Metabolic Profiling of Plasma in Patients with Irritable Bowel Syndrome after a 4-Week Starch- and Sucrose-Reduced Diet
Abstract
:1. Introduction
2. Results
2.1. Subject Characteristics
2.2. Clinical and Nutritional Effects of the SSRD Diet
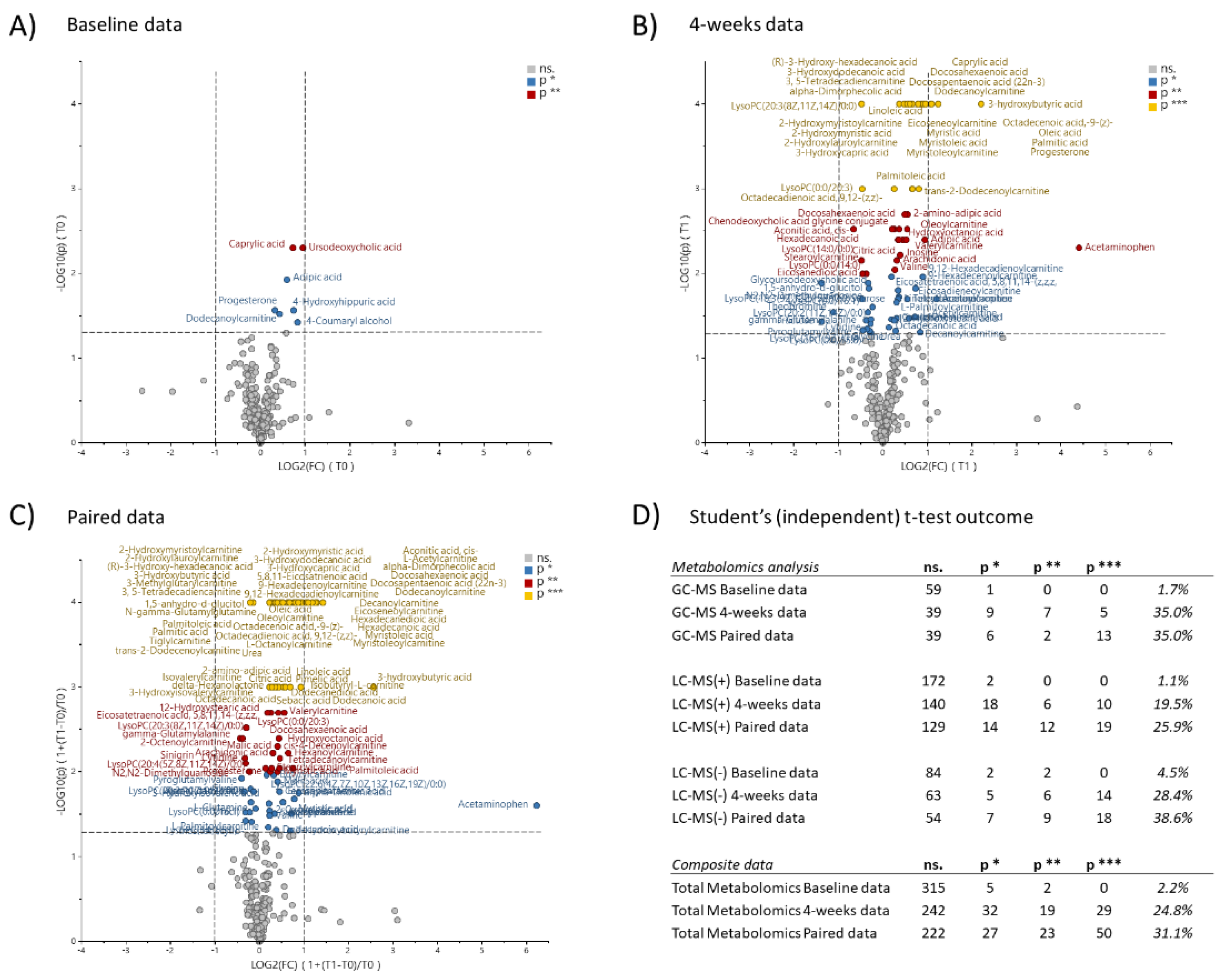
2.3. Metabolomic Effects of the SSRD Diet
2.3.1. Multivariate Analysis
2.3.2. Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Patients
4.3. Dietary Advice
4.4. Questionnaires
4.4.1. Study Questionnaire
4.4.2. Food Diaries
4.4.3. Rome IV Questionnaire
4.4.4. Irritable Bowel Syndrome-Symptom Severity Score
4.4.5. The Visual Analog Scale for Irritable Bowel Syndrome Questionnaire
4.5. Metabolomics
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, H.; Porter, J.; Gibson, P.R.; Barrett, J.; Garg, M. Review article: Implementation of a diet low in FODMAPs for patients with irritable bowel syndrome-directions for future research. Aliment. Pharmacol. Ther. 2019, 49, 124–139. [Google Scholar] [CrossRef] [Green Version]
- Nilholm, C.; Larsson, E.; Roth, B.; Gustafsson, R.; Ohlsson, B. Irregular Dietary Habits with a High Intake of Cereals and Sweets Are Associated with More Severe Gastrointestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1279. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.T.; Liu, S.; Li, H.C.; Zhang, Z.; Zhang, Q.; Chen, L.; Zhao, Y.; Chen, Y.; Gu, J.C.; Min, L.; et al. Identification of Gut Microbiota and Metabolites Signature in Patients With Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, V.; Homer, D.; Rigsbee, L.; Khamis, H.J.; Michail, S.; Raymer, M.; Reo, N.V.; Paliy, O. The networks of human gut microbe-metabolite associations are different between health and irritable bowel syndrome. ISME J. 2015, 9, 1899–1903. [Google Scholar] [CrossRef] [Green Version]
- Ponnusamy, K.; Choi, J.N.; Kim, J.; Lee, S.Y.; Lee, C.H. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J. Med. Microbiol. 2011, 60, 817–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Z.Y.; Xiong, L.S.; Zhuang, X.J.; Luo, M. The propionic acid and butyric acid in serum but not in faeces are increased in patients with diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2020, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Tana, C.; Umesaki, Y.; Imaoka, A.; Handa, T.; Kanazawa, M.; Fukudo, S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol. Motil. 2010, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Mujagic, Z.; Tigchelaar, E.F.; Zhernakova, A.; Ludwig, T.; Ramiro-Garcia, J.; Baranska, A.; Swertz, M.A.; Masclee, A.A.M.; Wijmenga, C.; van Schooten, F.J.; et al. A novel biomarker panel for irritable bowel syndrome and the application in the general population. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.X.; Zhang, Y.; Qin, G.; Li, K.M.; Wei, W.; Li, S.Y.; Yao, S.K. Altered profiles of fecal metabolites correlate with visceral hypersensitivity and may contribute to symptom severity of diarrhea-predominant irritable bowel syndrome. World J. Gastroenterol. 2019, 25, 6416–6429. [Google Scholar] [CrossRef]
- Miller, J.S.; Rodriguez-Saona, L.; Hackshaw, K.V. Metabolomics in Central Sensitivity Syndromes. Metabolites 2020, 10, 164. [Google Scholar] [CrossRef]
- Miles-Chan, J.L.; Dulloo, A.G.; Schutz, Y. Fasting substrate oxidation at rest assessed by indirect calorimetry: Is prior dietary macronutrient level and composition a confounder? Int. J. Obes. 2015, 39, 1114–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stellingwerff, T.; Spriet, L.L.; Watt, M.J.; Kimber, N.E.; Hargreaves, M.; Hawley, J.A.; Burke, L.M. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E380–E388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, M.; Puntschart, A.; Howald, H.; Mueller, B.; Mannhart, C.; Gfeller-Tuescher, L.; Mullis, P.; Hoppeler, H. Effects of dietary fat on muscle substrates, metabolism, and performance in athletes. Med. Sci. Sports Exerc. 2003, 35, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Matsuo, T. Effects of Short-Term Dietary Change from High-Carbohydrate Diet to High-Fat Diet on Storage, Utilization, and Fatty Acid Composition of Rat Muscle Triglyceride during Swimming Exercise. J. Clin. Biochem. Nutr. 2009, 44, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Pilegaard, H.; Keller, C.; Steensberg, A.; Helge, J.W.; Pedersen, B.K.; Saltin, B.; Neufer, P.D. Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J. Physiol. 2002, 541, 261–271. [Google Scholar] [CrossRef]
- Spriet, L.L. New Insights into the Interaction of Carbohydrate and Fat Metabolism During Exercise. Sports Med. 2014, 44, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noorbakhsh, H.; Yavarmanesh, M.; Mortazavi, S.A.; Adibi, P.; Moazzami, A.A. Metabolomics analysis revealed metabolic changes in patients with diarrhea-predominant irritable bowel syndrome and metabolic responses to a synbiotic yogurt intervention. Eur. J. Nutr. 2019, 58, 3109–3119. [Google Scholar] [CrossRef]
- James, S.C.; Fraser, K.; Young, W.; McNabb, W.C.; Roy, N.C. Gut Microbial Metabolites and Biochemical Pathways Involved in Irritable Bowel Syndrome: Effects of Diet and Nutrition on the Microbiome. J. Nutr. 2020, 150, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Nilholm, C.; Roth, B.; Ohlsson, B. A Dietary Intervention with Reduction of Starch and Sucrose Leads to Reduced Gastrointestinal and Extra-Intestinal Symptoms in IBS Patients. Nutrients 2019, 11, 1662. [Google Scholar] [CrossRef] [Green Version]
- Nilholm, C.; Larsson, E.; Sonestedt, E.; Roth, B.; Ohlsson, B. Assessment of a 4-Week Starch- and Sucrose-Reduced Diet and Its Effects on Gastrointestinal Symptoms and Inflammatory Parameters among Patients with Irritable Bowel Syndrome. Nutrients 2021, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Choosing Your Foods—A Basic Dietary Guide for People with CSID. Available online: https://www.sucroseintolerance.com/choosing-your-foods (accessed on 27 May 2021).
- The National Food Agency, S. The AIVO Diet Computer Program from the National Food Agency. Available online: https://www.mashie.com/sv/loesningar/vaara-produkter/aivo/ (accessed on 30 November 2020).
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtsson, M.; Ohlsson, B.; Ulander, K. Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS-IBS). BMC Gastroenterol. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.R.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Jewison, T.; Su, Y.L.; Disfany, F.M.; Liang, Y.J.; Knox, C.; Maciejewski, A.; Poelzer, J.; Huynh, J.; Zhou, Y.; Arndt, D.; et al. SMPDB 2.0: Big Improvements to the Small Molecule Pathway Database. Nucleic Acids Res. 2014, 42, D478–D484. [Google Scholar] [CrossRef] [Green Version]
- Chickos, J.S.; Way, B.A.; Wilson, J.; Shaharuzzaman, M.; Laird, J.; Landt, M. Analysis of 3-hydroxydodecanedioic acid for studies of fatty acid metabolic disorders: Preparation of stable isotope standards. J. Clin. Lab. Anal. 2002, 16, 115–120. [Google Scholar] [CrossRef]
- Cansu, A.; Serdaroglu, A.; Biberoglu, G.; Tumer, L.; Hirfanoglu, T.L.; Ezgu, F.S.; Hasanoglu, A. Analysis of acylcarnitine levels by tandem mass spectrometry in epileptic children receiving valproate and oxcarbazepine. Epileptic Disord. 2011, 13, 394–400. [Google Scholar] [CrossRef]
- Cozma-Petrut, A.; Loghin, F.; Miere, D.; Dumitrascu, D.L. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J. Gastroenterol. 2017, 23, 3771–3783. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Whelan, K. The low FODMAP diet: Recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017, 66, 1517–1527. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef]
- Camilleri, M.; Lasch, K.; Zhou, W. Irritable Bowel Syndrome: Methods, Mechanisms, and Pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G775–G785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, P.S.A.; Luben, R.; Shrestha, S.S.; Khaw, K.T.; Hart, A.R. Dietary arachidonic and oleic acid intake in ulcerative colitis etiology: A prospective cohort study using 7-day food diaries. Eur. J. Gastroenterol. Hepatol. 2014, 26, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hontecillas, R.; Wannemeulher, M.J.; Zimmerman, D.R.; Hutto, D.L.; Wilson, J.H.; Ahn, D.U.; Bassaganya-Riera, J. Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. J. Nutr. 2002, 132, 2019–2027. [Google Scholar] [CrossRef]
- Palsson, O.S.; Whitehead, W.E.; van Tilburg, M.A.L.; Chang, L.; Chey, W.; Crowell, M.D.; Keefer, L.; Lembo, A.J.; Parkman, H.P.; Rao, S.S.C.; et al. Development and Validation of the Rome IV Diagnostic Questionnaire for Adults. Gastroenterology 2016, 150, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, A.; Forshed, J.; Nordstrom, A. Overlap in serum metabolic profiles between non-related diseases: Implications for LC-MS metabolomics biomarker discovery. Biochem. Biophys. Res. Commun. 2016, 478, 1472–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diab, J.; Hansen, T.; Goll, R.; Stenlund, H.; Jensen, E.; Moritz, T.; Florholmen, J.; Forsdahl, G. Mucosal Metabolomic Profiling and Pathway Analysis Reveal the Metabolic Signature of Ulcerative Colitis. Metabolites 2019, 9, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.Z.; Bourque, G.; Wishart, D.S.; Xia, J.G. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]


| Control Group (n = 22) | Intervention Group (n = 69) | Sig. (p) | ||||||
|---|---|---|---|---|---|---|---|---|
| Age (year) | 41.65 ± 15.03 | 48.49 ± 12.51 | 0.058 | ns. | ||||
| Disease duration (year) | 18.81 ± 13.83 | 21.33 ± 13.84 | 0.523 | ns. | ||||
| Women (n, %) | 19 (86.4) | 52 (75.4) | 0.381 | ns. | ||||
| Active Smokers (n, (%)) | 4 (18.2) | 5 (7.2) | 0.212 | ns. | ||||
| Diseases (n, %) | ||||||||
| Allergy | 5 (22.7) | 9 (13.0) | 0.314 | ns. | ||||
| Hypothyroid disease | 4 (18.2) | 6 (8.7) | 0.247 | ns. | ||||
| Asthma bronchialis | 3 (13.6) | 8 (11.6) | 0.723 | ns. | ||||
| Hypertension | 3 (13.6) | 7 (10.1) | 0.699 | ns. | ||||
| Depression | 2 (9.1) | 5 (7.2) | 0.674 | ns. | ||||
| Migraine | 0 (0) | 4 (5.8) | 0.569 | ns. | ||||
| Drug treatment (n, (%)) | ||||||||
| Antidepressants | 4 (18.2) | 11 (15.9) | 0.752 | ns. | ||||
| Levaxine | 4 (18.2) | 7 (10.1) | 0.451 | ns. | ||||
| Vitamin D | 4 (18.2) | 7 (10.1) | 0.451 | ns. | ||||
| Asthma inhalators | 3 (13.6) | 3 (4.3) | 0.150 | ns. | ||||
| Laxatives | 2 (9.1) | 9 (13.0) | 1 | ns. | ||||
| Proton pump inhibitor | 2 (9.1) | 9 (13.0) | 1 | ns. | ||||
| Hormonal treatment | 2 (9.1) | 6 (8.7) | 1 | ns. | ||||
| Folic acid | 2 (9.1) | 3 (4.3) | 0.591 | ns. | ||||
| Cobalamin | 2 (9.1) | 3 (4.3) | 0.591 | ns. | ||||
| Statins | 1 (4.5) | 5 (7.2) | 1 | ns. | ||||
| Physical activity (n, (%)) | 0.730 | ns. | ||||||
| No activity | 1 (4.5) | 9 (13.0) | ||||||
| <30 min | 5 (22.7) | 15 (21.7) | ||||||
| 30–60 min | 4 (18.2) | 11 (15.9) | ||||||
| 60–90 min | 3 (13.6) | 6 (8.7) | ||||||
| 90–120 min | 2 (9.1) | 12 (17.4) | ||||||
| >120 min | 7 (31.8) | 16 (23.2) | ||||||
| Baseline data | 4-week data | Paired data | Baseline data | 4-week data | Paired data | |||
| BMI (kg/m2) | 1.02 ± 0.06 | 0.972 ± 0.023 | 0.002 | (**) | ||||
| 25.39 ± 3.75 | 25.32 ± 4.54 | 0.945 | ns. | |||||
| 25.1 ± 3.87 | 25.9 ± 4.597 | 0.471 | ns. | |||||
| Food diaries (AIVO) | ||||||||
| Carbohydrates (g) | 0.921 ± 0.49 | 0.599 ± 0.388 | 0.002 | (**) | ||||
| 194.9 ± 78.31 | 169.3 ± 79.7 | 193.9 ± 64.52 | 104 ± 55.03 | |||||
| Starch (g) | 1.178 ± 0.973 | 0.447 ± 0.542 | 0.002 | (**) | ||||
| 79.34 ± 41.35 | 73.84 ± 43.25 | 82.62 ± 42.11 | 30.87 ± 31.67 | |||||
| Disaccharides (g) | 0.965 ± 0.708 | 0.544 ± 0.466 | 0.014 | (*) | ||||
| 45.53 ± 36.5 | 35.83 ± 23.93 | 41.76 ± 23.56 | 18.98 ± 15.4 | |||||
| Sucrose (g) | 0.966 ± 0.902 | 0.524 ± 0.851 | 0.040 | (*) | ||||
| 34.42 ± 34.41 | 22.66 ± 17.24 | 29.68 ± 21.28 | 10.15 ± 12.79 | |||||
| Fat (g) | 1.182 ± 0.537 | 1.384 ± 0.843 | 0.190 | ns. | ||||
| 65.35 ± 27.67 | 70.74 ± 28.2 | 73.12 ± 36.42 | 85.16 ± 45.67 | |||||
| Monounsaturated fat (g) | 1.253 ± 0.783 | 1.595 ± 1.367 | 0.278 | ns. | ||||
| 24.45 ± 11.22 | 26.49 ± 12.37 | 27.76 ± 14.62 | 33.90 ± 22.25 | |||||
| Saturated fat (g) | 1.148 ± 0.643 | 1.326 ± 0.928 | 0.415 | ns. | ||||
| 27.35 ± 13.63 | 28.77 ± 15.96 | 27.63 ± 16.65 | 29.07 ± 19.55 | |||||
| Monosaccharides (g) | 1.015 ± 0.885 | 1.295 ± 1.526 | 0.415 | ns. | ||||
| 24.66 ± 12.83 | 20.19 ± 14.53 | 24.81 ± 14.23 | 22.02 ± 16.62 | |||||
| Fibre (g) | 0.976 ± 0.361 | 1.06 ± 0.59 | 0.427 | ns. | ||||
| 17.46 ± 6.99 | 16.92 ± 9.27 | 20.45 ± 10.34 | 18.36 ± 8.09 | |||||
| Energy (kcal) | 1.001 ± 0.341 | 0.941 ± 0.372 | 0.502 | ns. | ||||
| 1696 ± 528.4 | 1641 ± 538.5 | 1797 ± 594.9 | 1569 ± 549.6 | |||||
| Protein (g) | 1.198 ± 0.553 | 1.253 ± 0.579 | 0.692 | ns. | ||||
| 64.91 ± 27.52 | 70.61 ± 24.72 | 72.89 ± 24.32 | 82.93 ± 30.02 | |||||
| Polyunsaturated fat (g) | 1.442 ± 0.748 | 1.535 ± 1.025 | 0.701 | ns. | ||||
| 8.150 ± 3.580 | 10.00 ± 3.761 | 10.74 ± 5.303 | 13.64 ± 8.051 | |||||
| Sugars (g) | 0.893 ± 0.578 | 0.838 ± 0.697 | 0.737 | ns. | ||||
| 73.62 ± 44.59 | 58.78 ± 38.52 | 71.53 ± 37.54 | 47.45 ± 32.44 | |||||
| Alcohol (g) | 0.862 ± 0.317 | 0.846 ± 0.395 | 0.867 | ns. | ||||
| 5.386 ± 13.17 | 2.914 ± 5.629 | 4.235 ± 8.82 | 3.648 ± 12.92 | |||||
| Questionnaire (IBS-SSS, VAS-IBS) | ||||||||
| Total IBS-SSS | 0.965 ± 0.223 | 0.561 ± 0.352 | <0.001 | (***) | ||||
| 288.6 ± 78.14 | 168.5 ± 104.4 | <0.001 | (***) | |||||
| 302.1 ± 62.59 | 301.1 ± 73.5 | 0.953 | ns. | |||||
| Abdominal pain | 1.044 ± 0.377 | 0.58 ± 0.485 | <0.001 | (***) | ||||
| 50.73 ± 22.88 | 49.55 ± 20.19 | 49.28 ± 20.44 | 27.29 ± 24.07 | |||||
| Bloating and flatulence | 0.843 ± 0.246 | 0.501 ± 0.436 | <0.001 | (***) | ||||
| 76.86 ± 21.61 | 65 ± 22.64 | 68.71 ± 25.12 | 33.74 ± 27.91 | |||||
| Intestinal symptoms influence on daily life | 1.01 ± 0.274 | 0.664 ± 0.58 | <0.001 | (***) | ||||
| 65.05 ± 16.21 | 64 ± 19.1 | 65.99 ± 22.53 | 39.5 ± 27.36 | |||||
| Diarrhea | 1.086 ± 1.188 | 0.491 ± 0.523 | 0.032 | (*) | ||||
| 35.86 ± 32.22 | 27.55 ± 28.19 | 47 ± 31.92 | 21.61 ± 25.21 | |||||
| Constipation | 0.775 ± 0.661 | 0.565 ± 0.421 | 0.082 | ns. | ||||
| 49.05 ± 28.09 | 32.77 ± 32.14 | 41.62 ± 35.15 | 22.28 ± 24.05 | |||||
| Vomiting and nausea | 1.062 ± 1.866 | 0.748 ± 0.914 | 0.29 | ns. | ||||
| 27.91 ± 23.9 | 23.91 ± 27.5 | 23.23 ± 26.28 | 13.87 ± 21.06 | |||||
| Psychological well-being | 1.152 ± 0.732 | 1.068 ± 1.967 | 0.845 | ns. | ||||
| 47.32 ± 26.51 | 45.27 ± 21.6 | 47.13 ± 25.5 | 35.97 ± 24.63 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenlund, H.; Nilholm, C.; Chorell, E.; Roth, B.; D’Amato, M.; Ohlsson, B. Metabolic Profiling of Plasma in Patients with Irritable Bowel Syndrome after a 4-Week Starch- and Sucrose-Reduced Diet. Metabolites 2021, 11, 440. https://doi.org/10.3390/metabo11070440
Stenlund H, Nilholm C, Chorell E, Roth B, D’Amato M, Ohlsson B. Metabolic Profiling of Plasma in Patients with Irritable Bowel Syndrome after a 4-Week Starch- and Sucrose-Reduced Diet. Metabolites. 2021; 11(7):440. https://doi.org/10.3390/metabo11070440
Chicago/Turabian StyleStenlund, Hans, Clara Nilholm, Elin Chorell, Bodil Roth, Mauro D’Amato, and Bodil Ohlsson. 2021. "Metabolic Profiling of Plasma in Patients with Irritable Bowel Syndrome after a 4-Week Starch- and Sucrose-Reduced Diet" Metabolites 11, no. 7: 440. https://doi.org/10.3390/metabo11070440
APA StyleStenlund, H., Nilholm, C., Chorell, E., Roth, B., D’Amato, M., & Ohlsson, B. (2021). Metabolic Profiling of Plasma in Patients with Irritable Bowel Syndrome after a 4-Week Starch- and Sucrose-Reduced Diet. Metabolites, 11(7), 440. https://doi.org/10.3390/metabo11070440







