly obese, health-disparate sample. Public Health Nutr. 2017, 20, 25–29. [Google Scholar] [CrossRef]
- Fakhouri, T.H.I.; Jahren, A.H.; Appel, L.J.; Chen, L.; Alavi, R.; Anderson, C.A.M. Serum Carbon Isotope Values Change in Adults in Response to Changes in Sugar-Sweetened Beverage Intake. J. Nutr. 2014, 144, 902–905. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gibbons, H.; McNulty, B.; Nugent, A.; Walton, J.; Flynn, A.; Gibney, M.J.; Brennan, L. A metabolomics approach to the identification of biomarkers of sugar-sweetened beverage intake. Am. J. Clin. Nutr. 2015, 101, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, V.E.; Davy, B.M.; Wilburn, G.A.; Jahren, A.H.; Zoellner, J.M. Evaluation of a novel biomarker of added sugar intake (delta 13C) compared with self-reported added sugar intake and the Healthy Eating Index-2010 in a community-based, rural U.S. sample. Public Health Nutr. 2016, 19, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Moore, L.B.; Halliday, T.; Jahren, A.H.; Savla, J.; E Hedrick, V.; Marinik, E.L.; Davy, B.M. Short-term changes in added sugar consumption by adolescents reflected in the carbon isotope ratio of fingerstick blood. Nutr. Health 2018, 24, 251–259. [Google Scholar] [CrossRef]
- Logue, C.; Dowey, L.R.C.; Verhagen, H.; Strain, J.J.; O’Mahony, M.; Kapsokefalou, M.; Athanasatou, A.; Gallagher, A.M. A Novel Urinary Biomarker Approach Reveals Widespread Exposure to Multiple Low-Calorie Sweeteners in Adults. J. Nutr. 2020, 150, 2435–2441. [Google Scholar] [CrossRef]
- Logue, C.; Dowey, L.R.C.; Strain, J.J.; Verhagen, H.; McClean, S.; Gallagher, A.M. Application of Liquid Chromatography–Tandem Mass Spectrometry To Determine Urinary Concentrations of Five Commonly Used Low-Calorie Sweeteners: A Novel Biomarker Approach for Assessing Recent Intakes? J. Agric. Food Chem. 2017, 65, 4516–4525. [Google Scholar] [CrossRef]
- MacDougall, C.R.; E Hill, C.; Jahren, A.H.; Savla, J.; Riebl, S.K.; E Hedrick, V.; Raynor, H.; Dunsmore, J.C.; I Frisard, M.; Davy, B.M. The δ13C Value of Fingerstick Blood Is a Valid, Reliable, and Sensitive Biomarker of Sugar-Sweetened Beverage Intake in Children and Adolescents. J. Nutr. 2018, 148, 147–152. [Google Scholar] [CrossRef]
- Perng, W.; Tang, L.; Song, P.X.K.; Goran, M.; Rojo, M.M.T.; Cantoral, A.; Peterson, K.E. Urate and Nonanoate Mark the Relationship between Sugar-Sweetened Beverage Intake and Blood Pressure in Adolescent Girls: A Metabolomics Analysis in the ELEMENT Cohort. Metabolites 2019, 9, 100. [Google Scholar] [CrossRef]
- Votruba, S.B.; A Shaw, P.; Oh, E.J.; A Venti, C.; Bonfiglio, S.; Krakoff, J.; O’Brien, D.M. Associations of plasma, RBCs, and hair carbon and nitrogen isotope ratios with fish, meat, and sugar-sweetened beverage intake in a 12-wk inpatient feeding study. Am. J. Clin. Nutr. 2019, 110, 1306–1315. [Google Scholar] [CrossRef]
- Yun, H.Y.; Lampe, J.W.; Tinker, L.F.; Neuhouser, M.L.; Beresford, S.A.A.; Niles, K.R.; Mossavar-Rahmani, Y.; Snetselaar, L.G.; Van Horn, L.; Prentice, R.L.; et al. Serum Nitrogen and Carbon Stable Isotope Ratios Meet Biomarker Criteria for Fish and Animal Protein Intake in a Controlled Feeding Study of a Women’s Health Initiative Cohort. J. Nutr. 2018, 148, 1931–1937. [Google Scholar] [CrossRef]
- Yun, H.Y.; Tinker, L.F.; Neuhouser, M.L.; A Schoeller, D.; Mossavar-Rahmani, Y.; Snetselaar, L.G.; Van Horn, L.V.; Eaton, C.B.; Prentice, R.L.; Lampe, J.W.; et al. The Carbon Isotope Ratios of Serum Amino Acids in Combination with Participant Characteristics can be Used to Estimate Added Sugar Intake in a Controlled Feeding Study of US Postmenopausal Women. J. Nutr. 2020, 150, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Walter, P.J.; Garraffo, H.M.; Robien, K.; I Rother, K. Widespread sucralose exposure in a randomized clinical trial in healthy young adults. Am. J. Clin. Nutr. 2017, 105, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, L.O.; O’Grady, S.P.; Enright, L.E.; Murtaugh, M.; Sweeney, C.; Ehleringer, J.R. Evaluation of childhood nutrition by dietary survey and stable isotope analyses of hair and breath. Am. J. Hum. Biol. 2018, 30, e23103. [Google Scholar] [CrossRef] [PubMed]
- Nash, S.H.; Kristal, A.; Hopkins, S.E.; Boyer, B.B.; O’Brien, D. Stable Isotope Models of Sugar Intake Using Hair, Red Blood Cells, and Plasma, but Not Fasting Plasma Glucose, Predict Sugar Intake in a Yup’ik Study Population. J. Nutr. 2014, 144, 75–80. [Google Scholar] [CrossRef]
- Nash, S.H.; Kristal, A.R.; Boyer, B.B.; King, I.B.; Metzgar, J.S.; O’Brien, D.M. Relation between stable isotope ratios in human red blood cells and hair: Implications for using the nitrogen isotope ratio of hair as a biomarker of eicosapentaenoic acid and docosahexaenoic acid. Am. J. Clin. Nutr. 2009, 90, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Kraft, R.A.; Jahren, A.H.; Saudek, C.D. Clinical-scale investigation of stable isotopes in human blood: Delta13C and delta15N from 406 patients at the Johns Hopkins Medical Institutions. Rapid Commun. Mass Spectrom. 2008, 22, 3683–3692. [Google Scholar] [CrossRef]
- Nash, S.H.; Kristal, A.; Bersamin, A.; Hopkins, S.E.; Boyer, B.B.; O’Brien, D. Carbon and Nitrogen Stable Isotope Ratios Predict Intake of Sweeteners in a Yup’ik Study Population. J. Nutr. 2013, 143, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Shaw, P.A.; Oh, E.J.; Wooller, M.J.; Merriman, S.; Yun, H.Y.; Larsen, T.; Krakoff, J.; Votruba, S.B.; O’Brien, D.M. The carbon isotope ratios of nonessential amino acids identify sugar-sweetened beverage (SSB) consumers in a 12-wk inpatient feeding study of 32 men with varying SSB and meat exposures. Am. J. Clin. Nutr. 2021, 113, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, T.C. Rough Diamond: A Carbon Isotopic Biomarker of Added Sugar Intake. J. Nutr. 2020, 150, 2615–2616. [Google Scholar] [CrossRef]
- Hedrick, V.E.; Zoellner, J.M.; Jahren, A.H.; Woodford, N.A.; Bostic, J.N.; Davy, B.M. A Dual-Carbon-and-Nitrogen Stable Isotope Ratio Model Is Not Superior to a Single-Carbon Stable Isotope Ratio Model for Predicting Added Sugar Intake in Southwest Virginian Adults. J. Nutr. 2015, 145, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, D.A. A Novel Carbon Isotope Biomarker for Dietary Sugar. J. Nutr. 2013, 143, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, M.E.; Brosnan, J.T. Formate: The Neglected Member of One-Carbon Metabolism. Annu. Rev. Nutr. 2016, 36, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.K.; Wu, G.; Perkins-Veazie, P.; Spears, K.; Claypool, P.L.; Baker, R.A.; Clevidence, B.A. Watermelon consumption increases plasma arginine concentrations in adults. Nutritioon 2007, 23, 261–266. [Google Scholar] [CrossRef]
- Wójcik, O.P.; Koenig, K.L.; Zeleniuch-Jacquotte, A.; Costa, M.; Chen, Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 2010, 208, 19–25. [Google Scholar] [CrossRef]
- Goldberg, I.; Rokem, J. Organic and Fatty Acid Production, Microbial; Elsevier: Amsterdam, The Netherlands, 2009; pp. 421–442. [Google Scholar]
- Logue, C.; Dowey, L.C.; Strain, J.J.; Verhagen, H.; Gallagher, A.M. The Potential Application of a Biomarker Approach for the Investigation of Low-Calorie Sweetener Exposure. Proc. Nutr. Soc. 2016, 75, 216–225. [Google Scholar] [CrossRef]
- Ball, L.M.; Renwick, A.G.; Williams, R.T. The Fate of [14C]Saccharin in Man, Rat and Rabbit and of 2-Sulphamoyl[14C]benzoic Acid in the Rat. Xenobiotica 1977, 7, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Collings, A.J. Metabolism of Cyclamate and Its Conversion to Cyclohexylamine. Diabetes Care 1989, 12, 50–55. [Google Scholar] [CrossRef][Green Version]
- Renwick, A.G.; Thompson, J.P.; O’Shaughnessy, M.; Walter, E.J. The metabolism of cyclamate to cyclohexylamine in humans during long-term administration. Toxicol. Appl. Pharm. 2004, 196, 367–380. [Google Scholar] [CrossRef]
- Grice, H.; Goldsmith, L. Sucralose—An overview of the toxicity data. Food Chem. Toxicol. 2000, 38, S1–S6. [Google Scholar] [CrossRef]
- Wheeler, A.; Boileau, A.; Winkler, P.; Compton, J.; Prakash, I.; Jiang, X.; Mandarino, D. Pharmacokinetics of rebaudioside A and stevioside after single oral doses in healthy men. Food Chem. Toxicol. 2008, 46, S54–S60. [Google Scholar] [CrossRef]
- Huth, P.J.; Fulgoni, V.L.; Keast, D.R.; Park, K.; Auestad, N. Major food sources of calories, added sugars, and saturated fat and their contribution to essential nutrient intakes in the U.S. diet: Data from the national health and nutrition examination survey (2003–2006). Nutr. J. 2013, 12, 116. [Google Scholar] [CrossRef]
- Tasevska, N.; Runswick, S.A.; Welch, A.A.; McTaggart, A.; Bingham, S.A. Urinary sugars biomarker relates better to extrinsic than to intrinsic sugars intake in a metabolic study with volunteers consuming their normal diet. Eur. J. Clin. Nutr. 2008, 63, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Tasevska, N.; Midthune, D.; Potischman, N.; Subar, A.F.; Cross, A.J.; Bingham, S.A.; Schatzkin, A.; Kipnis, V. Use of the Predictive Sugars Biomarker to Evaluate Self-Reported Total Sugars Intake in the Observing Protein and Energy Nutrition (OPEN) Study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 490–500. [Google Scholar] [CrossRef]
- Tasevska, N.; Midthune, D.; Tinker, L.F.; Potischman, N.; Lampe, J.W.; Neuhouser, M.L.; Beasley, J.; Van Horn, L.; Prentice, R.L.; Kipnis, V. Use of a Urinary Sugars Biomarker to Assess Measurement Error in Self-Reported Sugars Intake in the Nutrition and Physical Activity Assessment Study (NPAAS). Cancer Epidemiol. Biomark. Prev. 2014, 23, 2874–2883. [Google Scholar] [CrossRef] [PubMed]
- Tasevska, N. Urinary Sugars—A Biomarker of Total Sugars Intake. Nutrition 2015, 7, 5816–5833. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.C.; Tasevska, N.; Lentjes, M.; Griffin, J.L.; Sims, M.A.; Richardson, L.; Aspinall, S.M.; Mulligan, A.A.; Luben, R.; Khaw, K.-T. Association between sucrose intake and risk of overweight and obesity in a prospective sub-cohort of the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk). Public Health Nutr. 2015, 18, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.; Luben, R.; Welch, A.; Tasevska, N.; Wareham, N.; Khaw, K.T. Epidemiologic Assessment of Sugars Consumption Using Biomarkers: Comparisons of Obese and Nonobese Individuals in the European Prospective Investigation of Cancer Norfolk. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Louie, J.C. Methodology for the assessment of added/free sugar intake in epidemiological studies. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 271–277. [Google Scholar] [CrossRef]
- Nash, S.H.; Kristal, A.R.; Bersamin, A.; Choy, K.; Hopkins, S.E.; Stanhope, K.L.; Havel, P.J.; Boyer, B.B.; O’Brien, D.M. Isotopic estimates of sugar intake are related to chronic disease risk factors but not obesity in an Alaska native (Yup’ik) study population. Eur. J. Clin. Nutr. 2013, 68, 91–96. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.M. Stable Isotope Ratios as Biomarkers of Diet for Health Research. Annu. Rev. Nutr. 2015, 35, 565–594. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Madrid-Gambin, F.; Garcia-Aloy, M.; Andres-Lacueva, C.; Logue, C.; Gallagher, A.M.; Mack, C.; Kulling, S.E.; Gao, Q.; Praticò, G.; et al. Biomarkers of intake for coffee, tea, and sweetened beverages. Genes Nutr. 2018, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
| Study, Country, [Reference] | Number of Participants | Age Range (Years) | Dietary Assessment Method | Sample Type | Chemical Analytic Method | Analytic Approach | Candidate Biomarker of Food Intake/Metabolite |
|---|---|---|---|---|---|---|---|
| Davy et al. 2017, USA [31] | 301 | ≥18 | 24-h recall (×3) | Fasting fingerstick blood | NA-SIMS | Targeted | δ13C |
| Choy et al. 2013, USA [29] | 68 | 14–79 | 24-h recall (×4) | Red blood cells, hair | GC-IRMS | Targeted | δ13C–alanine |
| Davy et al. 2011, USA [30] | 60 | ≥21 | 4-d DR | fingerstick blood | NA-SIMS | Targeted | δ13C |
| Fakhouri et al. 2014, USA [32] | 144 | 25–79 | 24-h recall (×2) | Serum, after 8-h fast | IRMS | Targeted | δ13C |
| Hedrick et al. 2016, USA [34] | 216 | ≥18 | 24-h recall (×3) | Fasting fingerstick blood | IRMS | Targeted | δ13C |
| Nash et al. 2014, USA [45] | 68 | 14–79 | 24-h recall (×4) | Red blood cells, plasma, hair | IRMS | Targeted | δ13C |
| Votruba et al. 2019, USA [40] | 32 | 46.2 (10.5) a | 7-d DR | Plasma, hair, Red blood cells | IRMS | Targeted | δ13C |
| Liu et al. 2018, USA [35] | 33 | 12–18 | 24-h recall (×8) | Fasting fingerstick blood | NA-SIMS | Targeted | δ13C |
| Yun et al. 2018, USA [41] ** | 153 | 75 (4) a | 4-d DR | Serum | IRMS | Targeted | δ13C |
| Yun et al. 2020, USA [42] | 145 | 75 (73, 78) b | 4-d DR | Serum AAs | GC-IRMS | Targeted | δ13C–alanine |
| MacDougall et al. 2018, USA [38] | 126 | 6–11 | 24-h recall (×4) | Fingerstick blood | IRMS | Targeted | δ13C |
| Valenzuela et al. 2018, USA [44] | 212 | 9–16 | FFQ | Hair, Breath | GC-IRMS | Targeted | δ13C |
| Gibbons et al. 2015, Ireland [33] | 565 | ≥18 | 4-d DR | Urine | H-NMR | Untargeted | Formate, citrulline, taurine, and isocitrate |
| Perng et al. 2019, Mexico [39] | 242 | 8–14 | FFQ | Fasting serum | LC/MS | Untargeted | Girls: 5-methyl-tetrohydrofolate, phenylephrine, urate, nonanoate, deoxyuridine, and sn-glycero-3-phosphocholine Boys: 2-piperidinone, octanoylcarnitine, and catechol |
| Logue et al. 2020, NL [36] | 79 | 19–70 | 7-d DR | 24-h urine | LC-MS | Targeted | acesulfame-K, saccharin, cyclamate, and sucralose steviol glycosides |
| Logue et al. 2017, NL [37] | 21 | 25.7 (4.9) a | 7-d DR | Fasting spot and 24-h urine | LC-MS | Targeted | Acesulfame-K, saccharin, sucralose, cyclamate, and steviol glycosides |
| Sylvetsky et al. 2017, USA [43] | 18 | 18–35 | 7-d DR | Spot urine | LC/MS | Targeted | Sucralose |
| Compound/Metabolite | HMDB ID | Sample Type | Validation Criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a | 3b | 4 | 5 | 6 | 7 | 8 | Max. Points = 9 | References | |||
| δ13C | - | RBCs, plasma, breath, hair | Y | Y | Y | Y | Y | Y | Y | Y | U | 8 | [6,23,30,31,32,34,35,38,40,41,44,45,48,51] |
| δ13C of alanine | HMDB0000161 | Blood, serum, hair | Y | Y | Y | Y | Y | Y | Y | Y | U | 8 | [29,42,49,50,52] |
| Formate | HMDB0000142 | Urine | N | Y | Y | U | Y | U | U | Y | N | 4 | [33,53] |
| Citrulline | HMDB0000904 | Urine | N | Y | Y | U | Y | U | U | Y | N | 4 | [33,54] |
| Taurine | HMDB0000251 | Urine | N | Y | Y | U | Y | U | U | Y | N | 4 | [33,55] |
| Isocitrate | HMDB0000193 | Urine | N | Y | Y | U | Y | U | U | Y | N | 4 | [33,56] |
| 5-Methyl-tetrohydrofolate | HMDB0001396 | Serum | N | Y | U | U | Y | U | U | Y | N | 2 | [39] |
| Phenylephrine | HMDB0002182 | Serum | N | U | U | U | Y | U | U | Y | N | 2 | [39] |
| Urate | HMDB0000289 | Serum | N | U | U | U | Y | U | U | Y | N | 2 | [39] |
| Nonanoate | HMDB0031264 | Serum | N | U | U | U | Y | U | U | Y | N | 2 | [39] |
| Deoxyuridine | HMDB0000012 | Serum | N | U | U | U | Y | U | U | Y | N | 2 | [39] |
| Sn-glycero-3-phosphocholine | HMDB0000086 | Serum | N | U | U | U | Y | U | U | Y | N | 2 | [39] |
| 2-Piperidinone | HMDB0011749 | Serum | N | U | U | U | Y | U | U | Y | N | 2 | [39] |
| Cctanoylcarnitine | HMDB0000791 | Serum | N | U | U | U | Y | U | U | Y | N | 2 | [39] |
| Catechol | HMDB0240490 | serum | N | U | U | U | Y | U | U | Y | N | 2 | [39] |
| Compound/Metabolite | HMDB ID | Sample Type | Validation Criteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a | 3b | 4 | 5 | 6 | 7 | 8 | Max. Points = 9 | References | |||
| Acesulfame-K | HMDB0033585 | Urine | Y | Y | Y | U | Y | Y | U | Y | U | 6 | [36,57] |
| Saccharin | HMDB0029723 | Urine | Y | Y | Y | U | Y | Y | U | Y | U | 6 | [36,37,58] |
| Cyclamate | HMDB0031340 | Urine | Y | Y | Y | U | Y | Y | U | Y | U | 6 | [36,37,57,59,60] |
| Sucralose | HMDB0031554 | Urine | Y | Y | Y | U | Y | Y | U | Y | U | 6 | [36,43,57,61] |
| Steviol glycosides | HMDB0036707 | Urine | Y | Y | Y | U | Y | Y | U | Y | U | 6 | [14,37,57,62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muli, S.; Goerdten, J.; Oluwagbemigun, K.; Floegel, A.; Schmid, M.; Nöthlings, U. A Systematic Review of Metabolomic Biomarkers for the Intake of Sugar-Sweetened and Low-Calorie Sweetened Beverages. Metabolites 2021, 11, 546. https://doi.org/10.3390/metabo11080546
Muli S, Goerdten J, Oluwagbemigun K, Floegel A, Schmid M, Nöthlings U. A Systematic Review of Metabolomic Biomarkers for the Intake of Sugar-Sweetened and Low-Calorie Sweetened Beverages. Metabolites. 2021; 11(8):546. https://doi.org/10.3390/metabo11080546
Chicago/Turabian StyleMuli, Samuel, Jantje Goerdten, Kolade Oluwagbemigun, Anna Floegel, Matthias Schmid, and Ute Nöthlings. 2021. "A Systematic Review of Metabolomic Biomarkers for the Intake of Sugar-Sweetened and Low-Calorie Sweetened Beverages" Metabolites 11, no. 8: 546. https://doi.org/10.3390/metabo11080546
APA StyleMuli, S., Goerdten, J., Oluwagbemigun, K., Floegel, A., Schmid, M., & Nöthlings, U. (2021). A Systematic Review of Metabolomic Biomarkers for the Intake of Sugar-Sweetened and Low-Calorie Sweetened Beverages. Metabolites, 11(8), 546. https://doi.org/10.3390/metabo11080546