The Impact of Photorespiratory Glycolate Oxidase Activity on Arabidopsis thaliana Leaf Soluble Amino Acid Pool Sizes during Acclimation to Low Atmospheric CO2 Concentrations
Abstract
:1. Introduction
2. Results
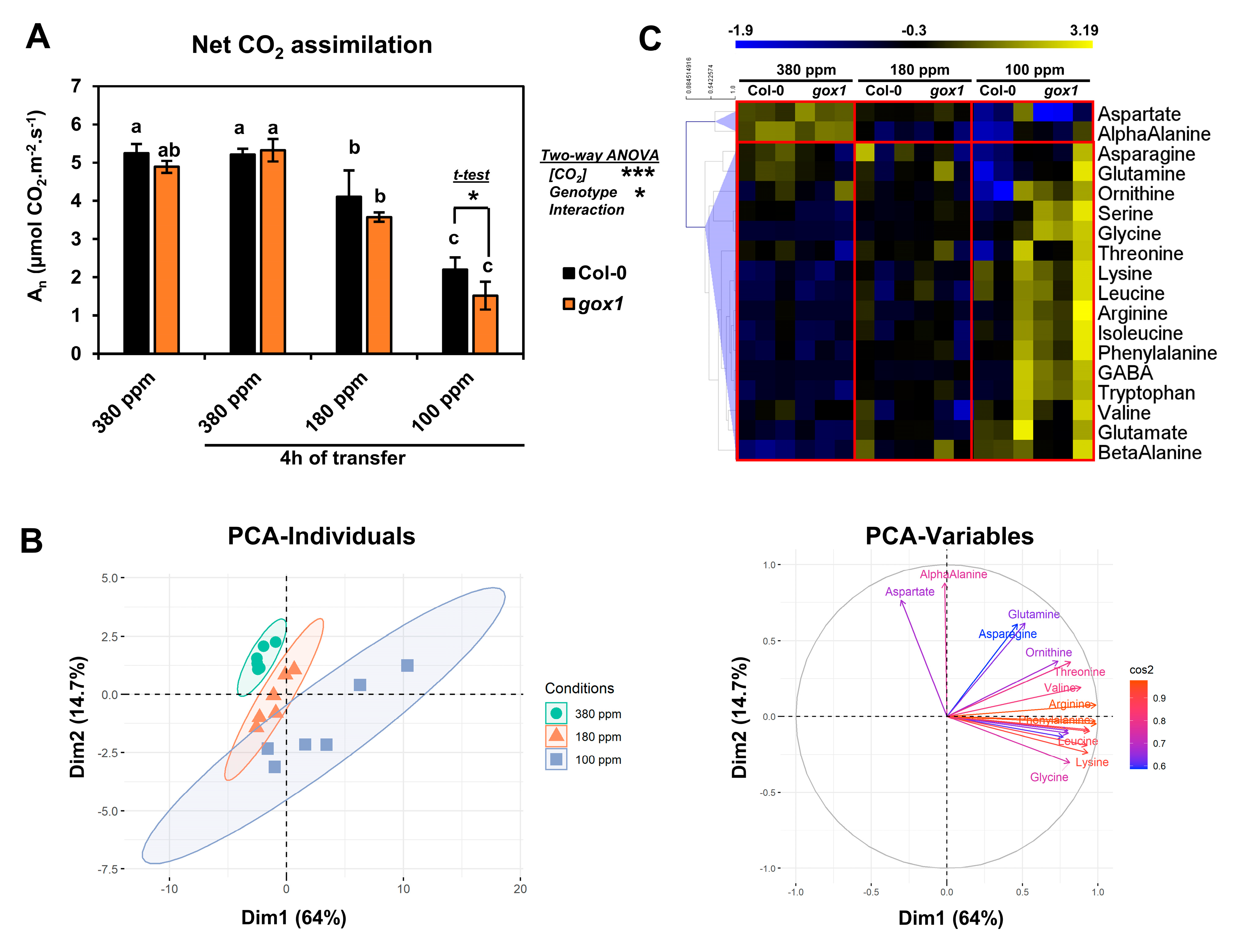
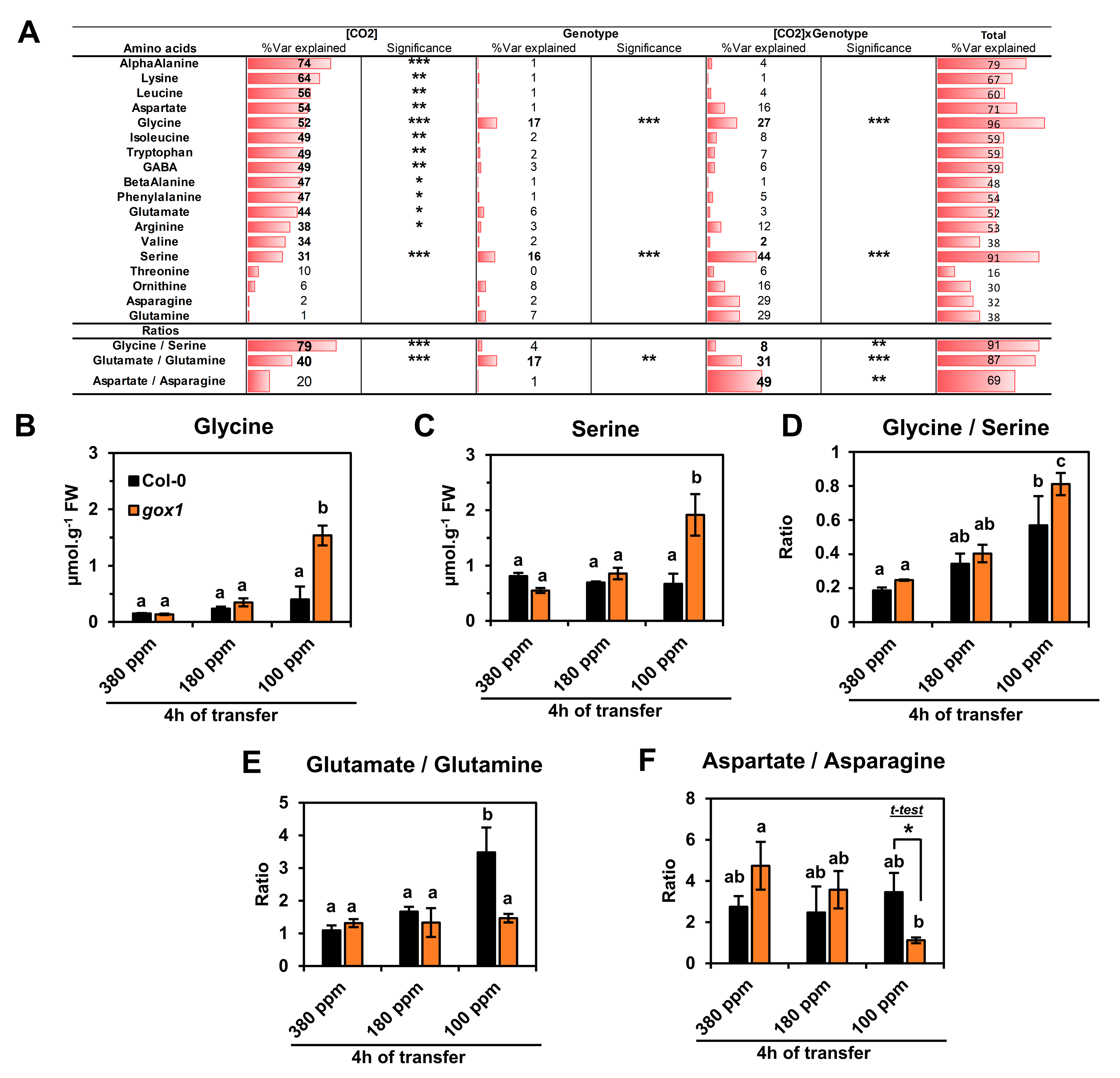
2.1. Impact of a Short-Term Low CO2 Treatment on Photosynthesis and Amino Acid Metabolism in the ARABIDOPSIS gox1 Mutant
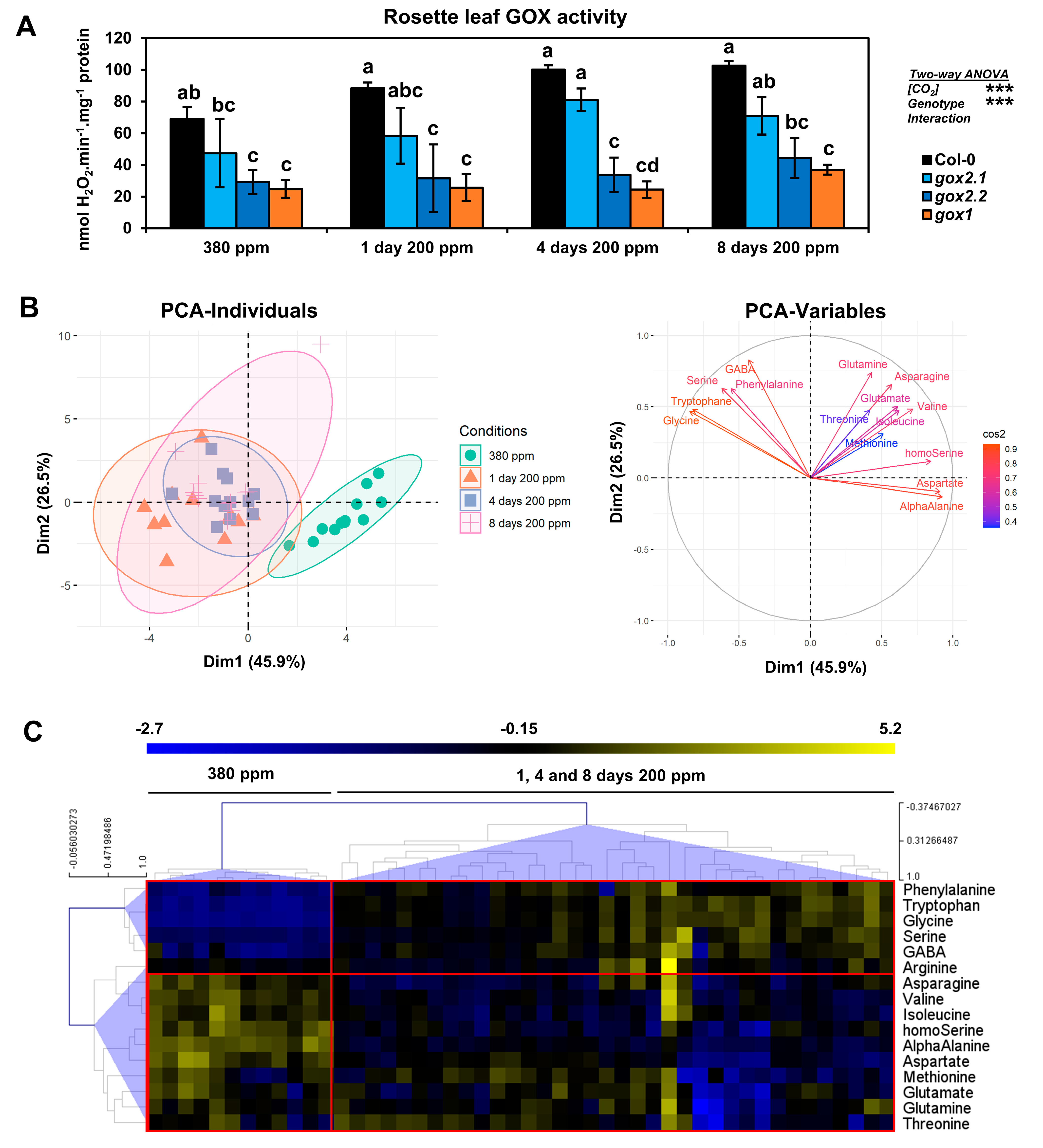
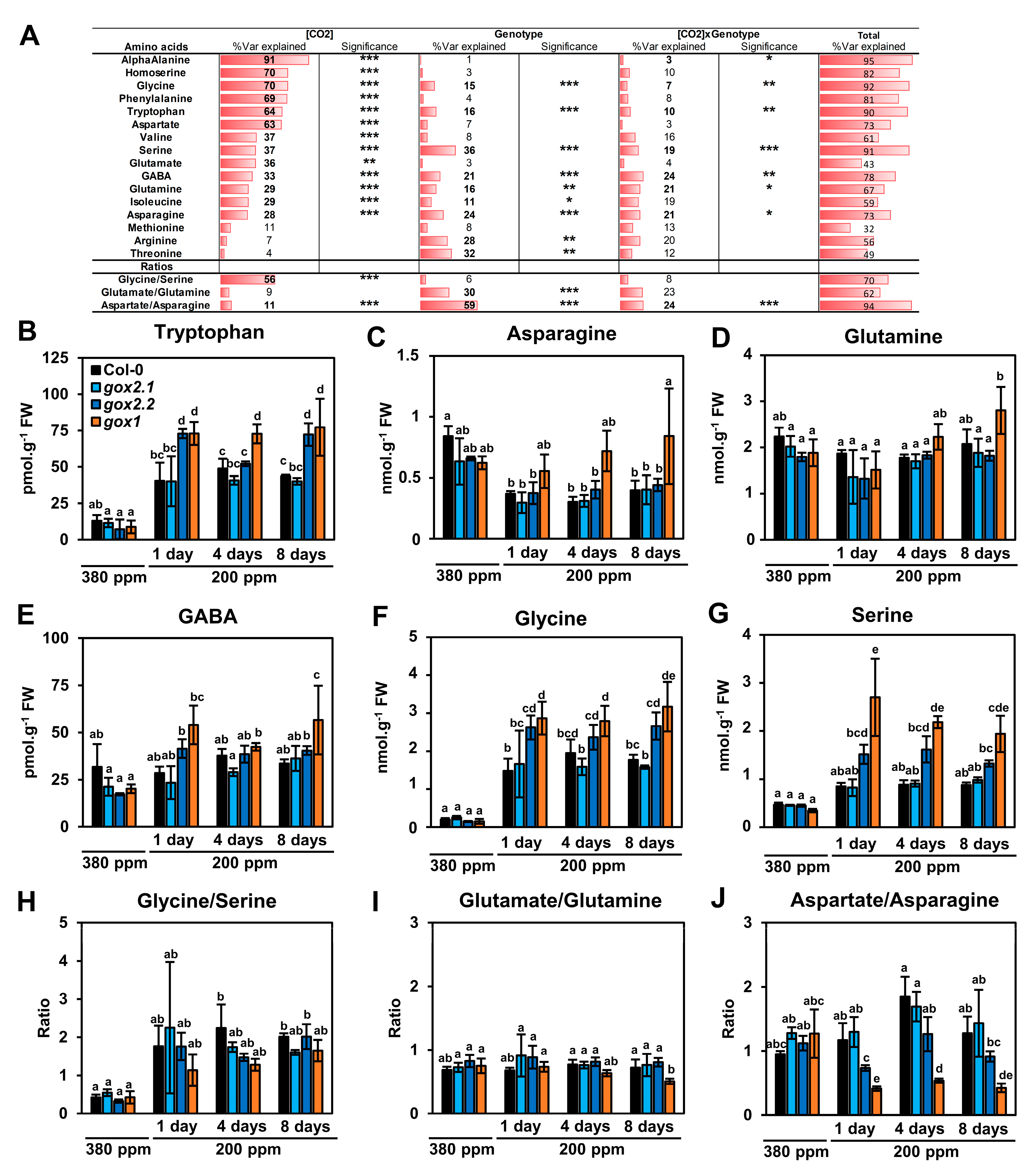
2.2. Impact of a Long-Term Low CO2 Treatment on Amino Acid Metabolism in gox1 and gox2 Mutants
3. Discussion
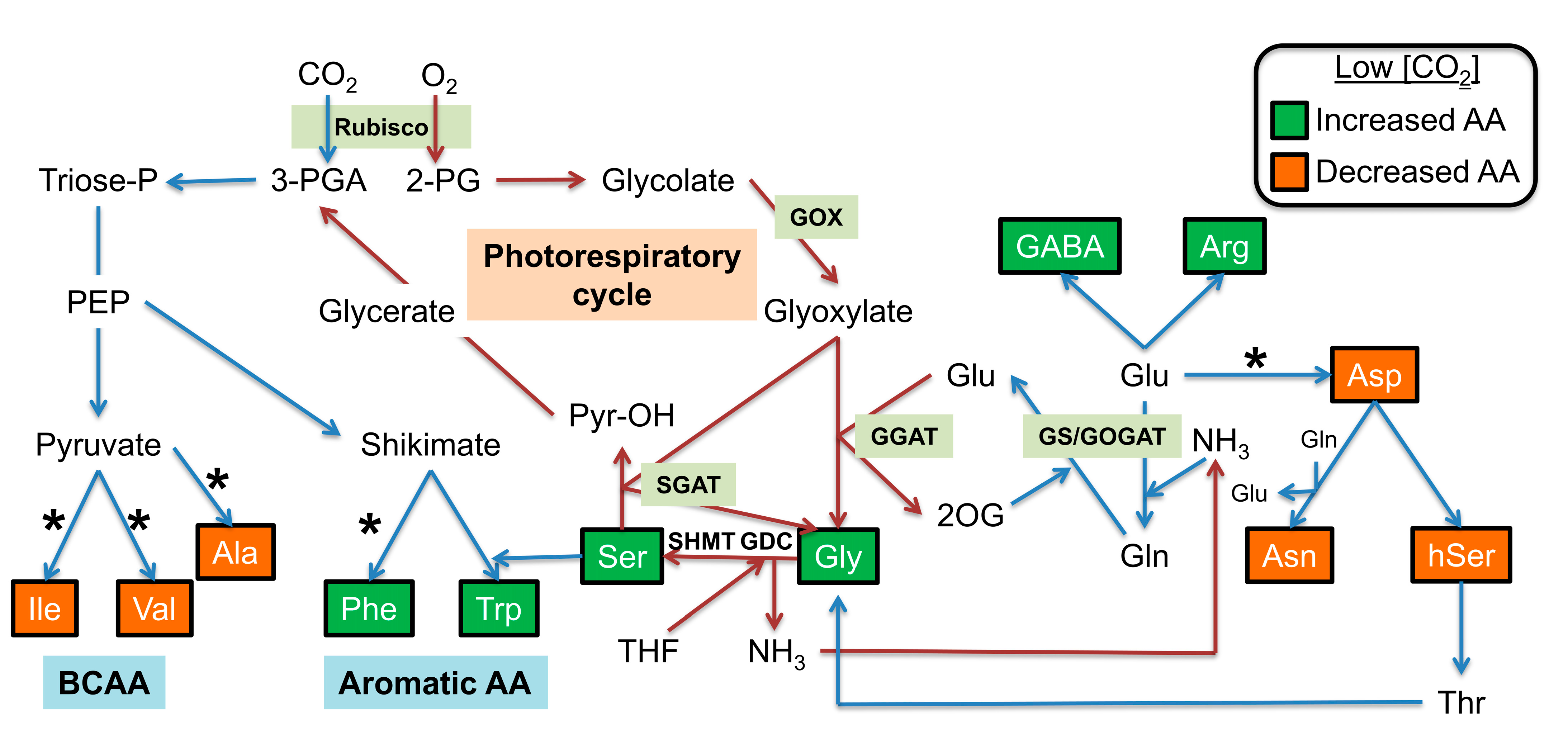
3.1. Minor Amino Acids: Arg, Aromatic and Branched-Chain Amino Acids under Low CO2 Conditions
3.2. Major Amino Acids under Low CO2 Conditions
3.3. Gly and Ser Become Major Amino Acids under Low CO2 Conditions and Accumulate More in Certain gox Mutants
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Low CO2 Exposure Experiments
4.3. Amino Acid Quantification
4.4. GOX Activity
4.5. Multivariate and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Caemmerer, S. Rubisco carboxylase/oxygenase: From the enzyme to the globe: A gas exchange perspective. J. Plant. Physiol. 2020, 252. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149. [Google Scholar] [CrossRef] [Green Version]
- Timm, S.; Hagemann, M. Photorespiration—How is it regulated and regulates overall plant metabolism? J. Exp. Bot. 2020, 71, 3955–3965. [Google Scholar] [CrossRef]
- Flugel, F.; Timm, S.; Arrivault, S.; Florian, A.; Stitt, M.; Fernie, A.R.; Bauwe, H. The photorespiratory metabolite 2-phosphoglycolate regulates photosynthesis and starch accumulation in arabidopsis. Plant Cell 2017, 29, 2537–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Berghuijs, H.N.C.; Yin, X.; Ho, Q.T.; Retta, M.A.; Nicolai, B.M.; Struik, P.C. Using a reaction-diffusion model to estimate day respiration and reassimilation of (photo)respired CO2 in leaves. New Phytol. 2019, 223, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Wallsgrove, R.; Turner, J.; Hall, N.; Kendall, A.; Bright, S. Barley mutants lacking chloroplast glutamine synthetasebiochemical and genetic analysis. Plant Physiol. 1987, 83, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Timm, S.; Bauwe, H. The variety of photorespiratory phenotypes—Employing the current status for future research directions on photorespiration. Plant Biol. 2013, 15, 737–747. [Google Scholar] [CrossRef]
- Timm, S.; Mielewczik, M.; Florian, A.; Frankenbach, S.; Dreissen, A.; Hocken, N.; Fernie, A.R.; Walter, A.; Bauwe, H. High-to-low CO2 acclimation reveals plasticity of the photorespiratory pathway and indicates regulatory links to cellular metabolism of arabidopsis. PLoS ONE 2012, 7, e42809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somerville, C.; Ogren, W. Photorespiration mutants of arabidopsis thaliana deficient in serine-glyoxylate aminotransferase activity. Proc. Natl. Acad. Sci. USA 1980, 77, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellero, Y.; Lamothe-Sibold, M.; Jossier, M.; Hodges, M. Arabidopsis thaliana ggt1 photorespiratory mutants maintain leaf carbon/nitrogen balance by reducing rubisco content and plant growth. Plant J. 2015, 83, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Dellero, Y.; Jossier, M.; Glab, N.; Oury, C.; Tcherkez, G.; Hodges, M. Decreased glycolate oxidase activity leads to altered carbon allocation and leaf senescence after a transfer from high CO2 to ambient air in arabidopsis thaliana. J. Exp. Bot. 2016, 67, 3149–3163. [Google Scholar] [CrossRef] [Green Version]
- Hodges, M.; Dellero, Y.; Keech, O.; Betti, M.; Raghavendra, A.S.; Sage, R.; Zhu, X.G.; Allen, D.K.; Weber, A.P. Perspectives for a better understanding of the metabolic integration of photorespiration within a complex plant primary metabolism network. J. Exp. Bot. 2016, 67, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Ros, R.; Munoz-Bertomeu, J.; Krueger, S. Serine in plants: Biosynthesis, metabolism, and functions. Trends Plant Sci. 2014, 19, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol. 2013, 15, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Bauwe, H.; Badger, M. Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem ii by suppression of repair but not acceleration of damage processes in arabidopsis. Plant Physiol. 2007, 144, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Blume, C.; Ost, J.; Muhlenbruch, M.; Peterhansel, C.; Laxa, M. Low CO2 induces urea cycle intermediate accumulation in arabidopsis thaliana. PLoS ONE 2019, 14, e0210342. [Google Scholar] [CrossRef]
- Abadie, C.; Bathellier, C.; Tcherkez, G. Carbon allocation to major metabolites in illuminated leaves is not just proportional to photosynthesis when gaseous conditions (CO2 and O2) vary. New Phytol. 2018, 218, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Abadie, C.; Lalande, J.; Limami, A.M.; Tcherkez, G. Non-targeted 13c metabolite analysis demonstrates broad re-orchestration of leaf metabolism when gas exchange conditions vary. Plant Cell Environ. 2020, 44, 13. [Google Scholar] [CrossRef]
- Timm, S.; Nunes-Nesi, A.; Parnik, T.; Morgenthal, K.; Wienkoop, S.; Keerberg, O.; Weckwerth, W.; Kleczkowski, L.A.; Fernie, A.R.; Bauwe, H. A cytosolic pathway for the conversion of hydroxypyruvate to glycerate during photorespiration in arabidopsis. Plant Cell 2008, 20, 2848–2859. [Google Scholar] [CrossRef] [Green Version]
- Timm, S.; Florian, A.; Jahnke, K.; Nunes-Nesi, A.; Fernie, A.R.; Bauwe, H. The hydroxypyruvate-reducing system in arabidopsis: Multiple enzymes for the same end. Plant Physiol. 2011, 155, 694–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, S. The impact of photorespiration on plant primary metabolism through metabolic and redox regulation. Biochem. Soc. Trans. 2020, 48, 2495–2504. [Google Scholar] [CrossRef]
- Esser, C.; Kuhn, A.; Groth, G.; Lercher, M.J.; Maurino, V.G. Plant and animal glycolate oxidases have a common eukaryotic ancestor and convergently duplicated to evolve long-chain 2-hydroxy acid oxidases. Mol. Biol. Evol. 2014, 31, 1089–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engqvist, M.K.; Schmitz, J.; Gertzmann, A.; Florian, A.; Jaspert, N.; Arif, M.; Balazadeh, S.; Mueller-Roeber, B.; Fernie, A.R.; Maurino, V.G. Glycolate oxidase3, a glycolate oxidase homolog of yeast l-lactate cytochrome c oxidoreductase, supports l-lactate oxidation in roots of arabidopsis. Plant Physiol. 2015, 169, 1042–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellero, Y.; Mauve, C.; Boex-Fontvieille, E.; Flesch, V.; Jossier, M.; Tcherkez, G.; Hodges, M. Experimental evidence for a hydride transfer mechanism in plant glycolate oxidase catalysis. J. Biol. Chem. 2015, 290, 1689–1698. [Google Scholar] [CrossRef] [Green Version]
- Gerhart, L.M.; Ward, J.K. Plant responses to low [CO2] of the past. New Phytol. 2010, 188, 674–695. [Google Scholar] [CrossRef]
- Brodribb, T. Dynamics of changing intercellular CO2 concentration (ci) during drought and determination of minimum functional ci. Plant Physiol. 1996, 111, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Pageau, K.; Lelandais, M.; Grandjean, O.; Kronenberger, J.; Valadier, M.H.; Feraud, M.; Jouglet, T.; Suzuki, A. Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol. 2006, 140, 444–456. [Google Scholar] [CrossRef] [Green Version]
- Gaufichon, L.; Masclaux-Daubresse, C.; Tcherkez, G.; Reisdorf-Cren, M.; Sakakibara, Y.; Hase, T.; Clement, G.; Avice, J.C.; Grandjean, O.; Marmagne, A.; et al. Arabidopsis thaliana asn2 encoding asparagine synthetase is involved in the control of nitrogen assimilation and export during vegetative growth. Plant Cell Environ. 2013, 36, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Timm, S.; Woitschach, F.; Heise, C.; Hagemann, M.; Bauwe, H. Faster removal of 2-phosphoglycolate through photorespiration improves abiotic stress tolerance of arabidopsis. Plants 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, C.M.; Senthil-Kumar, M.; Wang, K.; Ryu, C.M.; Kaundal, A.; Mysore, K.S. Glycolate oxidase modulates reactive oxygen species-mediated signal transduction during nonhost resistance in nicotiana benthamiana and arabidopsis. Plant Cell 2012, 24, 336–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Mauve, C.; Lamothe-Sibold, M.; Guerard, F.; Glab, N.; Hodges, M.; Jossier, M. Photorespiratory serine hydroxymethyltransferase 1 activity impacts abiotic stress tolerance and stomatal closure. Plant Cell Environ. 2019, 42, 17. [Google Scholar] [CrossRef]
- Novitskaya, L.; Trevanion, S.J.; Driscoll, S.; Foyer, C.H.; Noctor, G. How does photorespiration modulate leaf amino acid contents? A dual approach through modelling and metabolite analysis. Plant Cell Environ. 2002, 25, 15. [Google Scholar] [CrossRef] [Green Version]
- Abadie, C.; Boex-Fontvieille, E.R.; Carroll, A.J.; Tcherkez, G. In vivo stoichiometry of photorespiratory metabolism. Nat. Plants 2016, 2. [Google Scholar] [CrossRef]
- Abadie, C.; Lothier, J.; Boex-Fontvieille, E.; Carroll, A.; Tcherkez, G. Direct assessment of the metabolic origin of carbon atoms in glutamate from illuminated leaves using (13) c-nmr. New Phytol. 2017, 216, 1079–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadie, C.; Tcherkez, G. In vivo phosphoenolpyruvate carboxylase activity is controlled by CO2 and O2 mole fractions and represents a major flux at high photorespiration rates. New Phytol. 2019, 221, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Chrobok, D.; Law, S.R.; Brouwer, B.; Linden, P.; Ziolkowska, A.; Liebsch, D.; Narsai, R.; Szal, B.; Moritz, T.; Rouhier, N.; et al. Dissecting the metabolic role of mitochondria during developmental leaf senescence. Plant Physiol. 2016, 172, 2132–2153. [Google Scholar] [CrossRef] [Green Version]
- Dellero, Y.; Heuillet, M.; Marnet, N.; Bellvert, F.; Millard, P.; Bouchereau, A. Sink/source balance of leaves influences amino acid pools and their associated metabolic fluxes in winter oilseed rape (brassica napus l.). Metabolites 2020, 10, 150. [Google Scholar] [CrossRef]
- Dellero, Y. Manipulating amino acid metabolism to improve crop nitrogen use efficiency for a sustainable agriculture. Front. Plant Sci. 2020, 11, 1857. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araujo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Pires, M.V.; Pereira Junior, A.A.; Medeiros, D.B.; Daloso, D.M.; Pham, P.A.; Barros, K.A.; Engqvist, M.K.; Florian, A.; Krahnert, I.; Maurino, V.G.; et al. The influence of alternative pathways of respiration that utilize branched-chain amino acids following water shortage in arabidopsis. Plant Cell Environ. 2016, 39, 1304–1319. [Google Scholar] [CrossRef] [Green Version]
- Bloom, A.J.; Burger, M.; Asensio, J.S.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and arabidopsis. Science 2010, 328, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Lee, J.; Pandurangan, S.; Clarke, M.; Pajak, A.; Marsolais, F. Characterization of arabidopsis serine:Glyoxylate aminotransferase, agt1, as an asparagine aminotransferase. Phytochemistry 2013, 85, 30–35. [Google Scholar] [CrossRef]
- Clark, S.M.; Di Leo, R.; Dhanoa, P.K.; Van Cauwenberghe, O.R.; Mullen, R.T.; Shelp, B.J. Biochemical characterization, mitochondrial localization, expression, and potential functions for an arabidopsis gamma-aminobutyrate transaminase that utilizes both pyruvate and glyoxylate. J. Exp. Bot. 2009, 60, 1743–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, S.; Wittmiss, M.; Gamlien, S.; Ewald, R.; Florian, A.; Frank, M.; Wirtz, M.; Hell, R.; Fernie, A.R.; Bauwe, H. Mitochondrial dihydrolipoyl dehydrogenase activity shapes photosynthesis and photorespiration of arabidopsis thaliana. Plant Cell 2015, 27, 1968–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timm, S.; Florian, A.; Arrivault, S.; Stitt, M.; Fernie, A.R.; Bauwe, H. Glycine decarboxylase controls photosynthesis and plant growth. FEBS Lett. 2012, 586, 3692–3697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, V.; Brock Chatson, K.; Abrams, G.D.; King, J. 13c nuclear magnetic resonance detection of lnteractions of serine hydroxymethyltransferase with c1 -tetrahydrofoiate synthase and glycine decarboxylase complex activities in arabidopsis. Plant Physiol. 1996, 112, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modde, K.; Timm, S.; Florian, A.; Michl, K.; Fernie, A.R.; Bauwe, H. High serine:Glyoxylate aminotransferase activity lowers leaf daytime serine levels, inducing the phosphoserine pathway in arabidopsis. J. Exp. Bot. 2017, 68, 643–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havir, E.A.; McHale, N.A. A mutant of nicotiana sylvestris lacking serine:Glyoxylate aminotransferase. Plant Physiol. 1988, 87, 3. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.J.S.; Blackwell, R.D.; Joy, K.W.; Lea, P.J. Photorespiratory n donors, aminotransferase specificity and photosynthesis in a mutant of barley deficient in serine: Glyoxylate aminotransferase activity. Planta 1987, 172, 8. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Ann, V.J.; Lea, P.J.; Leegood, R.C. Serine: Glyoxylate aminotransferase exerts no control on photosynthesis. J. Exp. Bot. 1999, 50, 719–722. [Google Scholar] [CrossRef]
- Igarashi, D.; Tsuchida, H.; Miyao, M.; Ohsumi, C. Glutamate:Glyoxylate aminotransferase modulates amino acid content during photorespiration. Plant Physiol. 2006, 142, 901–910. [Google Scholar] [CrossRef] [Green Version]
- Oliver, D.J.; Sarojini, G. Regulation of Glycine Decarboxylase by Serine. In Progress in Photosynthesis Research; Springer: Dordrecht, The Netherlands, 1987; pp. 573–576. [Google Scholar] [CrossRef]
- Liepman, A.H.; Olsen, L.J. Peroxisomal alanine: Glyoxylate aminotransferase (agt1) is a photorespiratory enzyme with multiple substrates in arabidopsis thaliana. Plant J. 2001, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Kendziorek, M.; Paszkowski, A. Properties of serine:Glyoxylate aminotransferase purified from arabidopsis thaliana leaves. Acta Biochim. Biophys. Sin. 2008, 40, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Liepman, A.H.; Olsen, L.J. Alanine aminotransferase homologs catalyze the glutamate:Glyoxylate aminotransferase reaction in peroxisomes of arabidopsis. Plant Physiol. 2003, 131, 215–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Mao, X.; Ou, J.; Ye, N.; Zhang, J.; Peng, X. Distinct photorespiratory reactions are preferentially catalyzed by glutamate:Glyoxylate and serine:Glyoxylate aminotransferases in rice. J. Photochem. Photobiol. B 2015, 142, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Laubengayer, K.M.; Schauer, N.; Fernie, A.R.; Jander, G. Two arabidopsis threonine aldolases are nonredundant and compete with threonine deaminase for a common substrate pool. Plant Cell 2006, 18, 3564–3575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plant Metabolics Network. 2021. Available online: https://pmn.Plantcyc.Org/plant/new-image?Type=pathway&object=glysyn2-pwyonwww.plantcyc.org (accessed on 30 July 2021).
- Toujani, W.; Munoz-Bertomeu, J.; Flores-Tornero, M.; Rosa-Tellez, S.; Anoman, A.D.; Alseekh, S.; Fernie, A.R.; Ros, R. Functional characterization of the plastidial 3-phosphoglycerate dehydrogenase family in arabidopsis. Plant Physiol. 2013, 163, 1164–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascales-Minana, B.; Munoz-Bertomeu, J.; Flores-Tornero, M.; Anoman, A.D.; Pertusa, J.; Alaiz, M.; Osorio, S.; Fernie, A.R.; Segura, J.; Ros, R. The phosphorylated pathway of serine biosynthesis is essential both for male gametophyte and embryo development and for root growth in arabidopsis. Plant Cell 2013, 25, 2084–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benstein, R.M.; Ludewig, K.; Wulfert, S.; Wittek, S.; Gigolashvili, T.; Frerigmann, H.; Gierth, M.; Flugge, U.I.; Krueger, S. Arabidopsis phosphoglycerate dehydrogenase1 of the phosphoserine pathway is essential for development and required for ammonium assimilation and tryptophan biosynthesis. Plant Cell 2013, 25, 5011–5029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastmond, P.J.; Graham, I.A. Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci. 2001, 6, 487–498. [Google Scholar] [CrossRef]
- Charlton, W.L.; Johnson, B.; Graham, I.A.; Baker, A. Non-coordinate expression of peroxisome biogenesis, beta-oxidation and glyoxylate cycle genes in mature arabidopsis plants. Plant Cell Rep. 2005, 23, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.J.; Zelitch, I. Increasing photosynthesis by inhibiting photorespiration with glyoxylate. Science 1977, 196, 1450–1451. [Google Scholar] [CrossRef]
- Noctor, G.; Bergot, G.; Mauve, C.; Thominet, D.; Lelarge-Trouverie, C.; Prioul, J.-L. A comparative study of amino acid measurement in leaf extracts by gas chromatography-time of flight-mass spectrometry and high performance liquid chromatography with fluorescence detection. Metabolomics 2007, 3, 161–174. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. Factominer: An r package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Rohart, F.; Gautier, B.; Singh, A.; Le Cao, K.A. Mixomics: An r package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dellero, Y.; Mauve, C.; Jossier, M.; Hodges, M. The Impact of Photorespiratory Glycolate Oxidase Activity on Arabidopsis thaliana Leaf Soluble Amino Acid Pool Sizes during Acclimation to Low Atmospheric CO2 Concentrations. Metabolites 2021, 11, 501. https://doi.org/10.3390/metabo11080501
Dellero Y, Mauve C, Jossier M, Hodges M. The Impact of Photorespiratory Glycolate Oxidase Activity on Arabidopsis thaliana Leaf Soluble Amino Acid Pool Sizes during Acclimation to Low Atmospheric CO2 Concentrations. Metabolites. 2021; 11(8):501. https://doi.org/10.3390/metabo11080501
Chicago/Turabian StyleDellero, Younès, Caroline Mauve, Mathieu Jossier, and Michael Hodges. 2021. "The Impact of Photorespiratory Glycolate Oxidase Activity on Arabidopsis thaliana Leaf Soluble Amino Acid Pool Sizes during Acclimation to Low Atmospheric CO2 Concentrations" Metabolites 11, no. 8: 501. https://doi.org/10.3390/metabo11080501
APA StyleDellero, Y., Mauve, C., Jossier, M., & Hodges, M. (2021). The Impact of Photorespiratory Glycolate Oxidase Activity on Arabidopsis thaliana Leaf Soluble Amino Acid Pool Sizes during Acclimation to Low Atmospheric CO2 Concentrations. Metabolites, 11(8), 501. https://doi.org/10.3390/metabo11080501







